Abstract
Environmental limits for uncompensable heat stress, above which an imbalance between heat gain and heat loss forces body core temperature upward (i.e., the upper limits of the prescriptive zone), are unknown for children. To determine these limits, 7 lean and 7 obese 9- to 12-year-old heat-acclimated boys performed four randomized trials each on separate days to determine the critical water vapor pressure (Pcrit) forcing an upward inflection of body core temperature at several ambient temperatures. Subjects walked continuously on a treadmill at 30% maximal aerobic capacity at a constant dry bulb temperature (Tdb = 34, 36, 38 or 42°C). After a 30-min equilibration period at 9 torr, ambient water vapor pressure increased approximately 1 torr every 5-min until a distinct breakpoint in the core temperature versus time curve was evident. Compared to the lean subjects, obese subjects had significantly lower environmental limits (P < 0.03) in warm environments (Pcrit, for lean vs. obese, respectively = 32.9 ± 0.7 vs. 30.3 ± 0.8 torr at Tdb = 34°C; 29.6 ± 0.6 vs. 27.2 ± 0.9 torr at Tdb = 36°C; 27.8 ± 0.6 vs. 24.7 ± 0.9 torr at Tdb = 38°C; 25.5 ± 0.7 vs. 24.5 ± 1.5 torr at Tdb = 42°C). These results suggest that separate critical environmental guidelines should be tailored to lean and obese children exercising in the heat.
Keywords: Children, Thermoregulation, Prescriptive zone, Psychrometric chart, Heat balance
Introduction
During exercise over a wide range of climatic conditions, elevations in body core temperature (Tc) are proportional to work load and independent of ambient conditions (Saltin and Hermansen 1966). At constant exercise intensity, Tc maintains a new steady state at this higher temperature. The range of environments over which this relationship holds true was termed the “prescriptive zone” (Lind 1963). As climatic heat stress increases, combinations of ambient temperatures and water vapor pressures (Pa) above this zone force Tc upward, due to an imbalance between heat gain and heat loss, resulting in uncompensable heat stress. No study to date has determined these critical environmental conditions defining the upper limit of the prescriptive zone for a given metabolic rate in exercising children.
The approach used in the few studies investigating a child’s tolerance to exercise in the heat was to determine the time in a fixed environment in which a child was unable to continue exercising due to either subjective criterion (nausea, headache, etc.) or measured physiological responses (rectal temperature >39°C or >90% of maximum heart rate) (Drinkwater et al. 1977; Haymes et al. 1975; Haymes et al. 1974). Exercising obese/overweight versus lean children display no difference in heat tolerance but a greater physiological strain as indexed by a higher Tc and heart rate (Haymes et al. 1975; Haymes et al. 1974). However, these studies did not take into account body surface area. Review of this data collectively gives an indication of the environmental conditions which might reduce a child’s exercise performance. From this information, organizations have developed position stands providing specific recommendations for setting restraints on activities at differing levels of climatic heat stress for exercising children (Armstrong et al. 1996; American Academy of Pediatrics: Committee on Sports Medicine and Fitness 2000). However, each study employed an approximately 10° increase in climatic conditions among trials, thus the environmental limit for uncompensable heat stress, above which an imbalance between heat gain and heat loss forces Tc upward, is difficult to determine. In addition, it is unclear if obese children exercising in the heat should have a different set of guidelines to follow compared to lean children.
The purpose of the present investigation was to determine, for the first time, the critical environmental heat stress limits for exercising, heat-acclimated lean and obese 9- to 12-year-old boys. It was hypothesized that during light-to-moderate intensity exercise in a warm environment, the critical environmental limits for heat-acclimated obese versus lean 9- to 12-year-old boys would be shifted downward on a psychrometric chart, towards lower critical water vapor pressure (Pcrit). The Pcrit was identified by a continuous rise in Tc and was defined as the critical ambient water vapor pressure above which thermal balance could not be maintained during exercise.
Methods
Subjects
This study was approved by the Institutional Review Board of The Pennsylvania State University. Seven lean and 7 obese 9- to 12-year-old boys volunteered to participate in this study. Lean and obese were defined as ≤20 and ≥25% body fat, respectively (Lohman 1987) as measured by whole body dual energy X-ray absorptiometry scan (model QDR 4500 W, Hologic, Waltham, MA, USA). Each subject and his parent/guardian were advised of all experimental procedures and associated risks before verbal assent was given by the child and a written informed consent was provided by the parent/guardian. All subjects were healthy, normotensive, and not taking any medications that could affect their cardiovascular or thermoregulatory responses. Preliminary screening included blood chemistry analysis (CHEM-24, complete blood count and lipid profile, Quest Diagnostics), and resting 12-lead electrocardiogram. During a maximal graded exercise test on a treadmill, subjects began at a self-selected speed to elicit a heart rate of ~140-to 150-bpm at 0% grade, followed by an increase in slope of 2% until two of the following four criteria were met: (1) a plateau in oxygen uptake (V̇o2 max) defined as an increase of ≤2.0 ml/(kg min); (2) a heart rate >195 bpm; (3) a respiratory exchange ratio >1.0; or (4) subjective indicators of fatigue such as hyperpnea, facial flushing, unsteady gait or refusal of the child to exercise further (Goran et al. 2000; Owens and Gutin 1999). Subjects completed a physical exam during which a clinician determined pubertal status according to the criteria of Tanner (1962). Subject characteristics are presented in Table 1.
Table 1.
Subject characteristics by group
| Lean boys | Obese boys | |
|---|---|---|
| n | 7 | 7 |
| Age, year | 11 ± 0.3 | 11 ± 0.2 |
| Height, cm | 152 ± 2 | 155 ± 1 |
| Weight, kg | 42 ± 2 | 54 ± 4* |
| AD, m2 | 1.33 ± 0.03 | 1.50 ± 0.05* |
| AD/mass, m2/kg | 0.032 ± 0.001 | 0.028 ± 0.001* |
| Body fat, % | 18 ± 1 | 33 ± 2* |
| LBM, kg | 32 ± 1 | 33 ± 1 |
| V̇o2 max, ml kg−1 min−1 | 49 ± 1 | 37 ± 2* |
| V̇o2 max, L/min−1 | 2.1 ± 0.1 | 2.0 ± 0.1 |
| V̇o2 max, ml LBM−1 min−1 | 65 ± 2 | 59 ± 1* |
Values are means ± SE. AD DuBois surface area, LBM lean body mass, V̇o2 maxmaximal aerobic capacity
Significantly different from lean boys, P < 0.05
Experimental design
Before the experimental trials began, each subject completed six 70-min acclimation sessions (exercise + heat exposures) on separate days. Exercise at 30% of maximal aerobic capacity (V̇o2 max) alternated between a treadmill (Precor USA C962) and cycle ergometer (Monark Ergo-medic 818E) for three 20-min bouts interspersed with 5-min rests at 38°C, 50% relative humidity. During school recess and spontaneous playtime, children spend a majority of the time participating in light-to-moderate intensity activities (Ridgers et al. 2005); thus this exercise intensity was chosen because it reasonably simulates the workload typical of a child during spontaneous physical exertion. Experiments were conducted in the summer months; thus subjects were partially heat-acclimatized due to routine outdoor activities. Each subject completed six exercise in the heat bouts in order to attain further heat-acclimation from the partially heat-acclimatized state. For all experimental trials the time between each scheduled test day was no more than 2 days. Body weight was measured before and after each exposure and during rest periods, and subjects were given water to maintain body weight by replacing all water lost through sweat. All subjects attained heat-acclimation, operationally defined by a similar final Tc for two consecutive sessions and by a leveling off of Tc within the last exercise bout (all subjects completed six trials). Heat-acclimation testing procedures have been previously described in detail (Dougherty et al. 2009).
Following acclimation, subjects completed four separate tests on separate days in randomized order to determine the Pcrit for the upward inflection of Tc at four distinct dry bulb temperatures (Tdb = either 34, 36, 38 or 42°C). For all experimental trials the time between each scheduled test day was no more than 2 days. The Pcrit was identified by a continuous rise in Tc for a minimum of 10 min and was defined as the critical ambient water vapor pressure above which thermal balance could not be maintained during exercise. All subjects in both groups completed the tests at Tdb = 34, 36, and 38°C. Seven lean and five obese subjects completed the tests at Tdb = 42°C. Three obese subjects also completed tests at Tdb = 28°C, in which all subjects were able to sustain exercise within the prescriptive zone at >90% relative humidity. Therefore, trials at Tdb = 28°C were discontinued due to the inability to further increase the Pa. Subjects were encouraged to stay well-hydrated the day before each trial.
Testing procedures
Subjects were asked to refrain from caffeine consumption on each day of the experiment and reported to the lab at least 2 h after a meal. After providing a urine sample, the subject was instrumented with a Polar® heart rate monitor to measure heart rate, a handheld recorder (CT2000) to continuously measure Tc attached to the subject via a belt and pouch, and weighed (Seca 770, accuracy ±50 g) wearing only shorts (all subsequent weights were taken wearing shorts only). Next the subject entered the preconditioned environmental chamber where skin thermocouples were attached.
During each test, the subject walked continuously on a treadmill, for up to 2.5 h at 30% V̇o2 max (for justification of exercise intensity see “Experimental design”). Tdb was held constant while Pa increased approximately 1 torr every 5 min, after a 30-min equilibration period at 9 torr. There was no forced air movement in the programmable environmental chamber and air velocity measured near the active subject with an anemometer was 0.25 m/s. The experiment ended when the subject completed the protocol (i.e., a distinct breakpoint in the Tc versus time curve was evident), or if the Tc exceeded 39°C, the subject experienced adverse signs (nausea, dizziness, etc.), or if the subject desired to stop. After exiting the chamber at the conclusion of the experiment, a post-experiment urine sample was obtained. Last, the subject was given water to replace sweat loss during the trial.
Measurements
Temperature
A minimum of 8 h before each test, subjects swallowed an ingestible temperature sensor (CorTemp, HQ Inc., Palmetto, FL, USA) for the measurement of Tc. The sensor is a single-use, pill-shaped electronic device that contains a telemetry system, a microbattery, and a quartz crystal whose frequency of vibration is linearly related to temperature. Each temperature sensor was calibrated by the manufacturer, which provides a serial number that is programmed into a handheld recorder (CT2000) ensuring an accuracy of 0.1°C. Each pill was used within 6 months from the date it was shipped by the manufacturer. Regarding the heat-acclimation trials, previous research has shown that during steady state exercise in a warm environment, the temperature and response time of the ingestible temperature sensor was intermediate between rectal and esophageal (O’Brien et al. 1998).
Prior to the Pcrit trials, a pilot study was conducted to compare the agreement among methods for Tc measurement (esophageal, rectal and ingestible temperature sensor) utilizing the same dynamic protocol as in the present study. One male and one female subject walk on a treadmill at 3.8 mph in an environmental chamber where the Tdb was held constant at 36°C while the Pa was increased approximately 1 torr every 5 min, after a 30-min equilibration period at 9 torr. The root mean square deviation (RMSD) was calculated to compare the agreement among methods. At each minute, the RMSD between rectal and ingestible pill temperature (average RMSD = 0.24) was smaller than esophageal and rectal temperature (average RMSD = 0.32) and the RMSD between esophageal and ingestible pill temperature (average RMSD = 0.16) was smaller than esophageal and rectal temperature. Therefore, we conclude that the ingestible pill is a valid method for Tc measurement under the present experimental conditions.
Skin temperature (Tsk) was measured with copper-constantan thermocouples affixed to the skin at four sites: triceps (Ttriceps), upper back (Tback), chest (Tchest), and thigh (Tthigh). Wet bulb (Twb) and Tdb temperatures were measured according to the specifications of ASHRAE (American Society of Heating, Refrigerating and Air-Conditioning Engineers). All heart rate, Tc, Tsk, Tdb, and Twb data were measured continually through the protocol and stored as 1-min averages using computer software (Labview) in conjunction with a data-acquisition system (National Instruments, Austin, TX, USA).
Blood pressure, maximal aerobic capacity, and body weight
Blood pressure by manual brachial auscultation (sphygmomanometry) was measured every 10 min. To ensure that each subject was working at the desired workload, expired air for the determination of V̇o2 was measured 30 min into the protocol for 5 min (TrueOne 2400 Metabolic Measurement System, ParvoMedics, Salt Lake City, UT, USA). Body weight was measured before and after each trial.
Urine
Urine volume was measured with a graduated cylinders and urine color was determined by holding each specimen container next to a validated color scale (Armstrong et al. 1994) in a well-lit room. The eight-color scale ranges from very pale yellow (#1) to brownish green (#8). Urine osmolality (freezing point depression, Advanced DigiMatic Osmometer Model 3D2), and specific gravity (Refractometer, Atago A300CL) were determined in triplicate.
Subjective ratings
During each experiment, ratings of perceived exertion ((RPE), Borg scale (Borg 1970)) and thermal sensation ((TS), using a 0–8 scale in which 0 = unbearable cold, 4 = thermoneutral, and 8 = unbearably hot (Young et al. 1987)) were measured every 10 min.
Determination of critical water vapor pressure
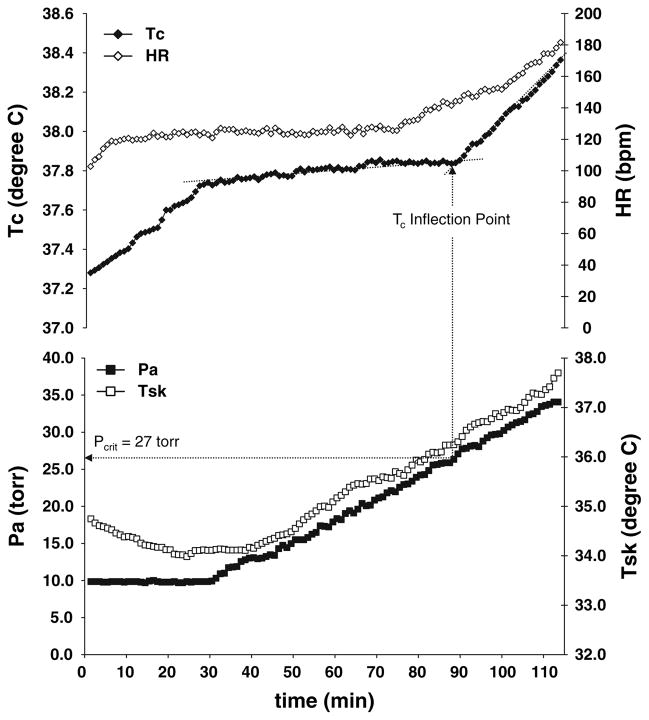
The methods used to determine Pcrit have been previously described (Kamon and Avellini 1976; Kamon et al. 1978; Kenney and Zeman 2002). The Tc, heart rate, Tsk and Pa data from a typical Pcrit test are illustrated in Fig. 1. Briefly, as subjects walked during the 30-min equilibration period, Tc increased and then began to plateau by approximately 40 min. At some point, the rising Pa pushed Tc past the prescriptive zone of thermal balance as evidenced by a distinct breakpoint in the Tc versus time curve where Tc began to rise again. To determine this inflection point, first a line was drawn from minute 30 between data points to denote the equilibrium slope. When the Tc versus time curve exhibited an increase in slope from the equilibrium slope, a second line was drawn from the point of departure of Tc from the first line. Pilot testing demonstrated that there is a 2-min lag in the ingestible temperature sensor compared to esophageal temperature response time (the point at which the second line deviated from the first line). Thus the Pa 2 min before the upward inflection point was defined as the Pcrit in the present study. Approximately 10–15 min prior to the Tc inflection point, an upward rise in heart rate (Kamon and Avellini 1976; Kamon et al. 1978; Kenney and Zeman 2002; Kenney et al. 1988) was evident in all tests.
Fig. 1.
A representative critical water vapor pressure (Pcrit) test illustrating the typical time course of body core temperature (Tc), heart rate (HR), and mean skin temperature (Tsk) responses to exercise and rising ambient water vapor pressure (Pa)
Reliability of Pcrit data
To test the reliability of the Pcrit data, tests were repeated by eight subjects in the present study on a separate day utilizing the same testing procedures previously described. Two subjects completed repeat trials at each of the Tdb = 34 (1 lean and 1 obese subject), 36 (1 lean and 1 obese subject), 38 (2 obese subjects), and 42 (1 lean and 1 obese subject) °C. In order to account for the repeated Pcrit tests, the time points at which each inflection point occurred were compared and a test–retest correlation was calculated, resulting in a correlation coefficient (r) of 0.99, with a slope of 0.97 (NS vs. 1.0) and an intercept of 3.66 (NS vs. 0).
Calculations
For all experimental trials, subjects wore shorts, socks and sneakers and therefore, no clothing corrections were made for this “semi-nude” state. Body surface area (AD) was estimated according to (DuBois and DuBois 1916) and AD/mass was calculated. A weighted mean Tsk (T̄sk°C) was calculated as
Sweating rate was calculated from the net change in body weight corrected for fluid consumption and urine excreted. Respiratory losses were considered negligible. Mean arterial pressure (MAP; torr) was calculated as
The wet bulb globe temperature (WBGT; °C) was calculated as
Metabolic rate (M; W/m2) was calculated from the respiratory exchange ratio (RER; unitless), V̇o2 (l/min) and AD (m2) as
External work (W; W/m2) was calculated from body mass (mb; kg), walking velocity (vw; m/min), fractional grade of the treadmill (fg) and AD as
Net metabolic heat production (Mnet; W/m2) was calculated as M−W.
Radiative and convective (R + C; W/m2) dry heat exchange was calculated as
where hr+c (W m−2°C−1) is the combined radiative and convective heat transfer coefficient and Tdb − Tsk denotes the temperature gradient between ambient air and the skin. For each subject, hr+c was calculated as
where 6.5 (treadmill speed; m/s)0.39 is the convective coefficient for treadmill walking (Nishi and Gagge 1970) and 4.7 is the radiative coefficient for indoor environments.
Heat storage (S; W/m2) was calculated as
where ΔTb/Δt is the change in mean body temperature (ΔTb; °C) measured over time (Δt; h) from minute 30 until a distinct breakpoint in the core temperature versus time curve was evident, and 0.97 W h kg−1 °C−1 is the specific heat of the body.
The ΔTb was calculated as
The heat balance equation was then used to solve for the evaporative heat loss required to match heat production (Ereq; W/m2)
The skin evaporative capacity (Esk; W/m2) for each trial was determined by multiplying the sweating rate by the specific heat of vaporization, 0.68 W h g−1.
The maximal evaporative capacity of the environment (Emax; W/m2) was calculated as
where 18.4 W m−2 torr−1 is the effective evaporative coefficient for heat-acclimatized males (Belding and Kamon 1973), air velocity (v; m s−1) was equal to 0.25 for this study, and Ps,sk − Pa (torr) is the gradient between saturated water vapor pressure of the skin (determined by Antoine’s equation (Parsons 2003)) and the air at the critical point. Skin wettedness (w; %) was calculated as Ereq/Emax.
Statistical analyses
Since no prior study has determined the critical environmental heat stress limits for exercising children, the sample size calculation was based upon data in adults (Kenney and Zeman 2002). Using a 2-sample t test power calculation in Minitab, the following values were used to determine that six subjects were needed per group: difference between means (Pcrit, torr) = 1.8; standard deviation = 0.9; power = 0.8; alpha = 0.05. Considering that some subjects may drop out of the study, we tested seven subjects per group.
A repeated measures analysis of covariance by SAS PROC MIXED was used to analyze the data. This linear mixed model took into account the correlated nature of the repeated measures. Group was treated as a fixed effect and subjects were treated as random effects. For data measured at four distinct Tdb values, the independent variables were group and Tdb. For data measured at four distinct Tdb values over different time points, group, time and Tdb were the independent variables. When making multiple comparisons, Bonferroni adjustments were used. Results were considered significant at P < 0.05.
Absolute versus relative exercise intensity
During exercise in the heat, whereas metabolic heat production is closely related to absolute exercise intensity, heat loss mechanisms are a function of relative exercise intensity. Thus, heat storage and the subsequent rise in Tc is dependent to some degree upon both absolute and relative intensities. In the present study, work at the same relative intensity was the logical choice in order to investigate differences in heat loss mechanisms between lean and obese boys. However, to investigate the impact of absolute versus relative intensity, four lean and three obese subjects repeated the first heat-acclimation trial which matched the absolute and relative workloads of the lean and obese groups (i.e. decreasing the workload for the lean group to match the obese group and increasing the workload for the obese group to match the lean group). The time between the completion of the last experimental trial (fourth Pcrit trial) and the repeat heat-acclimation trial was >2 months. The Bland–Altman approach to measuring agreements for repeated measures was used to determine the agreement of Tc between the first (relative exercise intensity) and repeated (absolute exercise intensity) trials using ±0.3°C as the physiological threshold for assessment. This threshold takes into account the anticipated standard deviation for Tc measurement in boys of this age (Bar-Or and Inbar 1977). The mean difference between the two trials was 0.01°C and the standard deviation of the difference between the two trials was 0.08°C. The 95% limits of agreement were −0.1503 to 0.1621. Therefore, when matched for absolute and relative exercise intensity, the difference in Tc was within acceptable limits and considered marginal under practical consideration. This suggests that other factors independent of exercise intensity contribute to significant differences observed in the present study.
Results
Six lean and two obese subjects were classified as pre-pubertal (Tanner stage 1), five obese subjects were classified as mid-pubertal (Tanner stage 2–4), and one lean subject was classified as late-pubertal (Tanner stage 5). As expected, obese subjects weighed more, had a higher AD, a lower AD/mass ratio, higher percent body fat, and a lower V̇o2 max (all P < 0.05; Table 1). Body fatness ranged from 14 to 20% in the lean subjects and from 28 to 45% in the obese subjects. There were no significant differences between groups in baseline Tc.
Compared to lean subjects, obese subjects had significantly lower M (lean vs. obese = 200 ± 3 vs. 164 ± 4 W/m2 at Tdb = 34°C; 196 ± 9 vs. 172 ± 4 W/m2 at Tdb = 36°C; 202 ± 8 vs. 167 ± 10 W/m2 at Tdb = 38°C; 217 ± 4 vs. 169 ± 4 W/m2 at Tdb = 42°C; all P < 0.001), and W performed (lean vs. obese = 7 ± 0.9 vs. 4 ± 0.2 W/m2 for all trials; all P < 0.003) during exercise at 30% V̇o2 max. Mnet, Ereq, and w during exercise at 30% V̇o2 max in each critical environment were significantly lower in obese compared to lean subjects (all P < 0.03; Table 2). The measured exercise intensity ranged from 30.2 ± 0.6 to 34.5 ± 0.5% for the lean subjects and 30.5 ± 0.5 to 34.3 ± 0.4% for the obese subjects across trials (P > 0.05). There was no difference between groups in R + C, S, (both Table 2) or ΔTb (lean vs. obese = 0.61 ± 0.10 vs. 0.48 ± 0.07°C at Tdb = 34°C; 0.52 ± 0.10 vs. 0.34 ± 0.06°C at Tdb = 36°C; 0.46 ± 0.06 vs. 0.43 ± 0.07°C at Tdb = 38°C; 0.51 ± 0.06 vs. 0.30 ± 0.07°C at Tdb = 42°C) in each critical environment. Compared to lean subjects, Emax was consistently significantly lower for the obese subjects in each critical environment (lean vs. obese = 100 ± 4 vs. 115 ± 4 W/m2 at Tdb = 34°C; 127 ± 4 vs. 142 ± 8 W/m2 at Tdb = 36°C; 149 ± 3 vs. 165 ± 7 W/m2 at Tdb = 38°C; 174 ± 6 vs. 182 ± 9 W/m2 at Tdb = 42°C; all P < 0.04). Obese subjects had a significantly lower relative (ml m−2 h−1) but not absolute (ml h−1) mean sweating rate which translated into a significantly lower Esk compared to lean subjects (both P < 0.04; Tables 2, 3).
Table 2.
Calculated heat balance variables in each critical environment
|
Mnet (W/m2) |
R + C (W/m2) |
S (W/m2) |
Esk (W/m2) |
Ereq (W/m2) |
w (%) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | |
| 34°C | 192 ± 3 | 161 ± 4* | −29 ± 2 | −23 ± 2 | 12 ± 2 | 10 ± 1 | 173 ± 11 | 124 ± 14* | 152 ± 5 | 128 ± 5* | 1.54 ± 0.09 | 1.12 ± 0.05* |
| 36°C | 189 ± 10 | 168 ± 3* | −5 ± 3 | −2 ± 3 | 14 ± 2 | 9 ± 1 | 188 ± 14 | 137 ± 20* | 170 ± 9 | 156 ± 4* | 1.34 ± 0.07 | 1.12 ± 0.07* |
| 38°C | 195 ± 8 | 163 ± 4* | 16 ± 2 | 20 ± 2 | 14 ± 1 | 16 ± 3 | 224 ± 10 | 177 ± 20* | 196 ± 7 | 167 ± 5* | 1.33 ± 0.07 | 1.03 ± 0.06* |
| 42°C | 209 ± 4 | 165 ± 4* | 60 ± 3 | 56 ± 3 | 23 ± 2 | 15 ± 2 | 241 ± 32 | 179 ± 15* | 246 ± 3 | 205 ± 8* | 1.43 ± 0.06 | 1.14 ± 0.08* |
Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C
Mnet, net metabolic heat production; R + C, dry heat exchange via radiation and convection; S, heat storage; Esk, evaporative cooling from the skin; Ereq, required evaporation to maintain heat balance; w, % skin wettedness, calculated as Ereq/Emax
Significant group difference at P < 0.05
Table 3.
Sweating rate
| Sweating rate (ml h−1) |
Sweating rate (ml·m−2·h−1) |
|||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| 34°C | 341 ± 27 | 260 ± 38 | 254 ± 16 | 182 ± 20* |
| 36°C | 371 ± 35 | 303 ± 44 | 276 ± 20 | 202 ± 29* |
| 38°C | 442 ± 28 | 390 ± 43 | 330 ± 14 | 260 ± 30* |
| 42°C | 477 ± 71 | 393 ± 21 | 354 ± 48 | 263 ± 29* |
Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C
Significant group difference at P < 0.05
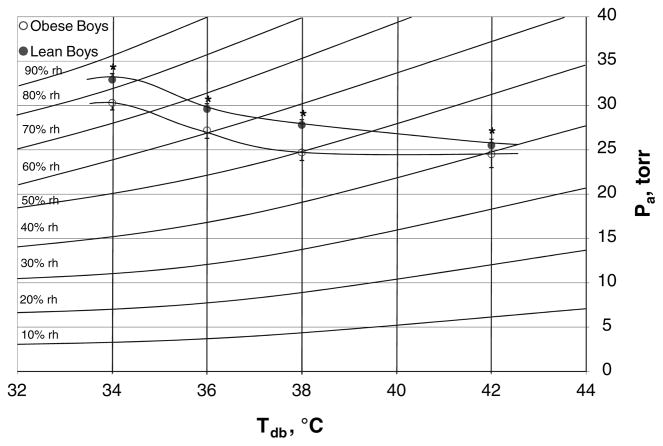
Obese subjects consistently had significantly lower critical environmental limits and a significantly higher (Ps,sk − Pa) in each warm environment compared to lean subjects (all P < 0.04; Table 4). These environmental thresholds are plotted on a standard psychrometric chart in Fig. 2. The WBGT was significantly lower in each critical environment for the obese versus lean subjects (P < 0.04). In each warm environment, at Pcrit there were no significant differences between groups in Tc or T̄sk. No pre or post urine variable was significantly different between groups. Likewise, there were no significant differences between groups in MAP at each time point across all Tdb.
Table 4.
Results from the determination of critical environmental limits and water vapor pressure gradients
|
Pcrit (torr) |
Twbcrit (°C) |
RHcrit (%) |
WBGTcrit (°C) |
Ps,sk − Pa (torr) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | Lean | Obese | |
| 34°C | 32.9 ± 0.7 | 30.3 ± 0.8* | 31.2 ± 0.3 | 30.1 ± 0.3* | 82 ± 2 | 76 ± 2* | 32.0 ± 0.2 | 31.3 ± 0.2* | 12.5 ± 0.5 | 14.3 ± 0.5* |
| 36°C | 29.6 ± 0.6 | 27.2 ± 0.9* | 30.3 ± 0.3 | 29.2 ± 0.4* | 66 ± 1 | 61 ± 2* | 32.0 ± 0.2 | 31.2 ± 0.3* | 15.9 ± 0.6 | 17.8 ± 1.0* |
| 38°C | 27.8 ± 0.6 | 24.7 ± 0.9* | 29.9 ± 0.3 | 28.5 ± 0.4* | 56 ± 1 | 50 ± 2* | 32.3 ± 0.2 | 31.3 ± 0.3* | 18.6 ± 0.4 | 20.6 ± 0.9* |
| 42°C | 25.5 ± 0.7 | 24.5 ± 1.5* | 29.7 ± 0.3 | 29.3 ± 0.7* | 41 ± 1 | 40 ± 3* | 33.4 ± 0.2 | 33.1 ± 0.4* | 21.7 ± 0.7 | 22.8 ± 1.1* |
Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C
Pcrit, the critical water vapor pressure; Twbcrit, critical wet bulb temperature; RHcrit, critical relative humidity; WBGTcrit, critical wet bulb globe temperature, Ps,sk − Pa; the gradient between saturated water vapor pressure of the skin and the air at the critical point
Significant group difference at P < 0.04
Fig. 2.
Critical environmental limits on a standard psychrometric chart. Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C. Compared to lean subjects, obese subjects consistently had significantly lower critical water vapor pressure (Pcrit) values in each warm environment. *Significant group difference at P < 0.03
Subjective responses during exercise at each Tdb are presented in Table 5. At the beginning of the exercise bout (10 min), TS and RPE were significantly higher in the obese versus lean subjects at Tdb = 36, 38 and 42°C (all P < 0.05). At minute 50 and the critical environment, obese subjects continued to rate perceived exertion and TS significantly higher compared to lean subjects in all conditions (all P < 0.05).
Table 5.
Subjective responses
| RPE |
TS |
|||
|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |
| 34°C | ||||
| 10 min | 9 ± 1.2 | 11 ± 1.3* | 4.7 ± 0.3 | 4.9 ± 0.3 |
| 50 min | 10 ± 1.2 | 13 ± 1.3* | 5.1 ± 0.4 | 5.4 ± 0.4* |
| Critical | 13 ± 2.0 | 16 ± 1.4* | 6.2 ± 0.5 | 6.8 ± 0.4* |
| 36°C | ||||
| 10 min | 9 ± 1.1 | 11 ± 1.4* | 4.5 ± 0.2 | 4.9 ± 0.3* |
| 50 min | 11 ± 1.5 | 14 ± 1.4* | 5.4 ± 0.4 | 5.9 ± 0.3* |
| Critical | 13 ± 2.0 | 15 ± 1.8* | 6.1 ± 0.5 | 7.0 ± 0.4* |
| 38°C | ||||
| 10 min | 9 ± 1.2 | 11 ± 1.5* | 4.6 ± 0.2 | 4.9 ± 0.4* |
| 50 min | 11 ± 1.7 | 13 ± 1.6* | 5.2 ± 0.4 | 5.9 ± 0.4* |
| Critical | 13 ± 2.0 | 15 ± 1.5* | 6.2 ± 0.6 | 6.6 ± 0.4* |
| 42°C | ||||
| 10 min | 9 ± 1.3 | 11 ± 1.8* | 4.9 ± 0.3 | 5.5 ± 0.4* |
| 50 min | 10 ± 1.5 | 14 ± 2.2* | 5.4 ± 0.4 | 6.6 ± 0.3* |
| Critical | 12 ± 1.8 | 16 ± 2.5* | 6.1 ± 0.6 | 7.4 ± 0.3* |
Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C
RPE rating of perceived exertion, TS thermal sensation, Critical critical environmental threshold
Significant group difference at P < 0.05
Discussion
The main finding of this study is that during light-to-moderate exercise at a similar relative intensity (30% V̇o2 max) in a warm environment, the critical environment limits for heat-acclimated obese versus lean 9- to 12-year-old children are shifted downward on a psychrometric chart, toward a lower Pcrit. Above these limits, thermal balance cannot be maintained, and a continuous rise in Tc is evident.
Psychrometric limits
For exercising children, position stands (American Academy of Pediatrics: Committee on Sports Medicine and Fitness 2000; Armstrong et al. 1996) recommending restricting activities at increasing levels of heat stress are based upon studies which determined the maximal tolerated time in a fixed environment (Drinkwater et al. 1977; Haymes et al. 1975; Haymes et al. 1974). The present study empirically defined critical environmental limits of uncompensable heat stress for 9- to 12-year-old heat-acclimated boys at a fixed relative exercise intensity based solely on thermal balance. These thermal limits are commonly displayed as lines on a psychrometric chart, which separate environmental zones of compensable and uncompensable heat stress (Fig. 2). Above these limit lines, an excessive rise in Tc is predicted as thermal equilibrium cannot be maintained. However, several points must be emphasized: (1) these limits apply only to situations where exercise intensity is low to moderate (approximately 30% V̇o2 max), (2) these limits are expanded by heat-acclimation and are likely to be lower for unacclimated boys (Kenney and Zeman 2002), and (3) it is difficult to discern how a radiant heat load and/or wind might impact these environmental thresholds. Thus, the recommendations presented should be used as an approximate guide rather than a strict rule.
The data presented are mean critical vapor pressures. However, in order to provide “safe” limits for 95% of the population, values 2 standard deviations below the mean would be appropriate. Since relative humidity values are more easily accessible and understood, Table 6 presents temperature and protective relative humidity combinations for 95% of the population (2 standard deviations below the mean). Another thermal index used to assess heat stress is WBGT, which was the focus of the American Academy of Pediatrics position stand (American Academy of Pediatrics: Committee on Sports Medicine and Fitness 2000). Critical WBGT values are presented in Table 4. However, the average of all individual critical WBGT values from the four trials which results in one overall WBGT value per group, 2 standard deviations below the mean, would be most user friendly. Therefore, protective WBGT values for 95% of the population, 2 standard deviations below the mean are lean = 30°C and obese = 29°C, which is in agreement with the recommendations set forth by the American Academy of Pediatrics.
Table 6.
Protective relative humidity
| Protective RH (%) |
||
|---|---|---|
| Lean | Obese | |
| 34°C | 72 | 66 |
| 36°C | 60 | 49 |
| 38°C | 50 | 40 |
| 42°C | 35 | 30 |
Values are means ± SE for 7 lean and 7 obese subjects at 34, 36 and 38°C and 7 lean and 5 obese subjects at 42°C
Protective RH, protective relative humidity for 95% of the population, 2 standard deviations below the mean
In response to an exercise-heat stress, heat-loss mechanisms attempt to defend Tc by increasing skin blood flow for convective heat transfer from core to periphery and sweating rate to enhance evaporative heat loss. The efficiency of these heat-dissipation mechanisms are governed by multiple factors including environmental conditions, AD/mass ratio, hydration status, and body fat. Havenith and van Middendorp (1990) found that in adults the percentage of body fat and the AD/mass ratio had the greatest influence on Tc and heat storage during exercise in warm/humid and hot/dry conditions. It is likely that not one but a combination of several factors contributed to the attenuated Pcrit values in the obese versus lean boys in the present study.
When environmental temperature is close to or above Tsk, dry heat exchange via radiation and convection, which is dependent upon the gradient between skin and Tdb, is minimal. In this environment, heat loss at a given metabolic rate occurs via evaporation of sweat. However, in high vapor pressure environments, evaporative cooling is limited due to the decreased water vapor pressure gradient between the saturated skin and the air. In the present study, dry heat exchange was not significantly different between groups. In these warm/humid environments, the evaporation of sweat, which was the primary means of heat dissipation, likely depends on the optimal sweating rate for a given unit of metabolic heat production and AD. Considering the time spent in less stressful environments earlier in the exposures and the inverse relationship between AD and sweat gland density (Bar-Or et al. 1968), the lower sweating rates per AD in the obese versus lean subjects might have been insufficient to maintain the evaporative heat loss necessary to match metabolic heat production. However, sweating rate is not synonymous with evaporation, and at the critical point at each Tdb, the estimated w values were >1.0 for both groups, suggesting dripped sweat which does not cool the skin. The calculated sweat rate is averaged over a long time period and the true sweating rate in the critical environment is unknown.
The metabolic heat generated during exercise is proportional to the active muscle mass (related to body mass) whereas the capacity for heat exchange with the environment is a function of AD. A larger individual has a greater AD, but a smaller individual has a greater AD/mass ratio. The larger individual, due to their smaller AD/mass, loses metabolic heat generated during exercise at a slower rate than a smaller person (Robinson 1942). Since adipose tissue has a lower specific heat of stored lipid and less water, less heat can be stored in adipose versus lean tissue before a rise in the tissue temperature is observed (Buskirk et al. 1969). Haymes et al. (1975) found no difference in heat storage, but a greater rise in Tc in obese versus lean, partially heat-acclimated 9- to 12-year-old boys during exercise in the heat at the same absolute intensity. During each trial in the present study, the earlier inflection in Tc in obese subjects with rising Pa despite similar S in both groups is in agreement with previous findings in this age group.
Forearm blood flow during exercise in the heat is lower in obese compared to lean adults (Vroman et al. 1983) which could impede the convection of heat by blood from core to the periphery. Although no study has investigated the skin blood flow response during exercise in the heat in obese versus lean children, nor was it measured in the present study, this could be another physiological liability contributing to the significantly lower Pcrit values in the obese compared to lean boys in the present study.
Subjective responses
Several RPE scales have been developed specifically for children to quantify their perceived physical effort during exercise (Eston and Lamb 2000). However, Borg’s established 6–20 RPE scale was used in the present study because (1) it is both valid and reliable for use with children aged 9 years and older (Mahon and March 1992; Bar-Or 1977), (2) obese children can rate their perceived exercise intensity both accurately and consistently (Bar-Or and Ward 1989) and (3) using Borg’s scale with children exercising in the heat can serve as a common denominator for comparison with adults in future studies. Surprisingly, very few studies have compared effort perception during exercise between lean and obese children, with no studies conducted in the heat. Ward et al. (1986) found a higher perception of effort, by 1.5–2 RPE units in obese compared to lean children during exercise. The present study indicates that an obese child’s perception of effort during exercise in the heat is significantly greater throughout the entire bout (some protocols lasting 2.5 h) compared to their lean counterparts. It is difficult to ascertain factors which may have contributed to the higher RPE values (i.e. increases in ventilation, metabolic rate, heart rate, Tsk, Tc, acidity, etc.) and to differentiate the magnitude of their impact. It is interesting to note that the obese children had significantly higher RPE values 10-min into the exercise bout. The significantly higher effort perception during exercise in the heat in obese versus lean children in the present study, suggests that obese children may require enhanced encouragement and support while exercising in the heat.
Protocol
The protocol utilized in the present study evolved from the one-first developed by Belding and Kamon (1973). Their time-intensive method determined Pcrit values for heat-acclimatized men exercising at different intensities and air speeds (up to ten separate exposures in a different environment, either semi-nude or clothed) at Tdb = 36°C while Pa was held constant for the full 2-h exposure. The most stressful ambient conditions in which a continuous rise in Tc was not observed was classified as the upper limit of thermal balance for that condition. As opposed to separate tests in each environment, Kamon and Avellini later refined this protocol by defining Pcrit values at several Tdb’s for heat-acclimated women by increasing Pa throughout each test (Kamon and Avellini 1976). Subsequent studies determined critical environmental limits for lightly (Kamon et al. 1978) and heavier (Kenney et al. 1988) clothed heat-acclimated and lightly clothed (Kenney and Zeman 2002), unacclimated men and women. The present study extends these critical environmental heat stress limits to a novel population of heat-acclimated children. Although the present study did not directly compare children and adults, it is interesting to consider how these critical environmental limits might differ between boys versus men. The critical environment at Tdb = 36°C for heat-acclimated semi-nude lean boys in the present study (mean ± SD = 29.6 ± 1.5 torr) is substantially lower than that of semi-nude heat-acclimatized men (Belding and Kamon 1973) (34 torr) exercising at a similar Mnet (approximately 190 W/m2). However, the air movement differed between studies (boys vs. men = 0.25 vs. 0.83 m/s). These critical environmental limits at Tdb = 36°C in the present study more closely resemble those determined for lightly clothed heat-acclimated men (mean ± SD = 30.6 ± 1.4 (Kamon et al. 1978)) exercising at a similar Mnet (approximately 190 W/m2). However, again the air movement differed between studies (boys vs. men = 0.25 vs. 1 m/s). Theoretically, compared to an adult during exercise in the heat, a child’s lower sweating rate per unit AD which could diminish the capacity for evaporative heat loss, lower cardiac output at a given oxygen uptake which could limit the transfer of heat from core to the periphery via blood flow and greater AD/mass ratio which might result in faster heat absorption, could place them at a thermoregulatory disadvantage (Falk 1998). However, as reviewed by Rowland, recent studies directly comparing physiological and thermoregulatory responses to exercise in the heat between children and adults fail to show thermoregulatory differences (Rowland 2008). More research is needed to elucidate if children are at an increased risk for heat-related illnesses compared to adults.
In summary, during light-to-moderate intensity exercise in a warm environment heat-acclimated obese compared to lean 9- to 12-year-old boys display attenuated critical environmental limits, above which a continuous rise in Tc is observed. This suggests that separate guidelines which set restraints on activities at differing levels of climatic heat stress should be tailored to obese and lean children exercising in the heat.
Acknowledgments
We are grateful to the children for their participation in this study and to the parents for supporting them. The technical assistance of Randy McCullough, Doug Johnson, and Jane Pierzga and data collection assistance of Allison Palaio, Matt Kenney, John Jennings, Samantha Wollman, Dave Nhan and Kristin Wielkiewicz is greatly appreciated. We thank the General Clinical Research Center nursing staff for their medical support. This study was supported by National Institute of Health Grants R01-AG-07004-14 (W. L. Kenney), M01-RR-10732 (General Clinical Research Center), the Graduate Student Research Endowment from the College of Health and Human Development, The Pennsylvania State University (K. A. Dougherty), and by the Carl V. Gisolfi Memorial Research Fund from the American College of Sports Medicine Foundation (K. A. Dougherty).
Footnotes
Communicated by George Havenith.
Conflict of interest statement None of the authors have relevant conflicts of interest to declare.
Contributor Information
Kelly Anne Dougherty, Email: kellydoc35@aol.com, Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Children’s Hospital of Philadelphia, 3535 Market Street, Room 1556, Philadelphia, PA 19104, USA.
Mosuk Chow, Department of Statistics, The Pennsylvania State University, University Park, PA, USA.
W. Larry Kenney, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA, USA.
References
- American Academy of Pediatrics: Committee on Sports Medicine, Fitness. Climatic heat stress and the exercising child and adolescent. American Academy of Pediatrics. Committee on Sports Medicine and Fitness. Pediatrics. 2000;106:158–159. [PubMed] [Google Scholar]
- Armstrong LE, Maresh CM, Castellani JW, Bergeron MF, Kenefick RW, LaGasse KE, Riebe D. Urinary indices of hydration status. Int J Sport Nutr. 1994;4:265–279. doi: 10.1123/ijsn.4.3.265. [DOI] [PubMed] [Google Scholar]
- Armstrong LE, Epstein Y, Greenleaf JE, Haymes EM, Hubbard RW, Roberts WO, Thompson PD. American College of Sports Medicine position stand. Heat and cold illnesses during distance running. Med Sci Sports Exerc. 1996;28:i–x. [PubMed] [Google Scholar]
- Bar-Or O. Age-related changes in exercise perception. In: Borg G, editor. Physical work and effort. Pergamon Press; Oxford: 1977. pp. 255–266. [Google Scholar]
- Bar-Or O, Inbar O. Relationship between perceptual and physiological changes during heat acclimatization in 8–10 year old boys. In: Lavallee H, Shephard R, editors. Frontiers of activity and child health: proceedings of the VIIth international symposium of paediatric work physiology. Editions du Pelican; Ottawa, Canada: 1977. pp. 205–214. [Google Scholar]
- Bar-Or O, Ward DS. Rating of perceived exertion in children. In: Bar-Or O, editor. Advances in pediatric sport sciences vol 3 biological issues. Human Kinetics; Champaign: 1989. pp. 151–168. [Google Scholar]
- Bar-Or O, Magnusson LI, Buskirk ER. Distribution of heat-activated sweat glands in obese and lean men and women. Hum Biol. 1968;40:235–248. [PubMed] [Google Scholar]
- Belding HS, Kamon E. Evaporative coefficients for prediction of safe limits in prolonged exposures to work under hot conditions. Fed Proc. 1973;32:1598–1601. [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Buskirk ER, Bar-Or O, Kollias J. Physiological effects of heat and cold. In: Wilson NL, editor. Obesity. Davis; Philadelphia: 1969. pp. 119–139. [Google Scholar]
- Dougherty KA, Chow M, Kenney WL. Responses of lean and obese boys to repeated summer exercise in the heat bouts. Med Sci Sports Exerc. 2009;41:279–289. doi: 10.1249/MSS.0b013e318185d341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater BL, Kupprat IC, Denton JE, Crist JL, Horvath SM. Response of prepubertal girls and college women to work in the heat. J Appl Physiol. 1977;43:1046–1053. doi: 10.1152/jappl.1977.43.6.1046. [DOI] [PubMed] [Google Scholar]
- DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- Eston RG, Lamb KL. Effort perception. In: Armstrong N, van Mechelen W, editors. Paediatric exercise science and medicine. Oxford University Press; Oxford: 2000. pp. 85–91. [Google Scholar]
- Falk B. Effects of thermal stress during rest and exercise in the paediatric population. Sports Med. 1998;25:221–240. doi: 10.2165/00007256-199825040-00002. [DOI] [PubMed] [Google Scholar]
- Goran M, Fields DA, Hunter GR, Herd SL, Weinsier RL. Total body fat does not influence maximal aerobic capacity. Int J Obes Relat Metab Disord. 2000;24:841–848. doi: 10.1038/sj.ijo.0801241. [DOI] [PubMed] [Google Scholar]
- Havenith G, van Middendorp H. The relative influence of physical fitness, acclimatization state, anthropometric measures and gender on individual reactions to heat stress. Eur J Appl Physiol Occup Physiol. 1990;61:419–427. doi: 10.1007/BF00236062. [DOI] [PubMed] [Google Scholar]
- Haymes EM, Buskirk ER, Hodgson JL, Lundegren HM, Nicholas WC. Heat tolerance of exercising lean and heavy prepubertal girls. J Appl Physiol. 1974;36:566–571. doi: 10.1152/jappl.1974.36.5.566. [DOI] [PubMed] [Google Scholar]
- Haymes EM, McCormick RJ, Buskirk ER. Heat tolerance of exercising lean and obese prepubertal boys. J Appl Physiol. 1975;39:457–461. doi: 10.1152/jappl.1975.39.3.457. [DOI] [PubMed] [Google Scholar]
- Kamon E, Avellini B. Physiologic limits to work in the heat and evaporative coefficient for women. J Appl Physiol. 1976;41:71–76. doi: 10.1152/jappl.1976.41.1.71. [DOI] [PubMed] [Google Scholar]
- Kamon E, Avellini B, Krajewski J. Physiological and biophysical limits to work in the heat for clothed men and women. J Appl Physiol. 1978;44:918–925. doi: 10.1152/jappl.1978.44.6.918. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Zeman MJ. Psychrometric limits and critical evaporative coefficients for unacclimated men and women. J Appl Physiol. 2002;92:2256–2263. doi: 10.1152/japplphysiol.01040.2001. [DOI] [PubMed] [Google Scholar]
- Kenney WL, Lewis DA, Armstrong CG, Hyde DE, Dyksterhouse TS, Fowler SR, Williams DA. Psychrometric limits to prolonged work in protective clothing ensembles. Am Ind Hyg Assoc J. 1988;49:390–395. doi: 10.1080/15298668891379954. [DOI] [PubMed] [Google Scholar]
- Lind AR. A physiological criterion for setting thermal environmental limits for everyday work. J Appl Physiol. 1963;18:51–56. doi: 10.1152/jappl.1963.18.1.51. [DOI] [PubMed] [Google Scholar]
- Lohman TG. The use of skinfold to estimate body fatness on children and youth. J Phys Edu Rec Dance. 1987;58:98–102. [Google Scholar]
- Mahon AD, Marsh ML. Reliability of the rating of perceived exertion at ventilatory threshold in children. Int J Sports Med. 1992;13:567–571. doi: 10.1055/s-2007-1024566. [DOI] [PubMed] [Google Scholar]
- Nishi Y, Gagge AP. Direct evaluation of convective heat transfer coefficient by naphthalene sublimation. J Appl Physiol. 1970;29:830–838. doi: 10.1152/jappl.1970.29.6.830. [DOI] [PubMed] [Google Scholar]
- O’Brien C, Hoyt RW, Buller MJ, Castellani JW, Young AJ. Telemetry pill measurement of core temperature in humans during active heating and cooling. Med Sci Sports Exerc. 1998;30:468–472. doi: 10.1097/00005768-199803000-00020. [DOI] [PubMed] [Google Scholar]
- Owens S, Gutin B. Exercise testing of the child with obesity. Pediatr Cardiol. 1999;20:79–83. doi: 10.1007/s002469900405. [DOI] [PubMed] [Google Scholar]
- Parsons KC. Human thermal environments: the effects of hot, moderate, and cold environments on human health, comfort, and performance. Taylor and Francis Group; New York: 2003. pp. 1–30. [Google Scholar]
- Ridgers ND, Stratton G, Fairclough SJ. Assessing physical activity during recess using accelerometry. Prev Med. 2005;41:102–107. doi: 10.1016/j.ypmed.2004.10.023. [DOI] [PubMed] [Google Scholar]
- Robinson S. The effect of body size upon energy exchange in work. Am J Physiol. 1942;36:363–368. [Google Scholar]
- Rowland TW. Thermoregulation during exercise in the heat in children: old concepts revisited. J Appl Physiol. 2008;105:718–724. doi: 10.1152/japplphysiol.01196.2007. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol. 1966;21:1757–1762. doi: 10.1152/jappl.1966.21.6.1757. [DOI] [PubMed] [Google Scholar]
- Tanner JM. Growth at adolescence. Blackwell Science; Oxford: 1962. The development of the reproductive system; pp. 28–39. [Google Scholar]
- Vroman NB, Buskirk ER, Hodgson JL. Cardiac output and skin blood flow in lean and obese individuals during exercise in the heat. J Appl Physiol. 1983;55:69–74. doi: 10.1152/jappl.1983.55.1.69. [DOI] [PubMed] [Google Scholar]
- Ward DS, Blimkie CJ, Bar-Or O. Rating of perceived exertion in obese adolescents. Med Sci Sports Exerc. 1986;18:S72. [Google Scholar]
- Young AJ, Sawka MN, Epstein Y, Decristofano B, Pandolf KB. Cooling different body surfaces during upper and lower body exercise. J Appl Physiol. 1987;63:1218–1223. doi: 10.1152/jappl.1987.63.3.1218. [DOI] [PubMed] [Google Scholar]