Summary
In obesity and the early stages of type 2 diabetes (T2D), pro-inflammatory cytokines are mildly elevated in the systemic circulation. This low-grade systemic inflammation exposes pancreatic islets to these circulating cytokines, but at levels ~100–1000x lower than seen within the islet during insulitis, which have not been well described. We examined mouse islets treated overnight with a low-dose cytokine combination commonly associated with inflammation (TNF-alpha, IL-1 beta, and IFN-gamma). We then examined islet function primarily using intracellular calcium ([Ca2+]i), a key component of insulin secretion and cytokine signaling. Cytokine-treated islets demonstrated several features that suggested dysfunction including excess [Ca2+]i in low physiological glucose (3mM), reduced responses to glucose stimulation, and disrupted [Ca2+]i oscillations. Interestingly, islets taken from young db/db mice showed similar disruptions in [Ca2+]i dynamics as cytokine-treated islets. Additional studies of control islets showed that the cytokine-induced elevation in basal [Ca2+]i was due to both greater calcium influx through L-type-calcium-channels and reduced endoplasmic reticulum (ER) calcium storage. Many of these cytokine-induced disruptions could be reproduced by SERCA blockade. Our data suggest that chronic low-grade inflammation produces circulating cytokine levels that are sufficient to induce beta-cell dysfunction and may play a contributing role in beta-cell failure in early T2D.
Keywords: oscillations, islets, cytokines, transplantation, inflammation, calcium, insulin, biphasic, beta cells, endoplasmic reticulum, ER stress
1. Introduction
Proinflammatory cytokines play a prominent role in the pathophysiology of type 1 diabetes (T1D) [1]. Tumor necrosis factor alpha (TNF-a), interleukin (IL)-1B, and interferon-gamma (IFN-g) are the most abundant pro-inflammatory cytokines from the infiltrating immune cells, and these cytokines act synergistically to inflict direct inhibitory and cytotoxic effects on pancreatic beta cells [1–4]. Increasing evidence suggests that inflammatory mediators also play a significant role in the loss of beta-cell mass during the latter stages of type 2 diabetes (T2D) [5,6] and may arguably link the pathologies of the T1D and T2D [6–8].
Prior to the onset of T2D, the metabolic stress of obesity causes adipocytes to begin to secrete low levels of TNF-a [9,10]. This is thought to stimulate endothelial cells and preadipocytes to secrete monocyte chemoattracting protein-1 (MCP-1), which in turn attracts macrophages to the adipose tissue [9,10]. Macrophages are the source of several additional proinflammatory cytokines that include TNF-a, IL-1B, and IFN-g [1–4]. This results in a low-grade, chronic, systemic inflammation that elevates certain proinflammatory cytokines to several fold over basal levels into the low pg/ml range (100–1000x less than observed in direct islet infiltration). Recent clinical studies have identified several cytokines, including IL-1B and TNF-a, as risk factors for subjects developing T2D based on elevated blood serum levels [11–13]. Whether cytokines at these low concentrations are sufficient to directly impact islet function is still unknown [14].
Intracellular calcium ([Ca2+]i) plays an important role in the signaling cascades of proinflammatory cytokines [15–17]. Cytokines can induce cell death by raising cytosolic calcium either through depletion of ER calcium [18–20] or by disrupting plasma membrane calcium flux [21]. In the NOD mouse model of T1D, increased expression of the low-voltage activated (LVA) T-type calcium channel was observed in beta-cells, resulting in elevated basal [Ca2+]i [22]. In addition, chronic treatment of beta cells from control mice with a combination of cytokines upregulated expression of this channel [22]. Also, exposing beta cells to serum isolated from T1D patients induced apoptosis, but not when the L-type channel blocker nifedipine was included in the media [23,24].
Even without causing cell death, cytokines can negatively impact beta-cell function. Cytokines directly reduce insulin secretion in response to glucose [1], in part, through their effects on [Ca2+]i handling since calcium is a key component of the insulin secretory pathway [25]. Reduced insulin release can exacerbate T2D in the face of greater insulin demand and can also exacerbate T1D in the face of progressive deterioration of beta-cell mass and function. Calcium is thus a potentially sensitive marker for assessing the effects of cytokine action on beta-cell function.
In this study, we examined the effects of cytokines at progressively lower concentrations to within the range of serum levels estimated from studies of patients with type 2 diabetes [11–13] in order to determine if mild cytokine exposure would disrupt islet function and/or induce islet damage. We subjected mouse islets overnight to the cytokines TNF-alpha, IL-1beta, and IFN-gamma, and then examined several aspects of islet calcium handling as measures of islet function. We also compared these cytokine-induced effects with the changes in islet function that occur during the early stages in disease progression in the db/db mouse model of T2D. Our findings suggest that low-dose cytokine treatment is sufficient to disrupt normal islet function, which may contribute to beta-cell loss under conditions of chronic low-grade systemic inflammation.
2. Materials and methods
2.1 Mice and islet isolation
All mice were housed in a pathogen-free facility in the Center for Comparative Medicine at the University of Virginia (UVA) for use in all studies. Male CD-1 mice at ages of 8–12 weeks were used for studies involving cytokines (Charles River Laboratories, MA). Additional studies were conducted using male Cg-m+/+Leprdb/J (db/db) mice as a model of T2D, with age-matched with heterozygous db/db mice as controls (Jackson Laboratories, Bar Harbor, ME). Studies were also repeated using age-matched C57BLKS/J (BKS) mice as controls.
Mice were euthanized according to IACUC approved protocol, and their pancreatic islets were isolated by collagenase digestion as previously described in detail [26]. Briefly, the pancreas was perfused though the common bile duct with 5mL of 1.4 mg/mL collagenase P (Roche Diagnostics, Indianapolis, IN), then removed and incubated at 37 °C for 8–11 minutes in 1 mL HBSS solution. Following incubation, pancreatic tissue was centrifuged and resuspended in Histopaque 1100 (Sigma-Aldrich, St. Louis, MO) and centrifuged again to separate islets from acinar tissue. All islets were incubated overnight in RPMI-1640 medium (Invitrogen) to allow sufficient recovery time from collagenase digestion before any experiments were performed.
2.2 Cytokines and drug treatments
The cytokines chosen for this study are used widely as a means of inducing inflammatory responses in islets [2,4,27,28]. Mouse cytokines (R&D Systems, Inc., Minneapolis, MN) were used at fractional concentrations of the following full dose: 10 ng/ml for TNF-alpha, 5 ng/ml for IL-1beta, and 100 ng/ml for IFN-gamma in PBS [29]. Islets were treated overnight with cytokines following a full day of recovery from the isolation procedure. Thapsigargin and cyclopiazonic acid, which promote the release of ER calcium, and nifedipine, used to block L-type voltage-gated calcium channels, were purchased from Sigma-Aldrich (St. Louis, MO) and prepared in 1000x stock concentration in DMSO (final DMSO <0.1%). All experimental tests were performed 1–2 days after isolation.
2.3 Intracellular calcium ([Ca2+]i)
[Ca2+]i was measured using the ratiometric [Ca2+]i indicator fura-2 AM using previously described methods [29,30]. Briefly, Cell Tracker Red CMTPX (Invitrogen), a membrane penetrating fluorescent probe, was used in order to distinguish cytokine-treated islets from control islets, thus enabling a simultaneous comparison of the two treatment groups [30]. Islets were loaded with 1 uM fura-2 AM and 0.2 uM CTR (30–40 min), washed, and then recorded with a Hamamatsu ORCA-ER camera (Hamamatsu Photonics, Japan) attached to an Olympus BX51WI fluorescence microscope (Olympus, Tokyo, Japan) using 340 and 380 nm excitation light and 510 nm emission. During each recording, islets were perifused in a small volume chamber (Warner Instruments, Hamden, CT) with KRB solution using a peristaltic pump (Gilson, France) at ~35°C by an in-line heater (Warner Instruments, Hamden, CT). Islets were incubated in 3 mM glucose for 15-min and then recorded to determine islet response to 28 mM glucose stimulation. Glucose-stimulated [Ca2+]i response (GSCa) was calculated as the change in islet [Ca2+]i as measured by fura-2 ratio (340/380 nm fluorescence) during the switch from 3 to 28 mM glucose. Data were analyzed with IP Lab software Version 4.0 (Scanalytics, Rockville, MD).
2.4 Cell death measurements
Islets were treated with 20 ug/ml concentration PI and incubated for 10 min. Islets were imaged once under brightfield illumination to determine the islet borders and imaged again to measure PI fluorescence using 535 nm excitation and 617 nm emission. It should be noted that PI is a fairly blunt indicator of cell death that does not distinguish between apoptosis and necrosis, so it is possible for islets to be apoptotic but not positive for PI staining. However, PI staining was used in this context to simply guide the selection of cytokine doses to induce islet dysfunction for these studies.
2.5 Islet insulin secretion
After overnight incubation, islets were tested for insulin secretion as described previously [29,30]. Briefly, islets were preincubated at 37 °C and 5% CO2 for 1 hour in a standard KRB solution, then washed and incubated in KRB supplemented with 3 mM glucose for 1 hour followed by a 1-hour treatment with KRB containing 28 mM glucose. The supernatant was collected after each treatment and insulin concentration in the supernatant was measured by an ELISA method (Mercodia, Uppsala, Sweden) according to the manufacturer’s instructions. The intra-assay variation was 3.6% and inter-assay variation was <10%.
2.6 Data analysis and statistics
[Ca2+]i patterns were smoothed with a 3-point moving average and analyzed initially by the pulse detection algorithm CLUSTER8, as used previously [29,31] using the following parameters: 10-sec minimum peak and minimum nadir size (2 points), 2.0 for t-score to detect peaks and nadirs, and no minimum value for peak amplitude. False positives were kept below 5% by using point-to-point noise in [Ca2+]i during 3 mM glucose (non-oscillatory glucose concentration) as a standard deviation for pulse detection. To improve the detection of possible false negatives, the data were reanalyzed using a t-score of 1, producing the same results. In a subset of recordings, we observed no significant difference between CLUSTER8 calculations of period and direct measurement from the start of one cycle to the start of the next, so the direct method was used. Oscillatory capacity was calculated as the percentage of islets with oscillatory activity among all islets recorded. Fisher’s exact test was used to compare oscillatory and non-oscillatory islets among db/db and het mice. A two-tailed t-test was used for all other two-group comparisons, with a p-value of p <0.05 used as an indication of statistical significance. Statistical analysis was performed using Prism version 4 software.
3. Results
3.1 Dose-response curve for cytokine-induced cell death
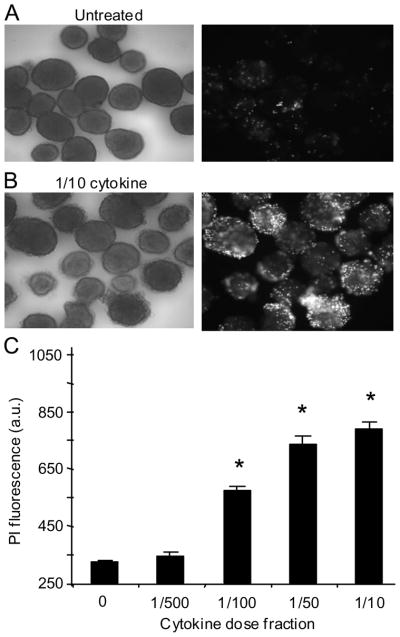
To first establish the effective dose range to induce islet dysfunction, islets were treated with a combination of cytokines at several different fractions of the following dose: 10 ng/ml TNF-alpha 5 ng/ml IL-1beta + 100 ng/ml IFN-gamma [29,30]. As shown in Figure 1A, untreated islets excluded propidium iodide (PI), a dye used to estimate cell death, whereas cytokine-treated islets showed substantial PI uptake, particularly at higher cytokine concentrations (Figure 1B). Cytokine-induced PI fluorescence increased in a dose-dependent manner as shown in Figure 1C with an EC50 of 0.0097 (~1/100 of the full dose). For subsequent experiments, we limited the cytokine concentrations to doses that were above (1/50), at (1/100), and below (1/500) the EC50 (see also Methods for caveats).
FIGURE 1.
Cytokine-induced islet cell death. A–B, Bright field image of control untreated islets (A, left panel) and corresponding PI fluorescence (A, right panel) compared with an image of islets treated overnight with 1/10 fractional dose of cytokines (B, left panel) and corresponding PI fluorescence (B, right panel). C, Islet PI fluorescence in response to a graded increase in cytokine concentrations. Each bar represents an average of 30–45 islets. Note that the pixel intensity of the background in this recording system was ~260 arbitrary units (a.u.). * indicates P < 0.001.
3.2 Low-dose cytokine effects on glucose-stimulated [Ca2+]i and insulin secretion
We investigated cytokine effects on islet function initially by measuring changes in [Ca2+]i in response to glucose stimulation. Typical responses to glucose stimulation are shown in Figure 2A for islets treated with a cytokine dose of 1/100, 1/500 or 0. Cytokines substantially elevated basal [Ca2+]i in 3 mM glucose and reduced peak stimulation during 28 mM glucose in these examples. We chose 28 mM glucose stimulation in order to elicit a maximal calcium response [32,33], which is needed to determine if cytokines reduce the full capacity to respond to glucose, Distinguishing between a decrease in stimulatory capacity and a shift in glucose sensitivity is more difficult using more physiological concentrations, such as 11 mM. As summarized in Figure 2B, these effects were consistent with mean values observed with 1/50, 1/100, and 1/500 cytokine doses, which ultimately reduced the overall [Ca2+]i response to glucose stimulation (Figure 2C).
FIGURE 2.
Glucose-stimulated calcium (GSCa) and insulin (GSIS) responses following cytokine treatment. A, Representative traces of [Ca2+]i changes in response to glucose stimulation for islets treated with 0, 1/500, or 1/100 cytokine dose. B, Mean [Ca2+]i values at 3 and 28 mM glucose following different cytokine doses (n= number of islets for each treatment). C, Mean change in calcium following glucose stimulation (n= same as in B). D, Insulin secretion from 50 islets per condition during incubation in 3 mM glucose for 1 hour (white bars) followed by incubation in 28 mM glucose for 1 hour (black bars). E, Mean increase in insulin secretion following glucose stimulation. F, Mean increase in glucose stimulation index. * indicates P < 0.05 difference between insulin levels at 3 vs. 28 mM glucose for each cytokine dose. A total of 7 replicates for each condition were used for insulin measurements.
In parallel studies using islets from the same mice, glucose stimulated insulin secretion (GSIS) was measured using the same glucose concentrations as for GSCa. As shown in Figure 2D, GSIS gradually declined but did not reach significance until the 1/50 dose, a ten-fold higher concentration than needed to detect significant changes in GSCa. Similarly, the total stimulated insulin release (calculated by subtracting stimulated insulin secretion from basal insulin secretion, Figure 2E) and the ratio between stimulated and basal insulin (also called the stimulation index, Figure 2F) did not differ significantly until the 1/50 dose. It should be noted that the inherently greater variability in measuring insulin and the smaller number of replicates could obscure a possible trend toward reduced GSIS correlated with increasing cytokine dose. Beta-cell insulin exocytosis therefore maybe impaired by low-dose cytokine treatment; the effects of cytokines on [Ca2+]i, however, were much more readily detectable by comparison.
3.3 ER and ion-channel contributions to cytokine effects on basal [Ca2+]i
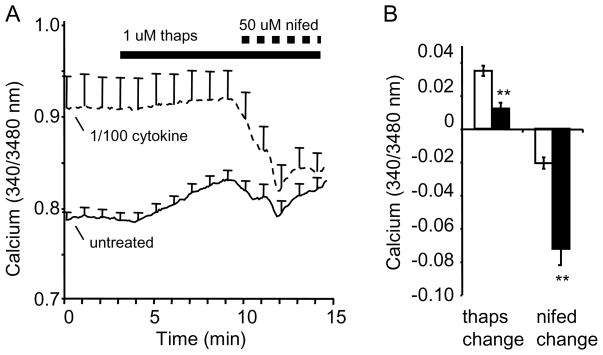
Cytokine effects were most striking on basal [Ca2+]i, even at the lowest cytokine dose used. To determine the mechanism underlying this effect, we investigated the two largest sources of [Ca2+]i: 1) plasma membrane calcium channels and 2) the endoplasmic reticulum (ER). As shown in Figure 3A, islets treated overnight with a 1/100 cytokine dose had substantially higher basal [Ca2+]i in 3 mM glucose than untreated islets. However, this gap narrowed due to a substantial rise in [Ca2+]i among untreated islets following thapsigargin treatment to promote ER calcium release. When L-type voltage-gated calcium-channels were additionally blocked by nifedipine treatment, the [Ca2+]i gap was largely eliminated due to a much larger drop in [Ca2+]i among cytokine-treated islets compared to untreated islets. Figure 3B shows that both ER (blocked by thapsigargin) and calcium influx (blocked by nifedipine) contribute to cytokine-induced increases in basal [Ca2+]i.
FIGURE 3.
Contributions of the endoplasmic reticulum and ion-channel activity to cytokine-induced changes in basal [Ca2+]i. A, Average of several traces from untreated (solid, n=9) and overnight cytokine-treated islets (dashed, n=8). Islets began in 3 mM glucose and were then treated with thapsigargin and then thapsigargin + nifedipine as shown. B, Mean change in [Ca2+]i during thapsigargin treatment and thapsigargin + nifedipine treatment. * indicates p<0.05.
3.4 Cytokines disrupt islet [Ca2+]i oscillations
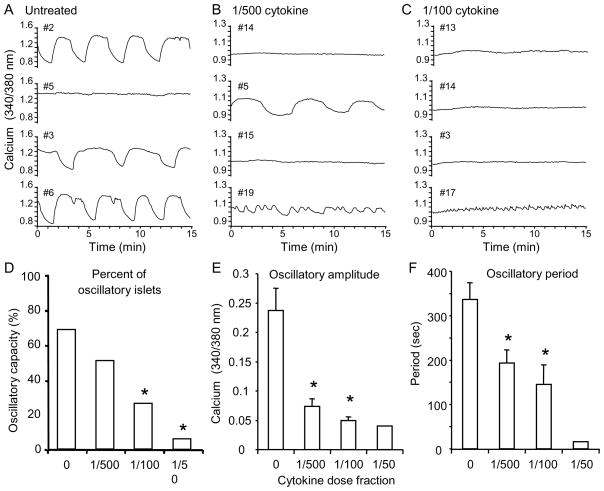
[Ca2+]i oscillations are endogenously produced in the presence of stimulatory (11 mM) glucose and are concurrent with pulses of insulin secretion [34]. Impaired pulsatile insulin secretion has been linked with T2D patients [35] and their non-diabetic relatives [36]. In our studies, untreated islets were typically oscillatory (34 of 49, 70%). As shown in the representative example in Figure 4A, all but one untreated islet (#5) showed robust and regular oscillatory activity. Overnight treatment with pro-inflammatory cytokines, however, reduced or eliminated oscillatory activity. As shown in the representative examples in Figure 4B, only 50% of islets showed any oscillatory activity at a 1/500 cytokine dose, and of those, only #5 showed the slow oscillations characteristic of the untreated islets. Islet #19 showed only rapid ~15 sec oscillations characteristic of electrically-driven bursts [33,37]. Oscillatory capacity was further degraded at the 1/100 cytokine dose, as shown by representative example in Figure 4C, in which only ~25% of islets displayed [Ca2+]i oscillations (represented by #17). These findings are summarized in Figure 4D-F for the various cytokine doses. Percent of oscillatory islets (Figure 4D), oscillatory amplitude (Figure 4E), and oscillatory period (Figure 4F) all declined in a dose-dependent manner.
FIGURE 4.
Cytokine-induced changes in islet oscillations. A–C, [Ca2+]i patterns during a 15-min record in steady-state 11 mM glucose for untreated islets (A) and for islets treated with a 1/500 cytokine dose (B) or 1/100 dose (C). D–F, The percentage of islets displaying oscillations (D), mean peak-to-peak amplitude of oscillations (E), and mean period of oscillations (F) declined with increased cytokine concentration. The number of oscillatory islets among total islets recorded was n=34/49 for untreated, n=22/45 for the 1/500 dose, n=11/45 for the 1/100 dose, and 1/22 for the 1/50 cytokine dose. * indicates P < 0.01. Note the different scales in (A–C).
3.5 The ER as a possible mediator of low-dose cytokine effects on [Ca2+]i oscillations
We further analyzed these data by determining the percentage of ‘fast oscillators’ as a possible indicator of disrupted beta-cell metabolism. Fast oscillations in insulin release are considered too frequent to be detected by standard blood sampling intervals in vivo and would thus be interpreted as irregular or non-rhythmic [38]. A 2-min cutoff was used to distinguish between the two forms of oscillations based on a previously published bimodal distribution [38]. As shown in Figure 5A, the percentage of fast islets increased with each increased cytokine dose (untreated: 5 fast, 24 slow; 1/500 cyto: 11 fast, 10 slow; 1/100 cyto: 8 fast, 3 slow; 1/50 cyto: 1 fast, 0 slow). Since the ER is a substantial calcium-storing organelle and a known cytokine target, we used thapsigargin to examine the ER as a possible mediator of the observed effects. Acute treatment of oscillatory islets with thapsigargin decreased the period of islet oscillations as shown by two representative examples in Figure 5B. Both fast (n=6) and slow (n=2) islets exhibited similar decreases in oscillatory period, as shown by others [39,40]. Among 8 islets treated with 1 uM thapsigargin, all islets showed a decrease in oscillatory period (mean period: 75 ± 15 sec to 31 ± 12 sec, n=8, p<0.05), indicating that these cytokine-induced effects on oscillations are consistent with disruptions in ER calcium handling. We observed even more substantial disruptions using another SERCA blocker, cyclopiazoinc acid (CPA, 50 uM). Acute CPA treatment induced elevated [Ca2+]i and a loss of slow oscillations as shown in Figure 5B (bottom panel, representative of n=8 islets).
FIGURE 5.
Cytokines or thapsigargin treatment similarly increase the frequency of islet oscillations. A, Relative percent of fast oscillations among all oscillatory islets at each cytokine dose. B, Two representative examples of islets treated with thapsigargin showing a shift from slow to fast oscillations. C, Example of 48-hour treatment with the SERCA inhibitor CPA showing impaired GSCa compared to untreated control islets. D, Summary of effects of thapsigargin (n=22 untreated, n=16 thapsigargin-treated, p<0.001) or CPA (n=17 untreated, n=11 CPA-treated, p<0.01) on peak change in [Ca2+]i during glucose stimulation.
We also examined long-term ER calcium deprivation as a possible mechanism for the reduced glucose sensitivity in cytokine-treated islets. As shown in Figure 5C, exposure to CPA for 48 hours impaired GSCa compared to untreated control islets. This effect was similar to overnight cytokine treatment, both in terms of elevated basal [Ca2+]i and reduced response to stimulation, though not as severely. As summarized in Figure 5D, both CPA and thapsigargin significantly reduced the GSCa compared to their respective controls, providing further evidence that the ER plays a role in cytokine-mediated islet dysfunction.
3.6 Cytokines induce islet dysfunction at levels consistent with low-grade systemic inflammation
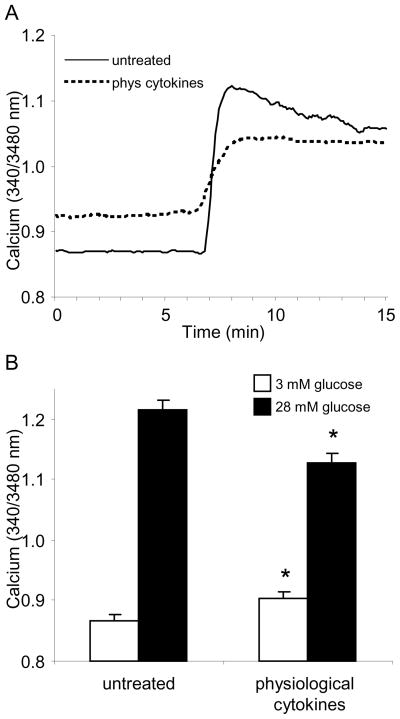
The cytokine concentrations typically found in the blood stream are somewhat lower than what has been presented thus far. Using estimates from the Handbook of Human Immunology as a guide [41] (see Table 17.1), we designed a cocktail containing 10 pg/ml TNF-a + 15 pg/ml IL-1B + 1 pg/ml IFN-g or untreated. As shown by the representative examples in Figure 6, the magnitude of cytokine effects on calcium handling in response to 28 mM glucose were slightly diminished, but the key features were quite similar. Elevated basal calcium and reduced peak were observed with the low pg/ml cytokine treatments.
FIGURE 6.
Cytokines can reproduce key inhibitory effects on islet function at the low physiological concentrations found in circulating blood. (A) Examples of GSCa traces for islets exposed overnight to “physiological” concentrations (10 pg/ml TNF-a + 15 pg/ml IL-1B + 1 pg/ml IFN-g) or untreated. (B) Mean values for basal and peak calcium values during glucose stimulation among cytokine-treated (n=40) and untreated (n=38) islets.
We also tested the individual effects of each of these low dose cytokine treatments. As shown in Figure 7A, 15 pg/ml of IL-1B elevated basal calcium levels and decreased peak glucose stimulation, as observed with the combination (see Figure 6). However, treatment with 10 pg/ml TNF-a (Figure 7B) and 1 pg/ml IFN-g (Figure 7C) had no significant effects on calcium handling compared to untreated islets. These results suggest that IL-1B is the key cytokine affecting islet calcium handing, however, it should be noted that these cytokines also act synergistically to negatively impact islet function [4,16,21,28].
FIGURE 7.
Effects of individual cytokines on islet calcium handling. (A-C) GSCa traces for islets exposed overnight to treatments of (A) 15 pg/mL IL-1B (B) 10 pg/mL TNF-a (C) 1 pg/mL IFN-g, compared to untreated islets. N=22–35 islets were used for each treatment condition. **, p<0.01; ***, p<0.001.
3.7 Islets from db/db mice show similarities to low-dose cytokine-treated islets
The data presented thus far demonstrate that very low doses of cytokines disrupt islet function in terms of GSCa, ER calcium handling, and oscillations. In order to compare these effects with a well-known model of type 2 diabetes, we examined islets from the db/db mouse during the early stages of the disease at 6 and 12 weeks of age using heterozygous db/db mice as controls. Db/db mice have immune deficiencies [42] and are more sensitive to proinflammatory cytokine actions [43]. Body weight and blood glucose levels were normal at 6 weeks, but db/db mice displayed elevated body weight and blood glucose by 12 weeks as summarized in Table 1. IL-1B levels were measured in parallel studies of db/db mice that utilized age-matched BKS mice as controls. IL-1B levels were significantly elevated for db/db mice at 6 weeks of age and similarly elevated at 12 weeks of age, though not significantly due to a low n (see Table 1).
TABLE 1.
Progressive islet dysfunction in db/db mice.
| measurement | 6 weeks | 12 weeks | ||
|---|---|---|---|---|
| control | db/db | control | db/db | |
| body weight (g) | 21.1 ± 0.8 N = 12 mice |
22.9 ± 1.1 N = 19 mice |
27.7 ± 0.4 N = 6 mice |
*40.7 ± 1.2 N = 14 mice |
| blood glucose (mg/dl) | 112.3 ± 5.4 N = 12 mice |
123.5 ± 10.9 N = 19 mice |
96.7 ± 10.5 N = 6 mice |
*344 ± 27.3 N = 14 mice |
| serum IL-1B (pg/ml) | 14.2 ± 5.0 N = 6 mice |
*42.6 ± 15.1 N = 7 mice |
2.8 ± 0.1 N = 4 mice |
52.0 ± 17.1 N = 3 mice |
| basal calcium (3G) | 0.815 ± 0.011 N = 25 islets |
0.817 ± 0.012 N = 23 islets |
0.776 ± 0.015 N = 14 islets |
*0.922 ± 0.022 N = 21 islets |
| peak calcium (28G) | 1.122 ± 0.013 N = 25 islets |
1.101± 0.010 N = 23 islets |
1.339 ± 0.026 N = 14 islets |
*1.126 ± 0.023 N = 21 islets |
| GSCa | 0.307 ± 0.019 N = 25 islets |
0.284 ± 0.014 N = 23 islets |
0.563 ± 0.038 N = 14 islets |
*0.204 ± 0.041 N = 21 islets |
| percent oscillating | 92% (13 of 14) | *42% (14 of 33) | 92% (45 of 49) | *17% (8 of 48) |
p < 0.05.
3G = 3 mM glucose, 28G = 28 mM glucose. Units for calcium measurements = fura-2 ratio (340/380 nm).
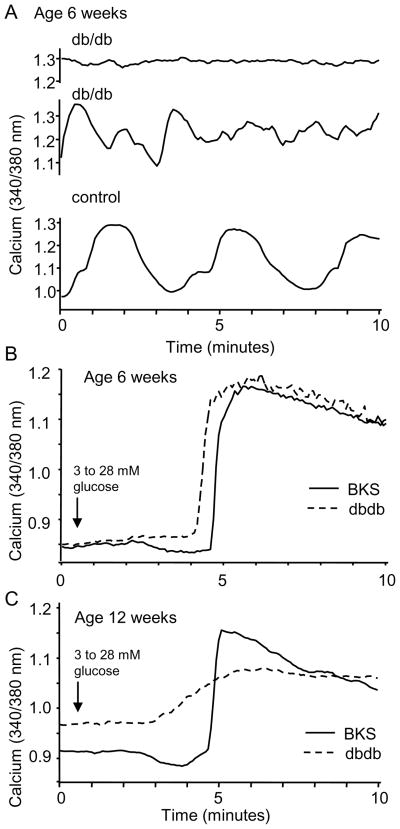
Using [Ca2+]i as a marker of islet health, the most striking differences between the db/db islets and heterozygous controls were observed in their oscillatory activity, basal [Ca2+]i levels, and response to glucose stimulation. Db/db islets at 6 weeks displayed either no oscillations or irregular patterns in comparison to control islets, as shown by the representative examples in Figure 7A. At 6 weeks of age, only 42% of db/db islets retained their oscillatory capacity in steady-state 11 mM glucose, whereas 92% of control islets displayed oscillations a shown in Table 1. By 12 weeks, oscillations were observed in only 17% of db/db islets compared to 92% of controls (see Table 1). These data suggest either a deficiency in the processes needed to generate oscillations or a shift in glucose sensitivity, such that normally oscillatory processes in the islet are saturated in 11 mM glucose, as suggested previously [44]. Despite the differences in oscillations, the GSCa among db/db islets was not significantly different from controls at 6 weeks of age (see Table 1). By 12 weeks of age, however, the GSCa among islets from db/db mice was significantly attenuated compared to controls and basal [Ca2+]i levels were elevated, as shown in Figure 7B and Table 1. These results echo the decreased oscillatory capacity, elevated basal calcium levels, and reduced maximal peak that were observed among cytokine-treated islets. A comparison of cytokine-treated, db/db, and control islets in Table 2 summarizes these signs of islet dysfunction.
TABLE 2.
Comparing islets from the db/db mouse with low-dose cytokine-treated islets.
| Oscillation parameter | cytokines | 6-week db/db | 12-week db/db |
|---|---|---|---|
| Basal Ca | |||
| GSCa peak | |||
| Percent Oscillation |
4. Discussion
Our findings suggest that cytokines at concentrations found circulating in the blood (low pg/ml) are sufficient in quantity to cause islet dysfunction by disrupting calcium handling in mouse islets. Calcium is a key component of the pathway that couples glucose stimulation with insulin secretion [25], but excess cytosolic calcium can lead to beta-cell dysfunction and cell death [15,16]. Exposure to serum from T1D patients has been shown to elevate basal calcium levels in clonal beta-cells [24] and to promote death in primary beta-cells in a calcium-dependent fashion [23]. Serum levels of proinflammatory cytokines are also elevated in the prediabetic stage of T1D and in first-degree relatives [45]. Among the many other well-established immunological factors involved with beta-cell destruction in T1D [46], these observations collectively suggest that circulating cytokines could contribute in a calcium-dependent manner to ongoing beta-cell death throughout the course of the disease [47] and following islet transplantation [48].
Low-grade systemic inflammation may also contribute to the pathology of T2D. Inflammation of the adipose tissue occurs prior to diabetes during the development of obesity. As adipocytes increase in size, they begin to secrete low levels of TNF-a, which can stimulate endothelial cells and preadipocytes to secrete monocyte chemoattracting protein-1 (MCP-1). This, in turn, attracts macrophages to the adipose tissue [6,9,10]. Macrophages are the sources of several additional proinflammatory cytokines that include TNF-a, IL-1B, IFN-g, IL-6, and others [6,9,10]. Due to infiltration of activated macrophages into adipose tissue, levels of these cytokines can increase in the systemic circulation several fold over basal levels [11–13]. Thus, cytokines produced by adipose and other tissues in response to obesity can reach the pancreatic islet by systemic circulation, albeit in low pg/ml concentrations, to potentially impact islet function.
We also show that low-grade inflammation in the db/db model of T2D correlates with reduced islet function. Our db/db mice also showed some signs of dysfunction (loss of oscillatory capacity) at least as early as 6-weeks of age, at which time body weight and blood glucose were still considered normal, with much greater declines in calcium handling observed by 12 weeks. Note that previous work by Roe et. al established similar declines in calcium handling as we report [44], although the disease progression was more rapid in their study. Our data also show that IL-1B levels are significantly elevated by 6 weeks of age in the db/db strain, so it is thus reasonable to suspect that these elevated cytokines can contribute to the deterioration of islet function between 6 and 12 weeks in db/db mice. Whether the cytokines are derived from adipose tissue or from other sources remains an open question. Regardless of the source, our data suggest that circulating levels of cytokines are sufficient to alter normal islet function and perhaps cause long-term damage under conditions of chronic inflammation in T2D. Of interest, recent work has shown that increasing levels of IL-1 receptor antagonist (also called anakinra) can reduce the deleterious effects of obesity in mice [49] and can reduce markers of systemic inflammation in patients with T2D [50].
We also found that cytokines elevated basal [Ca2+]i without concurrently increasing basal insulin secretion, suggesting a decoupling of the normal stimulus-secretion coupling. Although there are several circumstances in which insulin secretion can be stimulated without changes in [Ca2+]i [32,51] an increase in [Ca2+]i is normally coupled to an increase in insulin secretion [25]. Calcium microdomains are thought to play a role in regulating important cellular processes including insulin secretion, metabolism, and maintaining cell viability [52]. If cytokines are misdirecting calcium to inappropriate subcellular regions, then this could lead to problems with organelles that rely on dynamic changes in calcium such as the ER [17,19], nucleus [53,54] and mitochondria [55–58]. The mitochondria represent a particularly intriguing target since ATP produced in the mitochondria triggers KATP-channel mediated depolarization of the beta cell by increasing the ATP/ADP. Since cytokines disrupt mitochondrial function and promote oxidative stress [55–58], this may directly impact intracellular calcium and insulin release. Although not the focus of the present study, determining whether the mitochondrial effects of low-dose cytokines are linked with disruptions in calcium handling merits further investigation.
Another potential early warning of cytokine-mediated islet dysfunction is the progressive deterioration of endogenous [Ca2+]i oscillations. The loss of pulsatile secretion appears to be an early stage of T2D, with reduction in the amplitude and possibly frequency of the insulin pulses being linked to diabetic patients [59,60]. The close relatives of diabetic patients also demonstrate considerable degradation of pulsatile insulin secretion despite reporting clinically normal responses to glucose challenges and normal levels of insulin resistance [35,36]. Interestingly, diabetic patients were found to respond more effectively to pulsatile insulin delivery than to continuous delivery [61–63]. This is the first report to our knowledge describing detailed effects of cytokines on islet [Ca2+]i oscillations. The observed signs of dysfunction include: (a) fewer islets displaying oscillations, (b) reduced amplitude, and (c) decreased period (increased frequency) among the remaining oscillatory islets. These effects were caused, at least in part, by the disruption of slow oscillations, which resulted in small amplitude and high frequency rhythms (also known as ‘fast bursting’). The preferential disruption in the slow oscillations suggests disruptions in some aspect of beta-cell glucose metabolism/sensitivity [64], disruption in communication among cells within the islet [65–67], or possibly changes in other islet cell types like glucagon-secreting alpha cells [68,69]. The shift in oscillatory period is also consistent with the depletion of ER calcium [39,40]. Since normal healthy islets often display fast, slow, or mixed oscillations [33,37], it is premature to assume that fast oscillating islets are inherently dysfunctional. Oscillations are only present within a range of glucose concentrations [33], so changes in glucose sensitivity could also alter or eliminate oscillations. Our data suggest, however, that the slow form of oscillation is particularly sensitive to cytokine effects, which may be an early indicator islet stress.
It should be noted that cytokine signaling is complex and can result in markedly different effects depending on the dose, duration, and combination of cytokines involved. For example, short-term treatment with low-dose IL-1B improves the function of rat islets, whereas long-term, high dose treatment impairs islet function [70–72] and accelerates the development of T1D [73]. Low-dose TNF-a treatment has been shown to inhibit the autoimmune response in models of T1D [74–76], whereas TNF-a and IFN-g also have dose- and duration-dependent inhibitory effects on islet function in vitro [21,77]. Differences among duration and dose of treatment [78,79], species being tested [28,80], and combination of cytokines [4,27,28] are all important factors that likely contributed to the variable results among previous studies. Further, synergistic activity among multiple cytokines can alter or amplify signaling pathways [81], adding an additional layer of complexity to cytokine action. In our hands, low-dose cytokine treatment resulted in elevated basal calcium and reduced response to stimulation, which we interpret as indications of islet dysfunction. An alternative interpretation is that these calcium effects are the result of a protective response in normal islets, akin to the ER stress response that limits ER activity until the stressor is removed. It should also be noted that low-dose cytokines may have markedly different effects on islets from normal healthy individuals as compared with diabetes-prone individuals in which underlying beta-cell defects may exist.
In summary, our findings indicate that much lower concentrations of pro-inflammatory cytokines are sufficient to produce marked effects on islet function than perhaps previously thought. Disruptions in ER calcium homeostasis, plasma membrane calcium flux, and endogenous oscillations may thus be among the early signs of islet dysfunction that lead to the targeted destruction of beta cells. Because defects in beta-cell development and/or function are crucial to the development of T2D, any stressor, including low-grade systemic inflammation, may have magnified effects on islets from T2D-prone individuals. We suggest that cytokines, produced from distal tissues and carried through the circulatory system, are sufficient in concentration to disrupt normal islet function and possibly contribute to beta-cell failure, particularly in diabetes-prone individuals.
FIGURE 8.
Db/db islets show decreased oscillatory capacity and a decreased response to glucose stimulation. A, Representative examples of db/db islets (top and middle) and a control (bottom) in 11 mM glucose. B–C, Representative example of a db/db and control islet responding to acute glucose stimulation from 3 to 28 mM glucose at 6 weeks (B) and 12 weeks of age (C).
Acknowledgments
This work was supported by a Pilot and Feasibility Award of the UVA DERC (DK063609), NIH grant 1K01 DK081621 to C.S.N., and UVA Harrison Undergraduate Research Award to P.J. Mouse islets were acquired through the Cell and Islet Isolation Core facility at UVA (DK063609). A special thanks to Kathryn Corbin for technical support for this project. Thanks also to colleagues for their support and critiques, especially Drs. James D. Johnson, Richard Bertram, Arthur Sherman, and Les Satin.
Footnotes
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rabinovitch A, Suarez-Pinzon WL. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem Pharmacol. 1998;55:1139–1149. doi: 10.1016/s0006-2952(97)00492-9. [DOI] [PubMed] [Google Scholar]
- 2.Mandrup-Poulsen T, Bendtzen K, Dinarello CA, Nerup J. Human tumor necrosis factor potentiates human interleukin 1-mediated rat pancreatic beta-cell cytotoxicity. J Immunol. 1987;139:4077–4082. [PubMed] [Google Scholar]
- 3.Campbell IL, Iscaro A, Harrison LC. IFN-gamma and tumor necrosis factor-alpha. Cytotoxicity to murine islets of Langerhans. J Immunol. 1988;141:2325–2329. [PubMed] [Google Scholar]
- 4.Pukel C, Baquerizo H, Rabinovitch A. Destruction of rat islet cell monolayers by cytokines. Synergistic interactions of interferon-gamma, tumor necrosis factor, lymphotoxin, and interleukin 1. Diabetes. 1988;37:133–136. doi: 10.2337/diab.37.1.133. [DOI] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and beta-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32(Suppl 3):24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- 7.Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81:455–470. doi: 10.1007/s00109-003-0450-y. [DOI] [PubMed] [Google Scholar]
- 8.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 9.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasouli N, Kern PA. Adipocytokines and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93:S64–73. doi: 10.1210/jc.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pickup JC, Chusney GD, Thomas SM, Burt D. Plasma interleukin-6, tumour necrosis factor alpha and blood cytokine production in type 2 diabetes. Life Sci. 2000;67:291–300. doi: 10.1016/s0024-3205(00)00622-6. [DOI] [PubMed] [Google Scholar]
- 12.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA-J Am Med Assoc. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 13.Spranger J, Kroke A, Mohlig M, et al. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- 14.Donath MY, Schumann DM, Faulenbach M, Ellingsgaard H, Perren A, Ehses JA. Islet inflammation in type 2 diabetes: from metabolic stress to therapy. Diabetes Care. 2008;31(Suppl 2):S161–4. doi: 10.2337/dc08-s243. [DOI] [PubMed] [Google Scholar]
- 15.Levy J. Abnormal cell calcium homeostasis in type 2 diabetes mellitus: a new look on old disease. Endocrine. 1999;10:1–6. doi: 10.1385/ENDO:10:1:1. [DOI] [PubMed] [Google Scholar]
- 16.Lee MS, Chang I, Kim S. Death effectors of beta-cell apoptosis in type 1 diabetes. Mol Genet Metab. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 18.Oyadomari S, Takeda K, Takiguchi M, et al. Nitric oxide-induced apoptosis in pancreatic beta cells is mediated by the endoplasmic reticulum stress pathway. Proc Natl Acad Sci U S A. 2001;98:10845–10850. doi: 10.1073/pnas.191207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardozo AK, Ortis F, Storling J, et al. Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes. 2005;54:452–461. doi: 10.2337/diabetes.54.2.452. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Zhang H, Zhao B, Fei H. IL-1beta caused pancreatic beta-cells apoptosis is mediated in part by endoplasmic reticulum stress via the induction of endoplasmic reticulum Ca2+ release through the c-Jun N-terminal kinase pathway. Mol Cell Biochem. 2009;324:183–190. doi: 10.1007/s11010-008-9997-9. [DOI] [PubMed] [Google Scholar]
- 21.Chang I, Cho N, Kim S, et al. Role of calcium in pancreatic islet cell death by IFN-gamma/TNF-alpha. J Immunol. 2004;172:7008–7014. doi: 10.4049/jimmunol.172.11.7008. [DOI] [PubMed] [Google Scholar]
- 22.Wang L, Bhattacharjee A, Fu J, Li M. Abnormally expressed low-voltage-activated calcium channels in beta-cells from NOD mice and a related clonal cell line. Diabetes. 1996;45:1678–1683. doi: 10.2337/diab.45.12.1678. [DOI] [PubMed] [Google Scholar]
- 23.Juntti-Berggren L, Larsson O, Rorsman P, et al. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993;261:86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- 24.Conroy SJ, Green I, Dixon G, et al. Evidence for a sustained increase in clonal beta-cell basal intracellular Ca2+ levels after incubation in the presence of newly diagnosed Type-1 diabetic patient sera. Possible role in serum-induced inhibition of insulin secretion. J Endocrinol. 2002;173:53–62. doi: 10.1677/joe.0.1730053. [DOI] [PubMed] [Google Scholar]
- 25.Mears D. Regulation of insulin secretion in islets of Langerhans by Ca(2+)channels. J Membr Biol. 2004;200:57–66. doi: 10.1007/s00232-004-0692-9. [DOI] [PubMed] [Google Scholar]
- 26.Carter JD, Dula SB, Corbin KL, Wu R, Nunemaker CS. A Practical Guide to Rodent Islet Isolation and Assessment. Biol Proced Online. 2009 doi: 10.1007/s12575-009-9021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corbett JA, Sweetland MA, Wang JL, Lancaster JR, Jr, McDaniel ML. Nitric oxide mediates cytokine-induced inhibition of insulin secretion by human islets of Langerhans. Proc Natl Acad Sci U S A. 1993;90:1731–1735. doi: 10.1073/pnas.90.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eizirik DL, Sandler S, Welsh N, et al. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994;93:1968–1974. doi: 10.1172/JCI117188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jahanshahi P, Wu R, Carter JD, Nunemaker CS. Evidence of diminished glucose stimulation and endoplasmic reticulum function in nonoscillatory pancreatic islets. Endocrinology. 2009;150:607–615. doi: 10.1210/en.2008-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crim WS, Wu R, Carter JD, et al. AGI-1067, a novel antioxidant and anti-inflammatory agent, enhances insulin release and protects mouse islets. Mol Cell Endocrinol. 2010 doi: 10.1016/j.mce.2010.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunemaker CS, Satin LS. Comparison of metabolic oscillations from mouse pancreatic beta cells and islets. Endocrine. 2004;25:61–67. doi: 10.1385/ENDO:25:1:61. [DOI] [PubMed] [Google Scholar]
- 32.Heart E, Corkey RF, Wikstrom JD, Shirihai OS, Corkey BE. Glucose-dependent increase in mitochondrial membrane potential, but not cytoplasmic calcium, correlates with insulin secretion in single islet cells. Am J Physiol Endocrinol Metab. 2006;290:E143–E148. doi: 10.1152/ajpendo.00216.2005. [DOI] [PubMed] [Google Scholar]
- 33.Nunemaker CS, Bertram R, Sherman A, Tsaneva-Atanasova K, Daniel CR, Satin LS. Glucose modulates [Ca2+]i oscillations in pancreatic islets via ionic and glycolytic mechanisms. Biophys J. 2006;91:2082–2096. doi: 10.1529/biophysj.106.087296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilon P, Henquin JC. Distinct effects of glucose on the synchronous oscillations of insulin release and cytoplasmic Ca2+ concentration measured simultaneously in single mouse islets. Endocrinology. 1995;136:5725–5730. doi: 10.1210/endo.136.12.7588329. [DOI] [PubMed] [Google Scholar]
- 35.O’Rahilly S, Turner RC, Matthews DR. Impaired pulsatile secretion of insulin in relatives of patients with non-insulin-dependent diabetes. N Engl J Med. 1988;318:1225–1230. doi: 10.1056/NEJM198805123181902. [DOI] [PubMed] [Google Scholar]
- 36.Nyholm B, Porksen N, Juhl CB, et al. Assessment of insulin secretion in relatives of patients with type 2 (non-insulin-dependent) diabetes mellitus: evidence of early beta-cell dysfunction. Metabolism. 2000;49:896–905. doi: 10.1053/meta.2000.6737. [DOI] [PubMed] [Google Scholar]
- 37.Bertram R, Sherman A, Satin LS. Metabolic and Electrical Oscillations: Partners in Controlling Pulsatile Insulin Secretion. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00359.2007. [DOI] [PubMed] [Google Scholar]
- 38.Nunemaker CS, Zhang M, Wasserman DH, et al. Individual mice can be distinguished by the period of their islet calcium oscillations: is there an intrinsic islet period that is imprinted in vivo? Diabetes. 2005;54:3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 39.Gilon P, Arredouani A, Gailly P, Gromada J, Henquin JC. Uptake and release of Ca2+ by the endoplasmic reticulum contribute to the oscillations of the cytosolic Ca2+ concentration triggered by Ca2+ influx in the electrically excitable pancreatic B-cell. J Biol Chem. 1999;274:20197–20205. doi: 10.1074/jbc.274.29.20197. [DOI] [PubMed] [Google Scholar]
- 40.Tamarina NA, Kuznetsov A, Rhodes CJ, Bindokas VP, Philipson LH. Inositol (1,4,5)-trisphosphate dynamics and intracellular calcium oscillations in pancreatic beta-cells. Diabetes. 2005;54:3073–3081. doi: 10.2337/diabetes.54.11.3073. [DOI] [PubMed] [Google Scholar]
- 41.O’Gorman MRG, Donnenberg AD. Handbook of Human Immunology. Boca Raton, FL: CRC Press; 2008. p. 624. [Google Scholar]
- 42.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 43.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–9. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 44.Roe MW, Philipson LH, Frangakis CJ, et al. Defective glucose-dependent endoplasmic reticulum Ca2+ sequestration in diabetic mouse islets of Langerhans. J Biol Chem. 1994;269:18279–18282. [PubMed] [Google Scholar]
- 45.Hussain MJ, Maher J, Warnock T, Vats A, Peakman M, Vergani D. Cytokine overproduction in healthy first degree relatives of patients with IDDM. Diabetologia. 1998;41:343–349. doi: 10.1007/s001250050913. [DOI] [PubMed] [Google Scholar]
- 46.Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(Suppl 2):S52–61. doi: 10.2337/diabetes.54.suppl_2.s52. [DOI] [PubMed] [Google Scholar]
- 47.Eizirik DL, Colli ML, Ortis F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat Rev Endocrinol. 2009;5:219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 48.Huang X, Moore DJ, Ketchum RJ, et al. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr Rev. 2008;29:603–630. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sauter NS, Schulthess FT, Galasso R, Castellani LW, Maedler K. The antiinflammatory cytokine interleukin-1 receptor antagonist protects from high-fat diet-induced hyperglycemia. Endocrinology. 2008;149:2208–2218. doi: 10.1210/en.2007-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsen CM, Faulenbach M, Vaag A, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 51.Ammala C, Ashcroft FM, Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993;363:356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- 52.Parekh AB. Ca2+ microdomains near plasma membrane Ca2+ channels: impact on cell function. J Physiol (Lond) 2008;586:3043–3054. doi: 10.1113/jphysiol.2008.153460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicotera P, Zhivotovsky B, Orrenius S. Nuclear calcium transport and the role of calcium in apoptosis. Cell Calcium. 1994;16:279–288. doi: 10.1016/0143-4160(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 54.Romanelli P, Heit G, Chang SD, Martin D, Pham C, Adler J. Cyberknife radiosurgery for trigeminal neuralgia. Stereotact Funct Neurosurg. 2003;81:105–109. doi: 10.1159/000075112. [DOI] [PubMed] [Google Scholar]
- 55.Barbu A, Welsh N, Saldeen J. Cytokine-induced apoptosis and necrosis are preceded by disruption of the mitochondrial membrane potential (Deltapsi(m)) in pancreatic RINm5F cells: prevention by Bcl-2. Mol Cell Endocrinol. 2002;190:75–82. doi: 10.1016/s0303-7207(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 56.McDaniel ML, Kwon G, Hill JR, Marshall CA, Corbett JA. Cytokines and nitric oxide in islet inflammation and diabetes. Proc Soc Exp Biol Med. 1996;211:24–32. doi: 10.3181/00379727-211-43950d. [DOI] [PubMed] [Google Scholar]
- 57.Sandler S, Bendtzen K, Borg LA, Eizirik DL, Strandell E, Welsh N. Studies on the mechanisms causing inhibition of insulin secretion in rat pancreatic islets exposed to human interleukin-1 beta indicate a perturbation in the mitochondrial function. Endocrinology. 1989;124:1492–1501. doi: 10.1210/endo-124-3-1492. [DOI] [PubMed] [Google Scholar]
- 58.Maedler K, Storling J, Sturis J, et al. Glucose- and interleukin-1beta-induced beta-cell apoptosis requires Ca2+ influx and extracellular signal-regulated kinase (ERK) 1/2 activation and is prevented by a sulfonylurea receptor 1/inwardly rectifying K+ channel 6.2 (SUR/Kir6.2) selective potassium channel opener in human islets. Diabetes. 2004;53:1706–1713. doi: 10.2337/diabetes.53.7.1706. [DOI] [PubMed] [Google Scholar]
- 59.Lang DA, Matthews DR, Burnett M, Turner RC. Brief, irregular oscillations of basal plasma insulin and glucose concentrations in diabetic man. Diabetes. 1981;30:435–439. doi: 10.2337/diab.30.5.435. [DOI] [PubMed] [Google Scholar]
- 60.Polonsky KS, Given BD, Hirsch LJ, et al. Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 61.Bratusch-Marrain PR, Komjati M, Waldhausl WK. Efficacy of pulsatile versus continuous insulin administration on hepatic glucose production and glucose utilization in type I diabetic humans. Diabetes. 1986;35:922–926. doi: 10.2337/diab.35.8.922. [DOI] [PubMed] [Google Scholar]
- 62.Komjati M, Bratusch-Marrain P, Waldhausl W. Superior efficacy of pulsatile versus continuous hormone exposure on hepatic glucose production in vitro. Endocrinology. 1986;118:312–319. doi: 10.1210/endo-118-1-312. [DOI] [PubMed] [Google Scholar]
- 63.Koopmans SJ, Sips HC, Krans HM, Radder JK. Pulsatile intravenous insulin replacement in streptozotocin diabetic rats is more efficient than continuous delivery: effects on glycaemic control, insulin-mediated glucose metabolism and lipolysis. Diabetologia. 1996;39:391–400. doi: 10.1007/BF00400670. [DOI] [PubMed] [Google Scholar]
- 64.Tornheim K. Are metabolic oscillations responsible for normal oscillatory insulin secretion? Diabetes. 1997;46:1375–1380. doi: 10.2337/diab.46.9.1375. [DOI] [PubMed] [Google Scholar]
- 65.Sherman A, Rinzel J. Model for synchronization of pancreatic beta-cells by gap junction coupling. Biophys J. 1991;59:547–559. doi: 10.1016/S0006-3495(91)82271-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Calabrese A, Zhang M, Serre-Beinier V, et al. Connexin 36 controls synchronization of Ca2+ oscillations and insulin secretion in MIN6 cells. Diabetes. 2003;52:417–424. doi: 10.2337/diabetes.52.2.417. [DOI] [PubMed] [Google Scholar]
- 67.Benninger RK, Zhang M, Head WS, Satin LS, Piston DW. Gap junction coupling and calcium waves in the pancreatic islet. Biophys J. 2008;95:5048–5061. doi: 10.1529/biophysj.108.140863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu YJ, Vieira E, Gylfe E. A store-operated mechanism determines the activity of the electrically excitable glucagon-secreting pancreatic alpha-cell. Cell Calcium. 2004;35:357–365. doi: 10.1016/j.ceca.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 69.Grapengiesser E, Dansk H, Hellman B. Synchronization of pancreatic beta-cell rhythmicity after glucagon induction of Ca2+ transients. Cell Calcium. 2003;34:49–53. doi: 10.1016/s0143-4160(03)00021-6. [DOI] [PubMed] [Google Scholar]
- 70.Spinas GA, Hansen BS, Linde S, et al. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987;30:474–480. doi: 10.1007/BF00279615. [DOI] [PubMed] [Google Scholar]
- 71.Spinas GA, Mandrup-Poulsen T, Molvig J, et al. Low concentrations of interleukin-1 stimulate and high concentrations inhibit insulin release from isolated rat islets of Langerhans. Acta Endocrinol (Copenh) 1986;113:551–558. doi: 10.1530/acta.0.1130551. [DOI] [PubMed] [Google Scholar]
- 72.Spinas GA, Palmer JP, Mandrup-Poulsen T, Andersen H, Nielsen JH, Nerup J. The bimodal effect of interleukin 1 on rat pancreatic beta-cells--stimulation followed by inhibition--depends upon dose, duration of exposure, and ambient glucose concentration. Acta Endocrinol (Copenh) 1988;119:307–311. doi: 10.1530/acta.0.1190307. [DOI] [PubMed] [Google Scholar]
- 73.Wilson CA, Jacobs C, Baker P, et al. IL-1 beta modulation of spontaneous autoimmune diabetes and thyroiditis in the BB rat. J Immunol. 1990;144:3784–3788. [PubMed] [Google Scholar]
- 74.Satoh J, Seino H, Abo T, et al. Recombinant human tumor necrosis factor alpha suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest. 1989;84:1345–1348. doi: 10.1172/JCI114304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Satoh J, Seino H, Shintani S, et al. Inhibition of type 1 diabetes in BB rats with recombinant human tumor necrosis factor-alpha. J Immunol. 1990;145:1395–1399. [PubMed] [Google Scholar]
- 76.Jacob CO, Aiso S, Michie SA, McDevitt HO, Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990;87:968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dunger A, Cunningham JM, Delaney CA, et al. Tumor necrosis factor-alpha and interferon-gamma inhibit insulin secretion and cause DNA damage in unweaned-rat islets. Extent of nitric oxide involvement. Diabetes. 1996;45:183–189. doi: 10.2337/diab.45.2.183. [DOI] [PubMed] [Google Scholar]
- 78.Scarim AL, Heitmeier MR, Corbett JA. Irreversible inhibition of metabolic function and islet destruction after a 36-hour exposure to interleukin-1beta. Endocrinology. 1997;138:5301–5307. doi: 10.1210/endo.138.12.5583. [DOI] [PubMed] [Google Scholar]
- 79.Spinas GA, Palmer JP, Mandrup-Poulsen T, Andersen H, Nielsen JH, Nerup J. The bimodal effect of interleukin 1 on rat pancreatic beta-cells--stimulation followed by inhibition--depends upon dose, duration of exposure, and ambient glucose concentration. Acta Endocrinol. 1988;119:307–311. doi: 10.1530/acta.0.1190307. [DOI] [PubMed] [Google Scholar]
- 80.Kawahara DJ, Kenney JS. Species differences in human and rat islet sensitivity to human cytokines. Monoclonal anti-interleukin-1 (IL-1) influences on direct and indirect IL-1-mediated islet effects. Cytokine. 1991;3:117–124. doi: 10.1016/1043-4666(91)90031-8. [DOI] [PubMed] [Google Scholar]