Abstract
Rationale and Objectives
Despite overwhelming evidence of the importance of brain renin-angiotensin system (RAS), the very existence of intrinsic brain RAS remains controversial. We investigated the hypothesis that the brain (pro)renin receptor (PRR) is physiologically important in the brain RAS regulation and cardiovascular functions.
Methods and Results
PRR is broadly distributed within neurons of cardiovascular-relevant brain regions. The physiological functions of PRR were studied in the supraoptic nucleus (SON) since this brain region showed greater levels of PRR mRNA in the spontaneously hypertensive rats (SHR) compared with normotensive Wistar Kyoto rats (WKY). AAV-mediated overexpression of human PRR in the SON of normal rats resulted in increases in plasma and urine vasopressin (AVP), and decreases in H2O intake and urine output without any effects on mean arterial pressure (MAP) and heart rate (HR). Knockdown of endogenous PRR by AAV-shRNA in the SON of SHR attenuated age dependent increases in MAP and caused a decrease in HR and plasma AVP. Incubation of neuronal cells in culture with human prorenin and angiotensinogen resulted in increased generation of angiotensin I and II. Furthermore, renin treatment increased phosphorylation of ERK1/2 in neurons from both WKY and SHR; however, the stimulation was 50% greater in the SHR.
Conclusions
The study demonstrates that brain PRR is functional and plays a role in the neural control of cardiovascular functions. This may help resolve a long-held controversy concerning the existence of intrinsic and functional brain RAS.
Keywords: PRR, SON, cardiovascular homeostasis, MAP
Introduction
Evidence demonstrates that the renin-angiotensin system (RAS) is intrinsic to the brain and has an integral role in the neural control of cardiovascular functions1,2. Despite this, there is a continuous debate about the existence of an intrinsic brain RAS1,3. Skeptics have argued that brain levels of renin are too low to have any impact on angiotensin II formation and hence its actions3-5. In addition, discrepancies in the cellular localization of angiotensinogen, angiotensin converting enzyme (ACE), and angiotensin II type I receptors (AT1R) have made it difficult to explain the access of angiotensin II to cardiovascular-relevant neuronal circuits1. Discovery of (pro)renin receptor (PRR) may be a key to resolving this enigma.
PRR is a 350 amino acid transmembrane protein that binds prorenin or renin with comparable affinity6. PRR plays a dual role in the regulation of RAS activity: it binds prorenin/renin to facilitate angiotensin II formation locally; and the binding initiates an intracellular signaling cascade which is similar to those associated with angiotensin II mediated increases in pressor, proliferative, and fibrotic actions4, 6. These observations, coupled with recent data that PRR is highly expressed in cardiovascular-relevant brain regions and afftect neurons in vitro 7,8, have led us to propose the following hypothesis: brain PRR is involved in the regulation of central cardiovascular function and its dysregulation may contribute to hypertension. Our objective in this study was to evaluate this hypothesis.
Materials and Methods
An expanded Methods section is available in the online supplement at http://circres.ahajournals.org.
All the animal protocols were approved by the University of Florida Institutional Animal Care and Use Committee.
Six-week-old male Sprague-Dawley (SD) rats or SHR were implanted telemetry transducers into the abdominal aorta. Following 10 days recovery, animals were randomly divided into two groups. SD rats were used for bilateral injection into the SON (A/P: 1.4mm, D/V: 9.2mm, M/L: 1.8mm) with either AAV-hPRR or control virus, AAV-GFP; similarly, SHR were injected with either AAV-PRR--shRNA or control virus, AAV-Sc-shRNA. Mean arterial pressure (MAP) and heart rate (HR) were monitored; plasma and urinary AVP, and gene transduction were assayed as described in online Methods.
Results
First, we compared PRR mRNA levels in the brains of WKY and SHR since its expression is shown to be abundant in cardiovascular-relevant brain regions 7. Compared with WKY, PRR mRNA levels were 70%, 45% and 36% higher in the supraoptic nucleus (SON), the nucleus of the solitary tract (NTS) and the central amygdale (CA), respectively (Figure 1a). Other brain regions tested did not show significant differences between these two strains of rats. PRR immunoreactivity was primarily localized in NeuN (a marker for neuronal nuclei) positive cells; no significant staining of PRR was observed with GFAP positive cells, an astroglial marker (Figure 1b, c). Predominant neuronal localization of PRR is supported by our in vitro data. Neuronal cultures from WKY hypothalamus/brainstem regions showed a 2.7 fold higher PRR mRNA than comparable astroglial cultures. In addition, neuronal cultures from the SHR had ~2 fold greater PRR mRNA compared to WKY neurons (Online Figure I).
Figure 1. PRR in the brain of WKY and SHR.
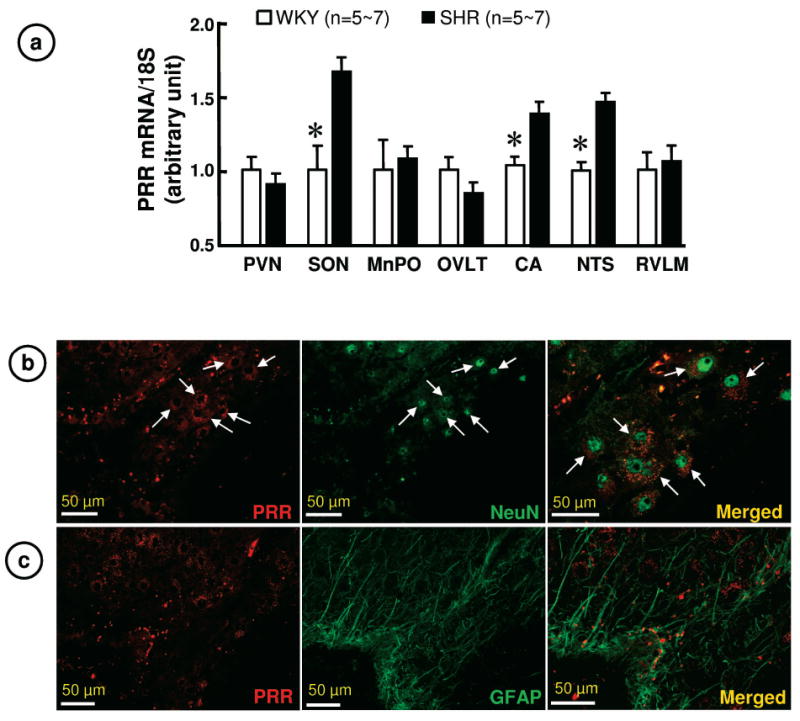
a. PRR mRNA in WKY and SHR brains. PVN: paraventricular nucleus; MnPO: median preoptic nucleus; OVLT: organum vasculosum of lamina terminals; RVLM: rostral ventrolateral medulla. *P< 0.05 vs. WKY.
b. Representative immunofluorescence micrographs using anti-PRR and anti-NeuN antibodies reveal co-localization of PRR with neurons in the SON.
c. Representative immunofluorescence micrographs using anti-PRR and anti-GFAP antibodies reveal that PRR is little present on astroglia in the SON.
Next, we studied the physiological role of PRR in the SON since this region exhibited the largest PRR expression difference between WKY and SHR. We used AAV-hPRR to increase PRR in normotensive rats and AAV-PRR-shRNA to knockdown its expression in the SHR. Bilateral microinjection of AAV-GFP showed a relatively restricted transduction of cells in the SON (Figure 2a). Transduction with AAV-hPRR demonstrated an increase in hPRR mRNA without influencing the levels of endogenous rat PRR (rPRR) (Figure 2b, c). Figure 3 shows that hPRR overexpression in the SON resulted in significant changes in the fluid homeostasis in SD rats. This increase was associated with a 21% decrease in daily water intake (PRR: 37.8±2, GFP: 48.0±2.4ml, p<0.05), 51% decrease in daily urine excretion (PRR: 9.7±1.8, GFP: 19.7±2.4ml, p<0.05), and 18% increase in urine osmolality (PRR: 2720±80, GFP: 2220±148 mosm/kg, p<0.05). Furthermore, plasma AVP was increased by 2 fold (PRR: 2.6±0.58, GFP: 1.2±0.2 pg/ml, p<0.05) while urine AVP increased by 3-fold (PRR: 74.9±17; GFP: 25.5±7.9 pg/ml, p<0.05). Further support for the role of PRR in AVP secretion is provided from the data showing colocalization of PRR on AVP neurons in the SON (Online Figure II). However, PRR overexpression in the SON had no effect on MAP and HR (Online Figure III).
Figure 2. Overexpression of hPRR in the SON of SD rats.
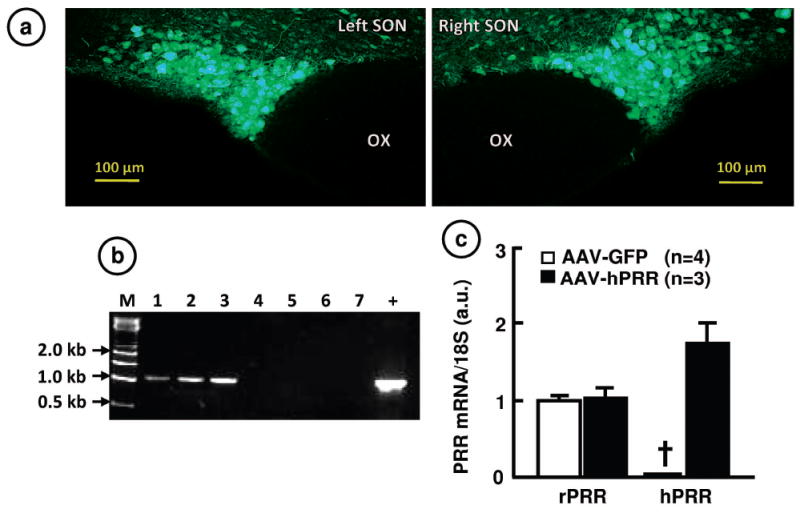
a. Representative fluorescence micrographs confirming AAV-mediated transduction of the SON with GFP. b. RT-PCR assay of AAV-mediated-hPRR transduction in the SON. M: DNA marker; 1-3, AAV-hPRR rats; 4-7, AAV-GFP rats; +, positive control. c. Quantitation of hPRR and rPRR mRNA from AAV-transduced SON. †, represents undetectable hPRR.
Figure 3. Effect of PRR overexpression in the SON of SD rats on fluid homeostasis.
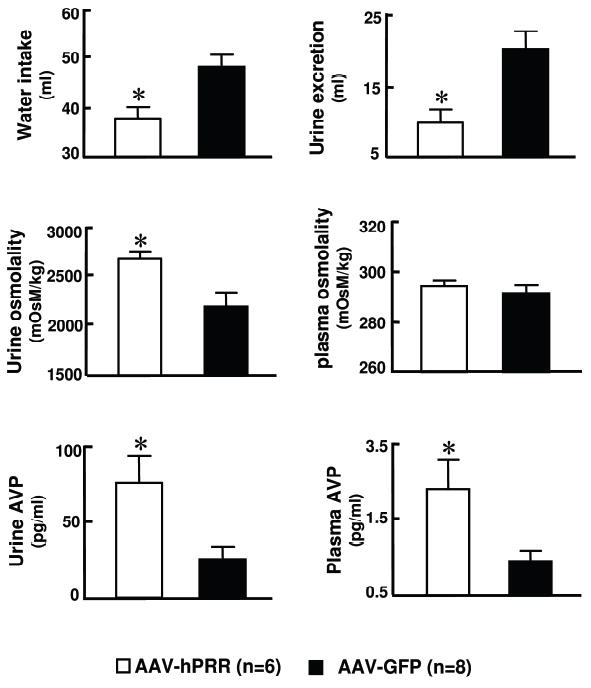
Twelve weeks following microinjection, H2O intake, urine excretion, urinary osmolality, plasma and urinary AVP of animals were measured. * P<0.05 vs. AAV2-GFP.
We constructed a shRNA targeted PRR which was cloned into the AAV vector. Infection of neuronal cultures with this PRR-shRNA caused ~60% decreases in both PRR mRNA and renin stimulation of ERK 1/2 phosphorylation (Online Figure IV). Transduction of SHR SON with PRR-shRNA attenuated the age dependent increases in MAP (ΔMAP) and caused a decrease in HR (ΔHR) compared to baseline MAP (PRR-shRNA: 124.6±7.6, Sc-shRNA: 121.2±3.0 mmHg) and HR (PRR-shRNA: 400.5.±5.1, Sc-shRNA: 388.6±5.9 bmp). Eight weeks following microinjection, ΔMAP was 22.4±3.9 mmHg in PRR-shRNA rats compared with 46.0±6.2 mmHg in Sc-shRNA animals. Similarly, ΔHR was decreased in shRNA treated SHR (PRR-shRNA: -81.1±9.0, Sc-shRNA: -51.2±6.9 bmp, P<0.05) (Figure 4a). These changes were associated with a 38% decrease in PRR mRNA in the SON. In addition, plasma AVP was decreased by 34% in PRR-shRNA animals (PRR-shRNA: 16.3±2.4, Sc-shRNA: 24.0±2.3 pg/ml, p<0.05) (Figure 4b). In contrast, transduction of WKY SON with PRR-shRNA failed to exert any effect on MAP (PRR-shRNA: 104.5±2.6, PRR-scRNA: 105.1± 9.0 mmHg, n=6 for each group).
Figure 4. Effect of PRR knockdown in the SON of SHR on cardiovascular function.
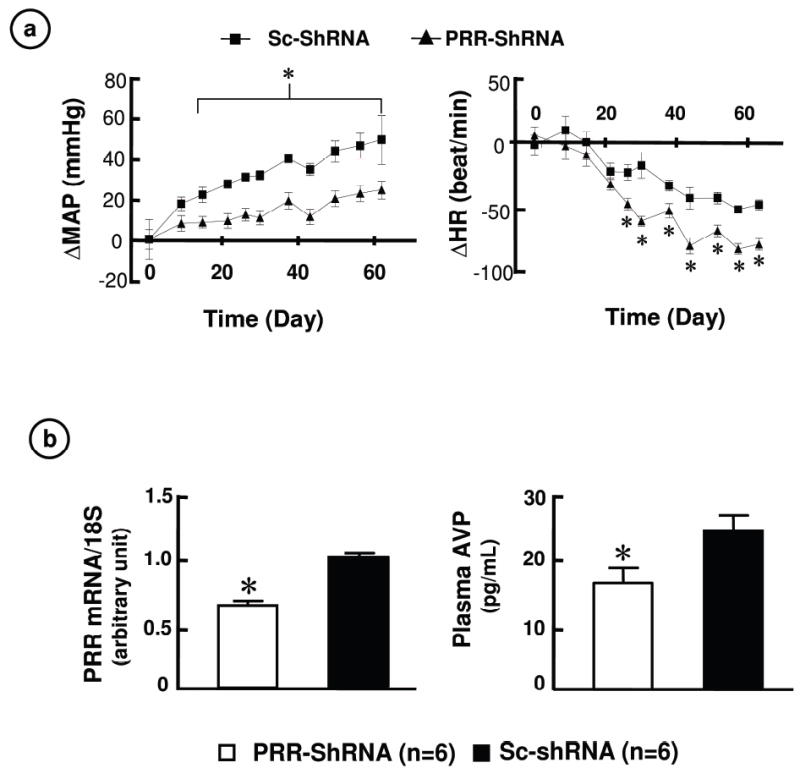
a. ΔMAP and ΔHR compared to baseline values following microinjection of either Sc-shRNA or PRR-shRNA into the SON. * P<0.05 vs. PRR-shRNA.
b. Effects of PRR-shRNA on PRR mRNA and plasma AVP level. * P<0.05 vs. Sc-shRNA.
Finally, we determined the cellular effects of prorenin and renin in neuronal cultures. A dose-and-time-dependent increase in angiotensin I and II formation was observed when the cultures were incubated with human prorenin and angiotensinogen (Online Figure V). Furthermore, incubation of neuronal cultures with renin in the presence of losartan, an AT1R blocker, resulted in a rapid and transient increase in phosphorylated ERK1/2 in both strains of rats although this stimulation was 2-fold greater in the SHR neurons (Online Figure VI).
Discussion
Our study is significant in a number of ways: (i) it demonstrates that PRR in the brain is physiologically relevant because its overexpression in the SON stimulates AVP secretion and alters fluid balance in normal rats. In addition, chronic knockdown of PRR causes a significant attenuation of MAP and HR in SHR. Thus, PRR may play a crucial role in the neuronal control of cardiovascular function by possibly regulating AVP secretion; (ii) PRR mediates the generation of angiotensin II and stimulates intracellular signaling, similar to its action in the periphery3, 6. Therefore, its presence further strengthens the presence of a functional intrinsic brain RAS; (iii) most previous studies implicating PRR’s functions are of pathophysiological states such as cardiovascular disease and diabetes, or from in vitro situations6, 9. Our study, provides data of functional PRR in a normotensive animal and demonstrates a beneficial outcome of PRR in hypertension by its chronic knockdown. Finally, our data demonstrate the role of SON in blood pressure control in the SHR model of hypertension.
PRR expression is widespread in brain cardioregulatory regions, consistent with the recent report of Contrepas et al7. However, our study is novel in that it shows that expression levels of PRR are higher in the SON of SHR compared to WKY controls. This increase appears to be genetically linked since it is maintained in primary neuronal cultures of prehypertensive SHR. It is pertinent to point out that these cultures closely mimic the changes observed in the RAS activity of adult SHR1. PRR are primarily localized on neuronal cells since PRR positive cells co-stain with NeuN positive cells Furthermore, GFAP positive cells exhibit little PRR staining. This conclusion is further supported by our observation that PRR levels are several fold lower in astroglial cultures than in neuronal cultures from the brains of both WKY and SHR.
Increased expression of PRR in the SHR SON appears to exert pathophysiological influences, a conclusion supported by our knockdown data. Long-term knockdown of PRR is associated with attenuation of hypertension development and a decrease in plasma AVP. Consistent with this data is our observation that overexpression of PRR in the SON of normotensive rat results in an increase in plasma and urinary AVP. However, increases in plasma AVP levels were not associated with an increase in MAP. There may be a number of explanations for this unexpected result: (i) increases in plasma AVP observed in this study were insufficient to affect MAP; (ii) redundant physiological mechanisms in normal rats may be able to overcome increases in plasma AVP; a view consistent with the current dogma that AVP appears to exert little effect on MAP in normal individuals and animals, (iii) circadian rhythmic release of AVP from the SON (rather than a continual increased secretion) is important in its cardiovascular effects 10. Transgenic overexpression of hPRR may not maintain this pattern of release, and (iv) PRR may inhibit sympathetic nervous system activity in normal rats, thus counteracting potentially hypertensive effects of circulating AVP.
Finally, our data show that the neuronal PRR has dual functions. First, by binding to renin/prorenin, it increases the generation of angiotensin II. This solid phase enzyme catalysis could provide thousands-fold higher rates of angiotensin formation than liquid phase occurring in the plasma. Thus, even a small amount of renin/prorenin would be capable of generating physiologically relevant concentrations of angiotensin II locally in the brain. We propose that this catalysis occurs on the surface of AT1R-expressing neurons. Evidence in support of this view are: (i) key components of angiotensin II actions, such as ACE and AT1R, are present in the neurons 1, 3. (ii) our preliminary data have shown that PRR and AT1R are co-localized on the SON neurons (data not presented). Second, binding of renin/PRR initiates signaling involving ERK1/2. These kinases have been shown to be involved in the regulation of neuronal activity1. This signaling would complement AT1R mediated neuromodulation.
Supplementary Material
Acknowledgments
Source of Funding This work was supported by grants of R37 HL33610, HL76312 and COBRE P20RR-017659.
Non-standard Abbreviations and Acronyms
- ACE
angiotensin converting enzyme
- AT1R
angiotensin II type I receptors
- CA
central amygdale
- HR
heart rate
- MAP
mean arterial pressure
- MnPO
median preoptic nucleus
- NTS
nucleus of the solitary tract
- OVLT
organum vasculosum of lamina terminals
- PVN
paraventricular nucleus
- PRR
(pro)renin receptor
- RAS
renin-angiotensin system
- RVLM
rostral ventrolateral medulla
- SHR
spontaneously hypertensive rats
- SON
supraoptic nucleus
- (WKY)
Wistar Kyoto rats
- AVP
vasopressin
Appendix
Novelty and Significance
What is known?
Prorenin receptor (PRR) is a new member of renin angiotensin (RAS) system. PRR can bind either renin or prorenin, and this binding facilitates angiotensin II (AngII) generation and stimulates signal transduction in the periphery.
What new information does this article contribute?
PRR is more highly expressed in the supraoptic nucleus (SON) of spontaneously hypertensive rats (SHR) than in normotensive Wistar Kyoto rats (WKY).
Overexpression of PRR in the SON increases vasopressin (AVP) secretion and alters fluid balance in normal rats.
Knockdown of this receptor significant attenuates the age-dependent increases in blood pressure and causes a decrease in heart rate in the SHR.
Summary
Our study shows that brain PRR is physiologically relevant since increase or decrease this receptor in the brain affects physiological status. In addition, our data show that brain PRR mediates AngII generation and stimulates signal transduction which supports the presence of a functional intrinsic brain RAS. Most previous studies implicating PRR’s functions are from pathophysiological states or from in vitro situations. Our study supports the concept that even under physiological conditions, the PRR contributes to cardiovascular regulation in a normotensive animal. Moreover, our results also support the idea that increased activity of the PRR contributes to hypertension, because arterial pressure was reduced by its chronic knockdown. Finally, our data suggests, for the first time, that SON plays an important role in the control of blood pressure.
Footnotes
Disclosures None
References
- 1.Cuadra AE, Shan Z, Sumners C, Raizada MK. A current view of brain renin-angiotensin system: Is the (pro)renin receptor the missing link? Pharmacol Ther. 2010;125:27–38. doi: 10.1016/j.pharmthera.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto S, Cassell MD, Beltz TG, Johnson AK, Davisson RL, Sigmund CD. Elevated blood pressure in transgenic mice with brain-specific expression of human angiotensinogen driven by the glial fibrillary acidic protein promoter. Circ Res. 2001;89:365–372. doi: 10.1161/hh1601.094988. [DOI] [PubMed] [Google Scholar]
- 3.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology (Bethesda) 2008;23:187–193. doi: 10.1152/physiol.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazartigues E. A map and new directions for the (pro)renin receptor in the brain: focus on “A role of the (pro)renin receptor in neuronal cell differentiation”. Am J Physiol Regul Integr Comp Physiol. 2009;297:R248–249. doi: 10.1152/ajpregu.00287.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lippoldt A, Fuxe K, Luft FC. A view of renin in the brain. J Mol Med. 2001;79:71–73. doi: 10.1007/s001090100215. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417–1427. doi: 10.1172/JCI14276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, Corvol P, Schwartz CE, Nguyen G. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol. 2009;297:R250–257. doi: 10.1152/ajpregu.90832.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol. 2008;93:701–708. doi: 10.1113/expphysiol.2008.041988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang J, Noble NA, Border WA, Owens RT, Huang Y. Receptor-dependent prorenin activation and induction of PAI-1 expression in vascular smooth muscle cells. Am J Physiol Endocrinol Metab. 2008;295:E810–819. doi: 10.1152/ajpendo.90264.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


