Abstract
Nitric oxide (NO) and NO-derived reactive nitrogen species (RNS) are present in the food vacuole (FV) of P. falciparum trophozoites. The product of PFL1555w, a putative cytochrome b5, localizes in the FV membrane, similar to what was previously observed for the product of PF13_0353, a putative cytochrome b5 reductase. These two gene products may contribute to NO generation by denitrification chemistry from nitrate and/or nitrite present in the erythrocyte cytosol. The possible coordination of NO to heme species present in the food vacuole was probed by resonance Raman spectroscopy. The spectroscopic data revealed that in situ generated NO interacts with heme inside the intact FVs to form ferrous heme nitrosyl complexes that influence intra-vacuolar heme solubility. The formation of heme nitrosyl complexes within the FV is a previously unrecognized factor that could affect the equilibrium between soluble and crystallized heme within the FV in vivo.
Keywords: Protozoa, Plasmodium falciparum, Malaria, Nitric oxide, Heme, Hemozoin, Antimalarials
Introduction
Malaria infection affects 40% of the world's population, mostly those living in the poorest countries. In 2006 alone, there were an estimated 247 million cases of malaria infection that caused nearly a million deaths worldwide (Mayor, 2008).
Although biochemical processes in the malaria parasite Plasmodium falciparum have been the object of intense scrutiny for more than a century, little is known about the biochemistry of nitric oxide (NO) in this organism. In our previous study, we established that the food vacuole (FV), also called digestive vacuole, is a site of NO-derived reactive nitrogen species (RNS) activity (Ostera et al., 2008) and reported the localization of a putative NADH-cytochrome b5 reductase associated with this organelle. We report here the identification of a potential molecular partner of the NADH-cytochrome b5 reductase, a putative cytochrome b5, which also localizes at the membrane of trophozoite FVs.
P. falciparum parasites are likely to generate NO and RNS from nitrate and/or nitrite that are abundantly present in the erythrocyte cytosol and may reach the FV during the process of hemoglobin uptake. Understanding the chemical interactions of NO with other molecules in the FV environment was one of the goals of this study, since the possible role of this radical in the malaria parasite is not known. Accordingly, we examined the direct coordination of NO to heme iron centers in heme species present in FVs isolated from P. falciparum 3D7 trophozoites.
Because of the well-known coordination chemistry of NO with heme, the presence of bioactive NO and NO-derived RNS in an organelle that contains a large quantity of heme raises the question of whether NO plays a defining role in the intravacuolar heme speciation. To investigate the molecular interactions of NO generated in P. falciparum parasites, we obtained Soret-excited resonance Raman (rR) spectra of isolated intact FVs. These experiments probed all heme species within the FVs, including soluble and insoluble forms of free heme as well as prosthetic hemes in any proteins associated with the FV. We found that NO diffusing into intact FVs formed nitrosyl complexes by directly coordinating with heme iron centers. Moreover, the rR spectra revealed that the reaction between hemozoin and NO disrupted the mutual heme coordination characteristic of the dimeric ferriprotoporphyrin IX, the building blocks of hemozoin. This behavior suggests that NO could form coordination complexes with vacuolar heme in live parasites as well. Thus, it gives rise to the hypothesis that in vivo NO might be one of the factors affecting the equilibrium between soluble and crystallized heme in the FV.
Materials and Methods
P. falciparum cultures
O+ erythrocytes for culture were processed using leukocyte reduction filters (Sepacell R-500, Baxter, Deerfield, IL). Washed erythrocytes were suspended in RPMI 1640 and stored at 4 °C. 3D7 parasites were used in all experiments. Asexual forms of the parasite were grown at either 5% or 2.5% hematocrit in RPMI 1640 medium (leukocyte filtered human O+ RBC) in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 0.5% Albumax II (Invitrogen, Carlsbad, CA), 2 mg/ml sodium bicarbonate (Gibco, Invitrogen, Carlsbad, CA), 0.10 mM hypoxanthine (Sigma-Aldrich, St Louis, MO), 25 mM HEPES pH 7.4 (Calbiochem, EMD Chemicals, Inc., Gibbstown, NJ) and 10 mg/L Gentamicin (Gibco, Invitrogen, Carlsbad, CA) at 37 °C in a 5% O2, 5% CO2, 90% N2 atmosphere. Gametocyte cultures were initiated at 6% hematocrit and 1% parasitemia (mixed stages) until parasites exhibited stress and initiated switching to gametocyte stages (approximately 4 days) after which the hematocrit was dropped to 3.6%. Gametocyte cultures were maintained for up to 17 days.
Investigation of NOS activity using [3H] L-arginine
NOS activity in 3D7 P. falciparum was measured using the NOSdetect Assay Kit (Stratagene, La Jolla, CA). Whole parasite experiments were performed with 2.6 × 107 magnetically purified trophozoites, obtained from approximately 10 mL of parasite culture. Parasite homogenates were obtained from 50 mL of Percol-sorbitol purified cultures, resuspended in the homogenization buffer supplied in the NOS activity kit and immersed for 30 seconds in a water-ice bath fitted with an ultrasonic probe. Protein concentration used in these assays was adjusted according to kit manufacturer's suggestion. [3H] L-arginine (1.0 mCi/mL) was purchased from Amersham (GE Healthcare UK Limited, Buckinghamshire, England). Rat cerebellum extract was used as positive control of NOS activity, with and without the addition of the NOS inhibitor N-nitro-L-arginine methyl ester (L-NAME) at the concentration suggested by the kit's manufacturer.
Preparation of antisera by DNA immunization
A previously published procedure for antisera production by DNA immunization (Oliveira et al., 2006) was followed. Briefly, cDNA from PFL1555w coding regions were cloned into the vector VR2001-TOPO (Vical, Inc. San Diego, CA). Constructs were sequenced to verify correct direction of the inserted coding sequences. DNA from selected plasmids was amplified, purified, concentrated and sterilized. Plasmid preparations were used to immunize Swiss Webster mice. Immune antisera were collected after a minimum of three consecutive immunizations, allowing a minimum of 2 weeks between immunizations.
Immunoblot analysis
A 3D7 P. falciparum protein extract was prepared using our published protocol (Ostera, et al., 2008). A 200-μg aliquot of this protein extract was loaded in a single well of a 2-D Bis-Tris SDS gel (Invitrogen, Carlsbad, CA) with MES running buffer under reducing conditions. After protein transfer, the membrane was blocked overnight with TBS/ 0.05% Tween in milk at 4 °C. Individual sera from different mice immunized with the PFL1555w constructs were incubated at a 1/50 dilution using a Mini-Protean Multi Screen (Bio-Rad, Hercules, CA). The membrane was then washed and incubated with a 1/10,000 dilution of anti-mouse AP-conjugated antibody (Zymed, San Francisco, CA) and developed with Western Blue alkaline phosphatase substrate (Promega, Madison, WI).
Immunofluorescence assays
Magnetically purified P. falciparum trophozoites were attached to coverslips, fixed and stained according to the published procedure (Ostera et al., 2008). Intra-erythrocytic parasites were reacted with a 1/25 dilution of immune antisera raised against PFL1555w coding sequences in a humid chamber at 37 °C for 1 hr. Goat anti-mouse IgG conjugated as Alexa 633 (Invitrogen, Carlsbad, CA) was used as secondary antibody at a 1/1000 dilution. Images were collected using a TCS-SP2 AOBS confocal microscope (Leica Microsystems GmbH) using a 63× oil immersion objective.
P. falciparum proliferation under limiting nitrate concentration
Custom-made RPMI 1640 without calcium nitrate was purchased from (Invitrogen, Carlsbad, CA). Calcium chloride (J.T. Baker Chemical Co., Phillipsburg, NJ) and sodium nitrate (Sigma-Aldrich, St. Louis, MO) were used to supplement the custom-made RPMI whenever necessary. Trophozoites were purified by magnetic separation using MACS separation columns (Myltenyi Biotech, Auburn, CA) and incubated with custom made RPMI with or without calcium chloride and sodium nitrate additions. Erythrocytes for cell culture were washed in custom-made nitrate-free RPMI with our without the addition of calcium chloride and sodium nitrate.
Resonance Raman spectroscopy of isolated food vacuoles
Resonance Raman spectra were recorded from isolated food vacuoles suspended in sodium phosphate buffer at pH 7.1. Spectra were excited with 406.7-nm emission from a Kr+ laser and scattered light was collected in the 135° back-scattering geometry. The spectrometer was calibrated using the Raman bands of toluene, DMF, acetone, and dimethylsulfoxide as external frequency standards. Spectra were recorded at ambient temperature from fluid suspensions in spinning 5-mm NMR tubes. Laser power at the sample ranged from 10 to 25 mW; at these powers no spectral artifacts due to photoinduced chemistry were observed. Food vacuoles that were tested for reaction with exogenous NO were flushed with nitrogen; the generation of NO was accomplished by sequential addition of Na14NO2 or Na15NO2 and Na2S2O4 to concentrations of 5 mM and 2 mM respectively. At these relative concentrations, nitrite was present in excess on the basis of redox equivalents.
Thus, with dithionite being the limiting reagent, the upper limit of the NO concentration was 4 mM. However, the solubility of NO in aqueous solution is only about 2 mM if the NO pressure over the solution is maintained at one atmosphere. As the reaction produced only enough NO to generate 6.5×10-3 atm of NO pressure in the sample tube, the NO concentration in the aqueous FV suspension was approximately 12 μM. This estimate is based on the assumption that the partitioning of NO between the solution and head space had come to equilibrium.
Results and Discussion
Genes that may be associated with RNS generation in P. falciparum
We have shown previously that NO and NO-derived RNS are not generated in trophozoite stage P. falciparum parasites through a NOS mechanism (Ostera et al., 2008). Recently, we confirmed that gametocytes, sexual forms of the P. falciparum parasite, had no detectable NOS activity either, judged by their inability to generate [3H] L-citrulline from [3H] L-arginine substrate (Fig S1). Although the P. falciparum genome does not contain a NOS coding sequence, it has been reported recently that it contains an arginase gene (PFI0320w), which has been implicated in the observed arginine depletion and ornithine accumulation during in vitro P. falciparum cultivation (Olszewski et al., 2009). Our previous findings provided the basis for the hypothesis that intraerythrocytic P. falciparum produce NO by a pathway involving a NADH-dependent cytochrome b5 reductase (Ostera et al., 2008). This cytochrome b5 reductase, coded in PF13_0353, suggests the presence of a redox partner, a cytochrome b5, which would be reduced by NADH in order to reduce the enzyme active site and start a cycle of nitrate/nitrite reduction. In accord with this prediction, an additional PlasmoDB search revealed the PFL1555w gene, a putative cytochrome b5. The complementary function of these two genes, PF13_0353 and PFL1555w, together with their similar expression patterns at comparable developmental stages (Fig S2) suggested that these two proteins could be co-expressed, forming part of a protein complex capable of delivering electrons to the active site of a nitrate or nitrite reductase.
To investigate the expression of the PFL1555w gene product and its localization in intraerythrocytic P. falciparum parasites, we raised antibodies against this gene using a DNA immunization protocol. Purified plasmid preparations were generated by cloning cDNA from PFL1555w coding regions into the DNA plasmid VR2001-TOPO (Oliveira et al., 2006).
Immunoblot analysis using antisera from mice immunized with PFL1555w revealed a band at approximately 15 kD, which is within the range predicted from the PFL1555w gene, estimated to be 18 kD (Fig 1). Immunofluorescence microscopy assays (IFA) using this anti-PFL1555w antiserum show fluorescence signals localized predominantly in the FV region of 3D7 trophozoites (Fig 2). Previously, we have shown that anti PF13_0353 antisera also displayed intense signals in the FV area of 3D7 parasites (Ostera et al., 2008). Taken together, these data indicate that the putative NADH-cytochrome b5 reductase and cytochrome b5, coded in PF13_0353 and PFL1555w respectively, are co-located with NO-derived RNS production. Thus they could be functionally associated with NO synthesis by nitrate or nitrite reduction in intraerythrocytic P. falciparum parasites. Interestingly, DAR-4M AM fluorescence of 3D7 trophozoites, established in nitrate-free buffer, is enhanced when trophozoites are incubated in buffer with the addition of nitrate (data not shown). Moreover, nitrite was found to be significantly lower in the erythrocytes of Plasmodium berghei-infected mice compared to uninfected controls (Gramaglia et al., 2006). Further biochemical studies are underway to confirm that NO and NO-derived nitrogen radicals are the result of denitrification reactions in the malaria parasite.
Figure 1. PFL1555w, putative cytochrome b5, a possible molecular partner of PF13_0353.
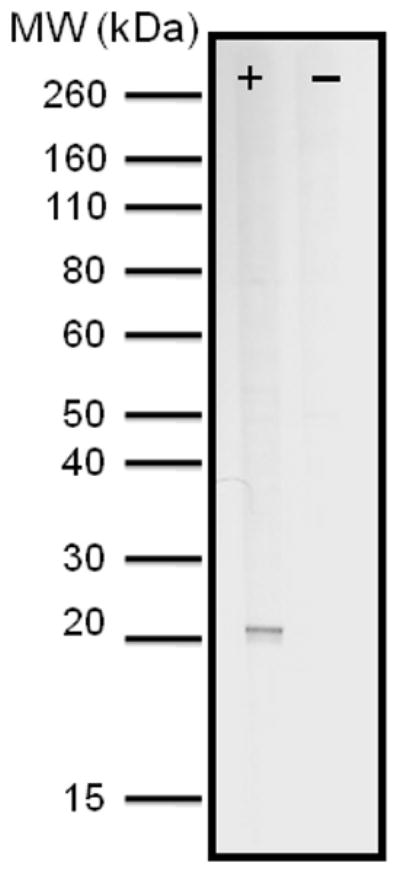
Immunoblot analysis using anti-PFL1555w showing one representative reactive sera. Predicted MW of the PFL1555w protein is 18 kD.
Figure 2. Immunofluorescence assay using anti-PFL1555w antisera.
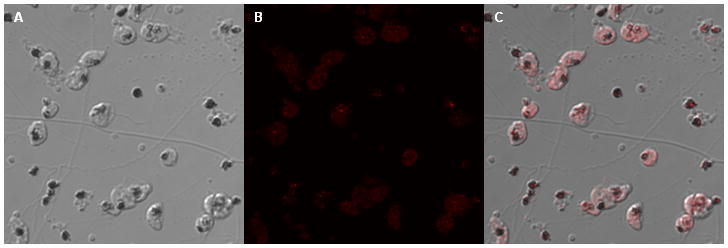
Purified P. falciparum trophozoites were incubated with a 1/25 dilution of antisera recognizing coding sequences of PFL1555w. Goat anti-mouse IgG conjugated with Alexa 633 was used as a secondary antibody. a:. DIC image of trophozoites treated with anti-PFL1555w antiserum, b: Alexa 633 signal recognizing anti-PFL1555w antibody, c: merged images of a and b.
P. falciparum growth in nitrate-free cultures
Nitrate, the substrate of a possible denitrification pathway, is abundantly present in human blood plasma (Ghasemi et al., 2008), the erythrocyte cytosol (Dejam et al., 2005) and RPMI 1640, the culture media utilized for P. falciparum culture, which contains 848 μM of calcium nitrate. To investigate if nitrate is a critical nutrient for P. falciparum parasites, we established cultures in custom-made RPMI 1640 that did not contain calcium nitrate, and was supplemented with calcium chloride to supply the equivalent dose of calcium present in RPMI 1640. Calcium nitrate-free RPMI 1640 with calcium chloride supplementation was also used to pre-wash the erythrocytes used in the test parasite cultures and to prepare the fully supplemented in vitro culture media.
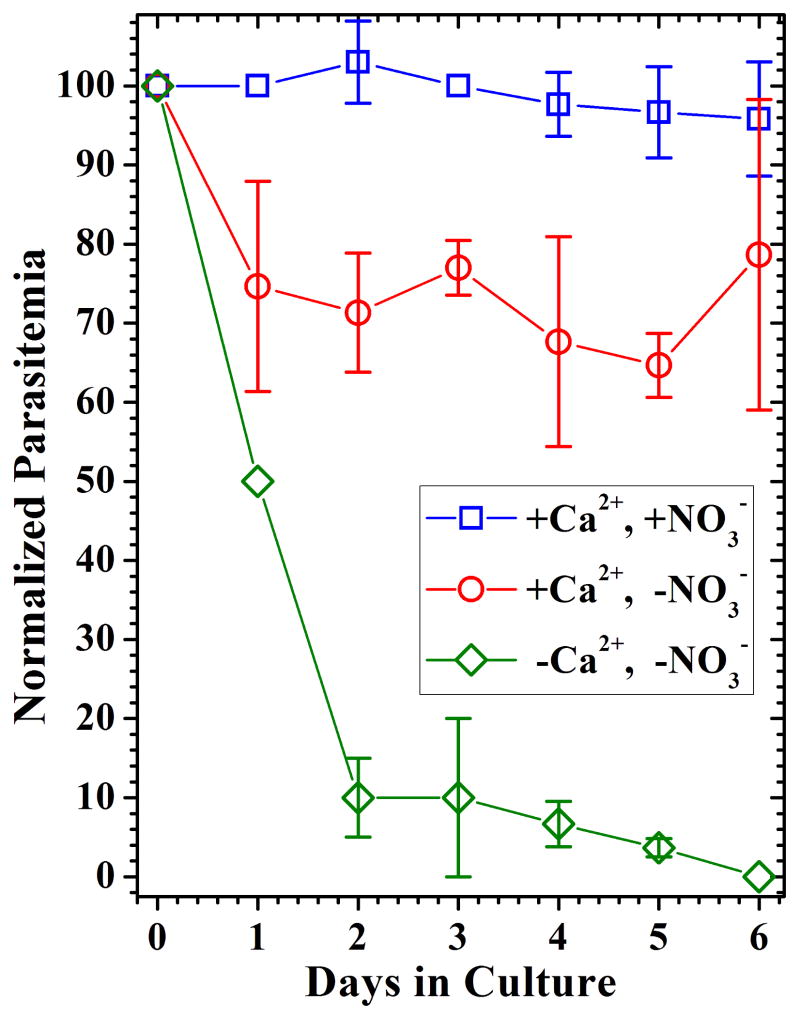
Our results shown that 3D7 parasites grown in calcium nitrate-free RPMI 1640 supplemented with calcium chloride only, developed with normal morphology but at reduced rates, ≈75% of control values, compared to parasites grown in culture media prepared with standard RPMI 1640 (Fig 3). Although parasite development was somewhat hindered under these culture conditions, we hypothesized that the proportion of parasites that developed did so by taking advantage of the nitrate/nitrite present in the erythrocyte's cytosol. Control cultures supplemented with both calcium chloride and sodium nitrate developed at a very similar rate compared to parasites grown in standard RPMI 1640. As anticipated, 3D7 parasites cultured using calcium nitrate-free RPMI without calcium or nitrate supplementation did not support parasite growth. Our observation that approximately 75% of P. falciparum parasites could develop in vitro in nitrate-free RPMI 1640, compared to parasites growing in regular culture media, suggested that there was enough nitrate and/or nitrite ions associated with the host cell to allow parasite development and that nitrate and/or nitrite, which could reach the FV through the process of hemoglobin uptake, are readily available substrates to the intraerythrocytic parasite.
Figure 3. P. falciparum cultures in nitrate free RPMI 1640.
Synchronized 3D7 cultures were incubated in Ca(NO3)2-free RPMI 1640 (◇), Ca(NO3)2-free RPMI 1640 supplemented with CaCl2 (○) and Ca(NO3)2-free RPMI 1640 supplemented with CaCl2 plus NaNO3 (□). Normalized parasitemia values compared to P. falciparum development in standard RPMI 1640 are shown for three experiments. Data points represent the sample mean for three independent experiments and the error bars indicate the sample standard deviation.
NO-dependent chemistry in the FV
While the function of NO in P. falciparum parasites is under investigation, additional evidence indicating that the FV is a center for the activity of RNS has been supplied by the observation of nitrated tyrosine residues in trophozoite and gametocyte FVs (data not shown).
Since this organelle is also the site of hemoglobin degradation and accumulation of large amounts of ferrous and/or ferric heme (protoporphyrin IX), we wanted to test whether NO might be involved in molecular interactions with soluble heme (monomeric and dimeric forms) or with insoluble hemozoin inside the food vacuole. Both ferric and ferrous hemes are known to form stable coordination complexes with NO (McCleverty, 2004). These interactions were probed by rR spectroscopy.
Resonance Raman spectroscopy is an inelastic light scattering method that provides information on structure and bonding within the heme as well as nonbonded interactions between the heme and its environment. Resonance Raman (rR) spectra of isolated trophozoite food vacuoles were recorded by exciting within the Soret band of the optical absorbance spectrum (406.7 nm). Isolated food vacuoles were used, instead of whole parasites, because hemoglobin had to be removed from the samples in order to avoid the spectral interference that it would have caused in the rR experiment. Food vacuoles were prepared using the method published by Goldberg and colleagues (Goldberg et al., 1990), as it preserves the vacuole's membrane integrity. The food vacuole supernatant was colorless at the end of the preparation but was nevertheless investigated by mass spectroscopy, which confirmed that no hemoglobin was present outside the FVs (data not shown) and ensured that heme rR features were attributable to heme located inside the FVs.
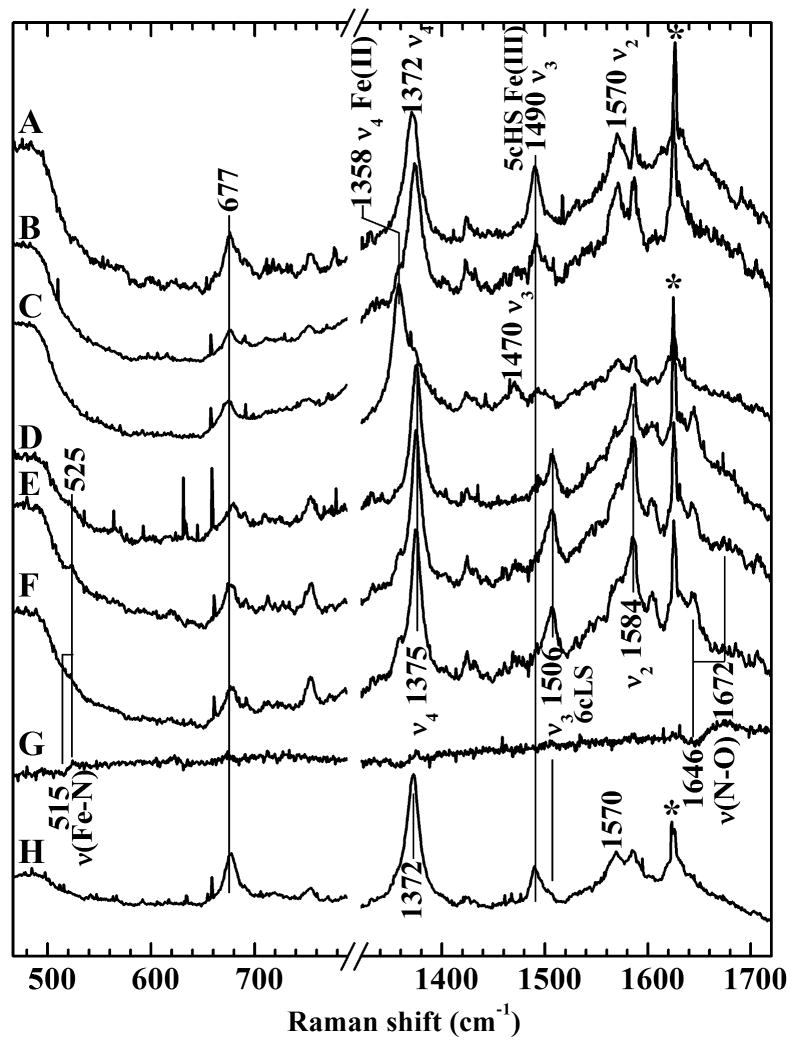
Fig 4A-C show rR spectra corresponding to FV under different control conditions, while Fig 4 D-G show rR of FV after in situ NO generation. These rR spectra were acquired from an aqueous suspension of hemozoin-containing food vacuoles that were harvested from 3D7 trophozoites. The frequencies (Raman shifts) of the observed bands are sensitive to the size of the porphyrin core, which is responsive to the iron oxidation number, its spin state, its coordination number and the identities of its axial ligand(s). Fig 4, trace A is the rR spectrum of FVs suspended in phosphate buffer saline (PBS) with no nitrate or nitrite added. These rR bands are typical of a five-coordinate ferric heme (Reynolds et al., 2009). The oxidation-state (or π* electron density) marker band (ν4) was observed at 1372 cm−1, consistent with ferric heme. The spin-state marker band (ν3) for the intact FVs was observed at 1491 cm−1, also in accord with a five-coordinate, high-spin heme. This signature most likely corresponds to the heme of micro-crystalline hemozoin. Trace B corresponds to the rR spectrum of the FV suspension in the presence of 5 mM NaNO2 alone and shows, similar to trace A, typical five-coordinate ferric heme bands. Trace C corresponds to the rR spectrum of the FV suspension after treatment with 2 mM dithionite and shows bands attributable to ferrous pentacoordinate high-spin heme. As expected, given the absence of any source of oxidized nitrogen, this spectrum contains no evidence for coordinated NO.
Figure 4. Resonance Raman spectra of hemozoin in food vacuoles isolated from P. falciparum.
All spectra were recorded with 406.7-nm excitation wavelength. A: spectrum of isolated food vacuoles, B: spectrum of anaerobic food vacuoles treated with 5 mM Na14NO2, C: spectrum of anaerobic food vacuoles treated with 2 mM Na2S2O4, D: spectrum of anaerobic food vacuoles under 1 atmosphere NO, E: spectrum of anaerobic food vacuoles treated with Na14NO2 and Na2S2O4, F: spectrum of anaerobic food vacuoles treated with Na15NO2 and Na2S2O4, G: difference spectrum obtained by digital subtraction of spectrum F from spectrum E, H: Sample from E opened to air. The sigmoidal difference features in trace G show the shifts of νFe–N and νN–O to lower frequencies in the spectrum of the 15NO isotopologue. The sharp lines marked with asterisks are due to Rayleigh scattering of plasma emission lines from the Kr + laser.
After establishing the Soret-excited rR signature of the intact FVs, we proceeded to investigate the possible occurrence of nitrosyl-heme complexes in that environment. As NO is a strongly coordinating ligand for iron, it is likely that at least some of any NO generated in vivo would coordinate intra-vacuolar heme and form a nitrosyl complex. Thus, we set out to determine whether these complexes could be directly observed by rR spectroscopy of isolated FVs. In order to identify whether rR bands in the spectra of FVs arise from one or more heme–NO complexes, it was necessary to record the spectra from samples containing NO with natural abundance isotopic composition (i.e. >99% 14N) and with NO having been generated from a 15N-enriched precursor. If heme–NO complexes are present at levels detectable by rR spectroscopy (>1 μM), they are expected to be identifiable by characteristic isotope shifts of their νFe–N and νN–O frequencies (Vogel et al., 1999; Ibrahim et al., 2006).
Two classes of heme–NO complexes are known. One comprises the so-called ferric heme nitrosyls, or {FeNO}6 hemes, where the superscripted six is the sum of the iron-based d electrons (five in the case of Fe(III)) and the single NO-based π* electron (Enemark and Feltham, 1974). These complexes can be either five-or six-coordinate, depending upon the nature and availability of ligands other than NO. The Fe–N stretching (νFe–N) frequencies of these complexes fall near 600 cm−1 (Linder et al., 2004). Their νN–O frequencies range from 1830 to 1940 cm−1. The second class, the {FeNO}7 or ferrous heme nitrosyls, are typically much more stable, with formation constants up to 106-fold greater than their ferric counterparts (Walker, 2005). Like {FeNO}6, the {FeNO}7 moiety also exists in both penta- and hexacoordinate environments. In heme proteins, six-coordinate NO complexes occur when a protein-based axial heme ligand is retained upon NO binding, and the five-coordinate NO complexes form when the protein-based axial ligand dissociates. The νFe–N frequencies of {FeNO}7 heme nitrosyls occur between 510 and 570 cm−1 while the N–O stretching (νN–O) frequencies are typically in the range of 1590-1685 cm−1 (Spiro et al., 2001).
The first of our rR experiments to investigate the possibility of heme nitrosyl complexes within the FV was carried out with vacuoles prepared from parasites cultured either in the presence of natural abundance nitrate or 15N-enriched nitrate. The purpose was to assess the possibility that any heme-NO complex produced in vivo might survive the isolation procedure. If so, rR difference spectra would show features attributable to isotope shifts in the Fe-NO and N-O stretching vibrations. Those difference spectra (not shown) contained no such difference bands and, therefore, no evidence for heme-NO complexes. Given the possibilities that (a) any heme-NO complexes formed within the parasite did not survive the isolation of FVs and/or (b) that any heme-NO complexes formed did not build up to a detectable concentration, the isolated vacuoles were treated with either exogenous, or in-situ generated NO to directly probe its chemistry with intra-vacuolar heme.
The high permeability of biological membranes to NO was exploited to introduce NO to the FVs in these experiments. Fig 4D shows the rR spectrum of a FV suspension after natural abundance NO was directly added to the sample by anaerobic transfer. Traces E and F of Fig 4 are the rR spectra of intact FV suspensions after introduction of 14NO and 15NO, respectively, via in situ reaction between a buffered stock solution of Na2S2O4 and either Na14NO2 or Na15NO2. Comparison of the rR spectra in Fig 4D and Fig 4E reveals that (a) both spectra are different from that of the untreated FVs, consistent with formation of a heme-NO complex, and (b) both methods of introducing NO yielded the same heme-NO complex, That complex was confirmed as a heme-nitrosyl by virtue of the 15NO isotope shifts in the frequencies of the Fe-NO and N-O stretching vibrations. These shifts are reported by the sigmoidal features in the difference spectrum shown in Fig 4G. The positive component of the low-frequency difference band corresponds to the Fe-14NO frequency, νFe–NO, at 525 cm−1, which shifts to 515 cm−1 (negative component of the difference band). A second band at 1672 cm−1 (positive difference amplitude) shifted to 1646 cm−1 (negative difference amplitude) with 15NO, verifying its assignment to the N–O stretching vibration, νN–O. These frequencies are typical of the Fe–N and N–O stretching modes of {FeNO}7 hemes (Spiro et al., 2001; Vogel et al., 1999; Ibrahim et al., 2006).
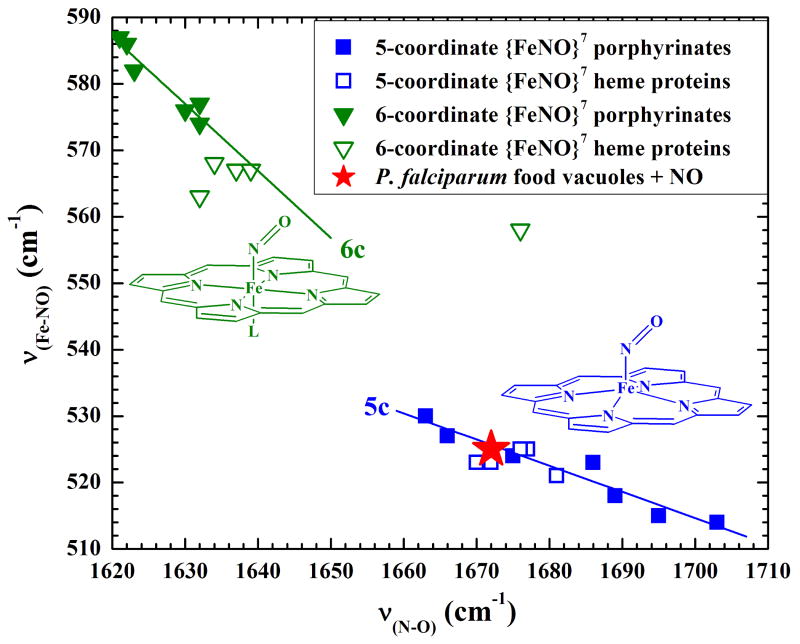
Hexacoordinate and pentacoordinate {FeNO}7 porphyrinates exhibit inverse correlations between the strengths of their Fe–N and their N–O bonds. Those correlations are revealed by experimental measurements that probe bond strength, including bond vibrational frequencies determined by rR spectroscopy. Thus, as νFe–N frequencies decrease among a series of {FeNO}7 porphyrinates, the corresponding νN–O frequencies increase. As seen in Fig. 5, five-coordinate and six-coordinate NO complexes of iron(II) porphyrinates, including hemes, fall on distinct correlation lines. Thus, the rR frequencies of the NO vibrational modes reveal that the {FeNO}7 complex formed upon treatment of intact FVs with NO is pentacoordinate, as it clearly falls on the five-coordinate νFe–N /νN–O correlation line.
Figure 5. Plot of the inverse correlation between the νFe–NO and νN–O frequencies of 5- and 6-coordinate iron (II) nitrosyl ({FeNO}7) porphyrinates.
The top left and bottom right lines correlate νFe–N and νN–O frequencies among a wide variety of 6-coordinate and 5-coordinate {FeNO}7 porphyrinates, including some in heme proteins, respectively. The red star indicates the position of the {FeNO}7 heme that was generated inside the P. falciparum food vacuoles upon their exposure to NO that was generated in situ by reduction of NaNO2 with Na2S2O4. Its position on the correlation line for 5-coordinate complexes is compelling evidence that it is a pentacoordinate ferrous heme nitrosyl complex.
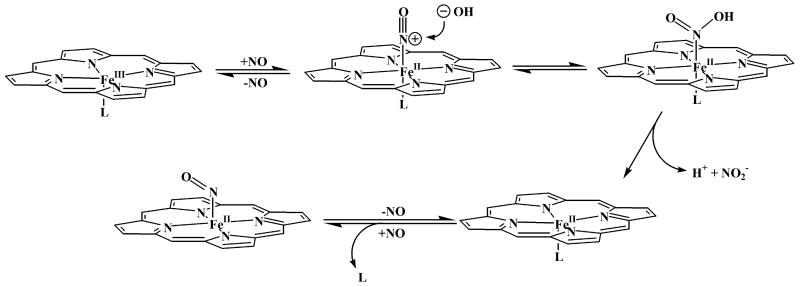
Given that virtually all of the heme in the FV is ferric, the appearance of reduced intravacuolar {FeNO}7 heme (ferrous heme nitrosyl) warrants further comment. Its production requires two steps. First NO must bind to the heme iron to form the {FeNO}6 (ferric heme nitrosyl) complex (Linder et al., 2004). That nitrosyl complex must then undergo a reductive nitrosylation reaction (Ford, 2002), as illustrated in Scheme 1. Briefly, this reaction involves nitrosylation of a nucleophile, typically hydroxide or water, which attacks the nitrogen atom of coordinated NO. This nucleophilic attack is followed by reductive elimination of nitrous acid, thereby leaving a ferrous iron center that reacts with another molecule of NO to give the {FeNO}7 complex. It is that complex whose rR spectrum is evident in traces D, E and F of Fig 4. It is important to note that reductive nitrosylation of iron porphyrinates is catalyzed by nitrite (Fernandez, et al., 2003; Fernandez and Ford, 2003; Jee and van Eldik, 2006), which is abundantly present in the erythrocyte cytosol and is likely to reach the FV through the process of hemoglobin uptake. Consequently, it is possible that the reductive nitrosylation reaction could occur at substantial rates, even under the acidic conditions of the vacuole, where hydroxide concentration is low and availability of NO is limited.
Scheme 1.
Illustration of the generalized reductive nitrosylation mechanism (adapted from Ford, 2002)
The in situ generation of 14NO and 15NO did not cause the vacuolar membrane to become detectably permeable to heme. The buffer solution in which the FVs were suspended remained colorless, as judged by its optical absorbance spectrum, throughout the rR experiment indicating that no heme “leaked” from the vacuoles. This result clearly indicates that in situ generated NO crossed the intact food vacuole membrane and formed a five-coordinate heme-NO complex within the vacuole. Furthermore, since the rR signature of hemozoin was no longer observable after treatment with NO, it is likely that the {FeNO}7 complex contained heme from hemozoin. The spectrum in Fig 4H shows that formation of the intra-vacuolar {FeNO}7 heme is reversible. This rR spectrum was recorded from the same sample as spectrum E after the sample was exposed to air overnight. It shows bands attributable to ferric protoporphyrin IX, as seen in Fig 4A and B.
In summary, the features of the rR spectra in Fig 4D-F clearly show the formation of a pentacoordinate {FeNO}7 heme complex upon treatment of intact FVs with NO. Additional information about the rR spectra can be found in Supp 3.
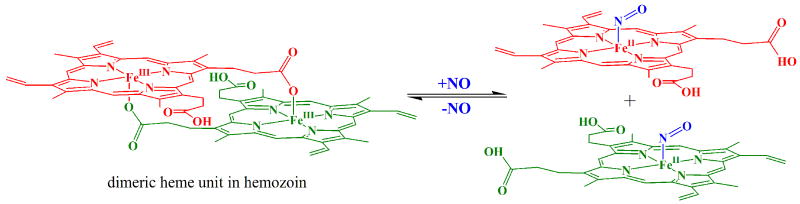
The lysosome-like FV, known for its proteolytic hemoglobin degradation, plays a crucial role in heme detoxification by facilitating the formation of microcrystalline hemozoin, wherein mutually coordinated dimers of ferriprotoporphyrin IX [Fe(III)PPIX] are crosslinked by intermolecular H-bonding between propionyl groups (Pagola, et al., 2000). These dimers comprise pentacoordinate heme in which the fifth heme ligand is the carboxylate group of a propionate substituent from the other heme molecule. As illustrated in Scheme 2, the formation of pentacoordinate heme–NO requires the disruption of those intradimer coordinative interactions that stabilize the hemozoin structure. Consequently, the soluble heme-NO complex that gave rise to the rR spectra in Fig. 4 D-F is likely the product of NO-driven dissociation of heme from hemozoin. While, to our knowledge, this is the first report of intravacuolar hemozoin solubilization by NO, it is not known whether solubilization of hemozoin crystallites is a significant phenomenon in vivo. On the other hand, it is also possible that the heme of the aforementioned heme–NO complex originated from soluble [Fe(III)PPIX]2 already present within the vacuole (Scheme 2). In either case, NO competes very effectively for the axial coordination sites that would otherwise become occupied by carboxylate groups from other Fe(III)PPIX molecules, thereby precluding the mutual coordination that characterizes polymerized heme in the malaria pigment. The NO complex that formed in this experiment is the ferrous heme nitrosyl, {FeNO}7, complex, which is produced in aqueous solution by reductive nitrosylation of a nucleophile, such as water or hydroxide (Ford, 2002; Fernandez et al., 2003; Jee and van Eldik, 2006). This same heme–NO complex was produced whether NO was generated in situ or added directly as NO gas to the vacuole suspension.
Scheme 2.
Illustration of hemozoin dissolution in the presence of excess NO.
These results give rise to the hypothesis that in vivo NO plays a role in intravacuolar heme solubility by regulating access of the heme propionate groups to the iron centers of other [Fe(III)PPIX} molecules. Although it is accepted that hemozoin formation is essential to P. falciparum heme detoxification, a small, albeit critical, soluble heme fraction is believed to be present in the FV under physiological conditions and does not affect parasite viability. This soluble heme fraction is relevant to antimalarial drug interactions with heme, since it is generally accepted that the aminoquinoline derivative chloroquine forms a soluble heme complex, thereby inhibiting its conversion to the aggregated, crystalline form. A recent review of the molecular nature of that interaction was inconclusive on whether this drug binds monomeric or dimeric heme forms (de Villiers et al., 2007). However, it is clear that the availability of soluble heme is critical to the antimalarial action of aminoquinolines. It has been proposed that, under physiological conditions, vacuolar pH, salt concentration and antimalarial drug concentration can affect the ratio between soluble and insoluble heme species in the FV (Ursos et al., 2001). This is the first time that a role for NO modulating heme solubility in the FV has been suggested.
Although the understanding of hemozoin self-assembly has been recently expanded by showing that it is favored in neutral, lipid-rich environments, molecular details of the hemozoin nucleation and propagation mechanisms remain to be clearly established under physiologically relevant conditions (Egan, 2008; Pisciotta et al., 2007). In this context, it is intriguing to consider that endogenously produced NO could play a role in heme speciation inside the FV. It is of particular interest to determine whether NO is involved in (a) the nucleation of hemozoin, a process that, unlike the epitaxial nucleation of the crystal at lipid surfaces, has never been observed in vitro (Egan, 2008), and/or (b) the propagation of hemozoin formation.
New insights into the mechanism of heme biomineralization could be relevant to diseases associated with other blood-feeding organisms as well. Recently, it has been demonstrated that organisms such as protozoans (Haemoproteus columbae), helminthes (Schistosoma mansoni), trematodes (Echinostosoma trivolis) and insects (Rhodnius prolixus and different species of triatomine insects) also digest hemoglobin and generate insoluble hemozoin from heme (Chen et al., 2001; Oliveira et al., 1999; Oliveira et al., 2000). These organisms are either agents or vectors of serious and highly prevalent diseases, such as schistosomiasis (Schistosoma mansoni) and Chagas disease (Rhodnius prolixus), that affect a large fraction of the world population. Physical and chemical studies have revealed that the heme-derived pigment found in all the above species is indistinguishable from P. falciparum hemozoin (Chen et al., 2001; Oliveira et al., 2005; Oliveira et al., 2007; Pisciotta et al., 2005). Thus new insights into factors affecting the solubility of heme species or heme detoxification products could contribute to a unified strategy in the fight against all of these medically relevant organisms.
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Thomas Wellems, Jesus Valenzuela, Alan Schechter and F. Ann Walker for their helpful discussions and contributions to this project. This research was supported by the Intramural Research Program of the NIAID, NIH and by NIH-NIAID (AI072719 to KRR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chen MM, Shi L, Sullivan DJ., Jr Haemoproteus and Schistosoma synthesize heme polymers similar to Plasmodium hemozoin and β-hematin. Molecular & Biochemical Parasitology. 2001;113:1–8. doi: 10.1016/s0166-6851(00)00365-0. [DOI] [PubMed] [Google Scholar]
- Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers K, Kaschula C, Egan T, Marques H. Speciation and structure of ferriprotoporphyrin IX in aqueous solution: spectroscopic and diffusion measurements demonstrate dimerization, but not μ-oxo dimer formation. Journal of Biological Chemistry. 2007;12:101–117. doi: 10.1007/s00775-006-0170-1. [DOI] [PubMed] [Google Scholar]
- Egan TJ. Recent advances in understanding the mechanism of hemozoin (malaria pigment) formation. Journal of Inorganic Biochemistry. 2008;102:1288–1299. doi: 10.1016/j.jinorgbio.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Enemark JH, Feltham RD. Principles of structure, bonding, and reactivity for metal nitrosyl complexes. Coordination Chemistry Reviews. 1974;13:339–406. [Google Scholar]
- Fernandez BO, Lorkovic IM, Ford PC. Nitrite catalyzes reductive nitrosylation of the water-soluble Ferri-Heme model FeIII(TPPS) to FeII(TPPS)(NO) Inorganic Chemistry. 2003;42:2–4. doi: 10.1021/ic020519r. [DOI] [PubMed] [Google Scholar]
- Fernandez BO, Ford PC. Nitrite catalyzes ferriheme reductive nitrosylation. Journal of the American Chemical Society. 2003;125:10510–10511. doi: 10.1021/ja036693b. [DOI] [PubMed] [Google Scholar]
- Ford PC, Lorkovic IM. Mechanistic aspects of the reactions of nitric oxide with transition-metal complexes. Chemistry Reviews. 2002;102:993–1017. doi: 10.1021/cr0000271. [DOI] [PubMed] [Google Scholar]
- Ghasemi A, Zahedi Asl S, Mehrabi Y, Saadat N, Azizi F. Serum nitric oxide metabolite levels in a general healthy population: relation to sex and age. Life Sciences. 2008;83:326–331. doi: 10.1016/j.lfs.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Goldberg DE, Slater AF, Cerami A, Henderson GB. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proceedings of the National Academy of Sciences U S A. 1990;87:2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, Intaglietta M, van der Heyde H. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nature Medicine. 2006;12:1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Xu C, Spiro TG. Differential sensing of protein influences by NO and CO vibrations in heme adducts. Journal of the American Chemical Society. 2006;128:16834–16845. doi: 10.1021/ja064859d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee JE, van Eldik R. Mechanistic studies on the nitrite-catalized reductive nitrosylation of highly charged anionic and cationic Fe(III) porphyrin complexes. Inorganic Chemistry. 2006;45:6523–6534. doi: 10.1021/ic0603104. [DOI] [PubMed] [Google Scholar]
- Jin Y, Nagai M, Nagai Y, Nagatomo S, Kitagawa T. Heme Structures of Five Variants of Hemoglobin M Probed by Resonance Raman Spectroscopy. Biochemistry. 2004;43:8517–8527. doi: 10.1021/bi036170g. [DOI] [PubMed] [Google Scholar]
- Linder DP, Rodgers KR, Banister J, Wyllie GRA, Ellison MK, Scheidt WR. Five-coordinate Fe(III)NO and Fe(II)CO porphyrinates: where are the electrons and why does it matter? Journal of the American Chemical Society. 2004;126:14136–14148. doi: 10.1021/ja046942b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S. WHO report shows that countries adopting preventive measures see big fall in malaria. British Medical Journal. 2008;337:1678. [Google Scholar]
- McCleverty JA. Chemistry of nitric oxide relevant to biology. Chemistry Reviews. 2004;104:403–418. doi: 10.1021/cr020623q. [DOI] [PubMed] [Google Scholar]
- Oliveira F, Kamhawi S, Seitz AE, Pham VM, Guigal PM, Fischer L, Ward J, Valenzuela JG. From transcriptome to immunome: identification of DTH inducing proteins from a Phlebotomus ariasi salivary gland cDNA library. Vaccine. 2006;24:374–390. doi: 10.1016/j.vaccine.2005.07.085. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, Silva JR, Dansa-Petretski M, de Souza W, Lins U, Braga CM, Masuda H, Oliveira PL. Haem detoxification by an insect. Nature. 1999;400:517–518. doi: 10.1038/22910. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, d'Avila JC, Torres CR, Oliveira PL, Tempone AJ, Rumjanek FD, Braga CM, Silva JR, Dansa-Petretski M, Oliveira MA, de Souza W, Ferreira ST. Haemozoin in Schistosoma mansoni. Molecular and Biochemical Parasitology. 2000;111:217–221. doi: 10.1016/s0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, Hempelmann E, Menezes D, Vannier-Santos MA, Oliveira PL, Ferreira ST. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Letters. 2005;579:6010–6016. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Oliveira MF, Gandara AC, Braga CM, Silva JR, Mury FB, Dansa-Petretski M, Menezes D, Vannier-Santos MA, Oliveira PL. Heme crystallization inn the midgut of triatomine insects. Comparative Biochemistry and Physiology. 2007;146:168–174. doi: 10.1016/j.cbpc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Olszewski K, Morrisey J, Wilinski D, Burns J, Vaydya A, Rabinowitz J, Llinas M. Host-parasite interactions revelealed by Plasmodium falciparum metabolomics. Cell Host & Microbe. 2009;5:191–199. doi: 10.1016/j.chom.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostera G, Tokumasu F, Oliveira F, Sa J, Furuya T, Teixeira C, Dvorak J. Plasmodium falciparum: Food vacuole localization of nitric oxide-derived species in intraerythrocytic stages of the malaria parasite. Experimental Parasitology. 2008;120:29–38. doi: 10.1016/j.exppara.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagola S, Stephens P, Bohle DS, Kosar A, Madsen S. The structure of malaria pigment-β-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- Pisciotta JM, Ponder EL, Fried B, Sullivan D. Hemozoin formation in Echinostoma trivolis rediae. International Journal of Parasitology. 2005;35:1037–1042. doi: 10.1016/j.ijpara.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, Bajad S, Shulaev V, Sullivan DJ., Jr The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochemistry Journal. 2007;402:197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MF, Ackley L, Blizman A, Lutz Z, Manoff D, Miles M, Pace M, Patterson J, Pozzessere N, Saia K, Sato R, Smith D, Tarves P, Weaver M, Sieg K, Lukat-Rodgers GS, Rodgers KR. Role of conserved Fa -helix residues in the native fold and stability of the kinase-inhibited oxy state of the oxygen-sensing FixL protein from Sinorhizobium meliloti. Archives of Biochemistry and Biophysics. 2009;485:150–159. doi: 10.1016/j.abb.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Spiro TG, Zgierski MZ, Kozlowski PM. Stereoelectronic factors in CO, NO and O2 binding to heme from vibrational spectroscopy and DFT analysis. Coordination Chemistry Reviews. 2001:219–221. 923–936. [Google Scholar]
- Ursos L, DuBay K, Roepe P. Antimalarial drugs influence the pH dependent solubility of heme via apparent nucleation phenomena. Molecular & Biochemical Parasitology. 2001;112:11–17. doi: 10.1016/s0166-6851(00)00342-x. [DOI] [PubMed] [Google Scholar]
- Vogel KM, Kozlowski PM, Zgierski MZ, Spiro TG. Determinants of the FeXO (X=C, N, O) vibrational frequencies in heme adducts from experiment and density functional theory. Journal of the American Chemical Society. 1999;121:9915–9921. [Google Scholar]
- Walker FA. Nitric oxide interaction with insect nitrophorins and thoughts on the electron configuration of the {FeNO}6 complex. Journal of Inorganic Biochemistry. 2004;99:216–236. doi: 10.1016/j.jinorgbio.2004.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.