Abstract
Indoleamine 2,3-dioxygenase (IDO) is an intracellular heme containing enzyme that is activated by proinflammatory cytokines, including interferon-γ (IFNγ), and metabolizes tryptophan along the kynurenine pathway. Activation of murine macrophages induces not only IDO but also nitric oxide synthase (iNOS), and the ensuing production of nitric oxide (NO) inhibits IDO. To determine the sensitivity of primary cultures of murine microglia to NO, microglia were stimulated with recombinant murine IFNγ (1 ng/ml) and lipopolysaccharide (LPS) (10 ng/ml). This combination of IFNγ + LPS synergized to produce maximal amounts of nitrite as early as 16 h. Steady-state mRNAs for both iNOS and IDO were significantly increased by IFNγ + LPS at 4 h post-treatment, followed by an increase in IDO enzymatic activity at 24 h. Murine microglia (> 95% CD11b+) were pretreated with the iNOS inhibitor, L-NIL hydrochloride, at a dose (30 μM) that completely abrogated production of nitrite. L-NIL had no effect on IDO mRNA at 4 h or IDO enzymatic activity at 24 h following stimulation with IFNγ + LPS. These data establish that IDO regulation in murine microglia is not restrained by NO, thereby permitting the accumulation of kynurenine and its downstream metabolites in the central nervous system.
Keywords: Nitric oxide, Kynurenine, Indoleamine Enzymatic Activity, Interferon-γ, Primary Microglia
Introduction
Indoleamine 2,3-dioxygenase (IDO) is an intracellular monomeric heme-containing enzyme that catalyzes the first and rate-limiting step that degrades the essential amino acid tryptophan in extrahepatic tissues (Rafice et al., 2009). IDO is induced during an immune responses in many types of cells, including those in the central nervous system (CNS) (Connor et al., 2008; Fujigaki et al., 2006; Guillemin et al., 2005; Jung et al., 2007). Activation of IDO causes degradation of the essential amino acid tryptophan to kynurenine and other metabolites of the kynurenine (Taylor and Feng, 1991). Besides its importance for the development of immunotolerance, activation of IDO is implicated in the pathophysiology of psychiatric disorders (Dantzer et al., 2008; Perez-De La Cruz et al., 2007; Widner et al., 2002).
IDO activation is regulated by many factors, an important one of which is nitric oxide (NO) (Thomas and Stocker, 1999). The inducible form of nitric oxide synthase iNOS is a calcium-independent enzyme that upon stimulation produces large quantities of NO (MacMicking et al., 1997). Activation of murine, but not human macrophages, increases the expression and activity of iNOS (Sethi and Dikshit, 2000). An important interaction between iNOS and IDO is that the product(s) of one pathway regulate(s) the enzyme of the other pathway (Oh et al., 2004; Oxenkrug, 2007; Samelson-Jones and Yeh, 2006). As a result of this crosstalk, NO is well known to inhibit IDO activity in many types of cells (Fujigaki et al., 2002; Hucke et al., 2004; Samelson-Jones and Yeh, 2006; Thomas et al., 1994).
Microglia act as resident mononuclear myeloid cells in the brain (Soulet and Rivest, 2008), and these resident CNS microglia are composed of heterogeneous populations (Schmid et al., 2009). Microglia are activated in most pathological conditions of the CNS and play an important role in sensing and propagating inflammatory signals in response to activation of the peripheral innate immune system (Hanisch and Kettenmann, 2007). Activated microglia are a major source of IDO activity in the brain (Kwidzinski et al., 2005). Along with the production of kynurenines, activated murine microglia also produce large amounts of NO via enhanced expression of iNOS (Cheret et al., 2008; Mir et al., 2008).
NO inhibition of IDO is apparently specific to both certain species and cell types. In macrophages, the inhibition of IDO by NO occurs at both the transcriptional (Alberati-Giani et al., 1997) and post-transcriptional levels (Hucke et al., 2004; Thomas et al., 1994). In sharp contrast to macrophages, murine microglial cell clones immortalized with the activated c-myc oncogene have been reported to be resistant to NO inhibition of IDO (Alberati-Giani et al., 1997; Alberati-Giani et al., 1996). Given the wide heterogeneity of macrophages and their dissimilarities with microglia (Ransohoff and Perry, 2009; Rivest, 2009), the objective of the present experiments was to determine whether expression of IDO in primary rather than transformed murine microglia are sensitive to inhibition by NO. We stimulated primary murine microglia with IFNγ and LPS in order to maximize activation of both iNOS and IDO pathways. We show that NO is unable to inhibit either expression of IDO transcripts or IDO enzymatic activity. These data support the conclusion that production of IDO by primary microglial cells from mice is not subject to regulation by endogenous NO.
Materials and Methods
Reagents
Fetal bovine serum (FBS; <0.25 EU/ml endotoxin), 0.25% trypsin, Dulbecco’s modified Eagle medium/high glucose (DMEM) containing 0.584 g/l glutamine and 4.5 g/l glucose, sodium pyruvate and antibiotics (100 U/ml penicillin, and 100 μg/ml streptomycin) were purchased from HyClone (Logan, UT). Nylon cell strainers (70 μm) were obtained from BD Falcon (Bedford, MA), whereas the Griess reagent for measuring nitric oxide (cat# G2930), as well as the CytoTox96 non-radioactive cytotoxicity kit (cat# G1781), was from Promega Corporation (Madison, USA). Recombinant murine IFNγ (cat# 315-05) was from PeproTech, Inc. (Rocky Hill, NJ). Purity of primary microglia was confirmed with a FITC-labeled anti-mouse CD11b (cat# 557396) using a FITC-labeled rat IgG2b isotype antibody as a control (cat# 553988; BD Biosciences Pharmingen (USA)). Protein was measured with a standard Bradford assay kit (cat# 500-0113, 0114, 0115). The protease inhibitor cocktail (cat# P2714), lipopolysaccharide (LPS) from Escherichia coli 0127:B8 (cat# L-3137), poly-L-lysine (cat# P4832) and other reagents and chemicals were obtained from Sigma Aldrich. (Sigma, USA). L-cell conditioned medium used to grow primary microglia was produced from L-929 cells (American Type Culture Collection, ATCC, cat# CCL-1™, Manassas, USA).
Preparation of primary murine microglia
Primary mixed glial cultures were established from brains of <2 day-old C57BL/6J pups using previously described techniques (Wang et al., 2010). Isolated microglia were collected and cultured in 20% (v:v) L929-cell conditioned medium (LCCM) for 7–10 days. Purity of microglia was confirmed as > 95% CD11b+ cells, as verified by flow cytometry using previously described techniques (26–27).
Primary microglia were treated with 1 ng/ml IFNγ + 10 ng/ml LPS in DMEM supplemented with 2% FBS following preliminary experiments to establish these concentrations as optimal for stimulating expression of IDO and iNOS. At various times following addition of IFNγ + LPS, supernatants were collected and stored at −80°C for measurement of nitrites. Cells were washed twice with cold PBS and stored at −80°C for isolation of IDO mRNA and measurement of IDO enzymatic activity.
Nitrite assay
The major breakdown product of NO in aqueous solutions is the stable derivative, nitrite (Kelm, 1999). The amount of NO released was estimated by measurement of nitrite in the supernatant of microglial conditioned medium using the Griess reagent. Briefly, 50 μl of each sample supernatant was incubated with an equal volume of sulfanilamide solution in duplicate wells of a 96-well plate at room temperature for 5–10 min. Then, 50 μl of N-1-napthylethylenediamine (NED) solution was added to all wells. After 10 min incubation at room temperature, optical density at 540 nm was measured with an OPTImax multiplate reader. Concentrations were calculated by comparison of absorptions with a standard (0.1 M sodium nitrite) curve (1.56, 3.13, 6.25, 12.5, 25, 50 and 100 μM).
Determination of IDO activity
IDO enzymatic activity was assessed by measuring the conversion of L-tryptophan into kynurenine in microglial cell lysates prepared with ice cold lysing buffer (140 mM KCl, 20 mM potassium phosphate buffer, pH 7.0, with a cocktail of protease inhibitors), as previously described (Lestage et al., 2002). Kynurenine was measured by high performance liquid chromatography with electrochemical detection (Wang et al., 2010).
Statistical analysis
Each experiment was replicated at least three times. Data were analyzed using a one-way (treatment) or two-way (pretreatment × treatment) ANOVA, followed by a post hoc pair wise multiple comparison using Fisher’s least significant difference test if the interaction was significant. Statistical significance was determined as P < 0.05. All data are presented as the mean ± SEM.
Results
IFNγ and LPS Synergize to Induce Nitrite Production in Primary Murine Microglia
Numerous reports have established that IFNγ alone can induce iNOS in myeloid cells, but these experiments often used high, pharmacological concentrations (~100 ng/ml) of IFNγ (Alberati-Giani et al., 1997). We therefore tested a low concentration of IFNγ (1 ng/ml) that synergizes with small amounts of LPS (10 ng/ml) to activate macrophages (Davila et al., 1990). As expected, no detectable nitrite could be detected in control cultures. At 16 h, no nitrite (<1.6 μM) was induced by IFNγ alone, 3.0 ± 0 μM by LPS alone and 15.4 ± 0.04 μM by the combination of IFNγ + LPS; at 24 h, these respective values were 1.4 ± 0.0 μM, 14.0 ± 0 μM and 28.9 ± 0.01 μM. In both time points, addition of 1 ng/ml of IFNγ increased (P<0.01) the amount of nitrite induced by 10 ng/ml of LPS. Subsequent experiments used these low doses of both IFNγ + LPS to induce iNOS and the subsequent accumulation of nitrite.
L-NIL Blocks Nitrite Production Induced by IFNγ + LPS in Primary Murine Microglia Without Modifying iNOS Expression
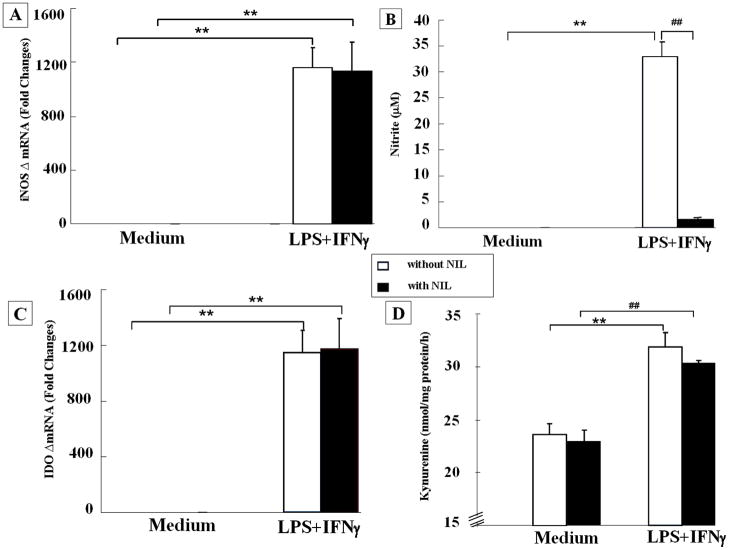
IFNγ + LPS strongly increased iNOS mRNA at 4 h post treatment. As expected, this increase was not affected by L-NIL (Fig. 1A). The increase in nitrite in culture medium at 24 h post IFNγ + LPS (p<0.01) was blocked by L-NIL (p < 0.01) (Fig. 1B). These data confirm the ability of L-NIL to inhibit iNOS enzymatic activity induced by IFNγ + LPS in primary mouse microglia.
Figure 1.
Microglia are resistant to nitric oxide inhibition of indoleamine 2, 3 dioxygenase. Primary mouse microglia were primed with IFNγ (1 ng/ml) and triggered with LPS (10 ng/ml) in the presence or absence of iNOS inhibitor L-NIL (30 μM) for 4 h and 24 h, respectively. (A) Co-stimulation with IFNγ + LPS strongly enhanced iNOS mRNA (Ct cycle for IFNγ + LPS treatment was 19 ± 0.4) compared to constitutive iNOS mRNA in primary microglia incubated in medium alone (Ct cycle was 30 ± 0.5). L-NIL had no effect on expression of iNOS transcripts (Ct cycle for IFNγ + LPS treatment with L-NIL was 19 ± 0.2). (B) IFNγ + LPS significantly increased nitrate accumulation 24 h later, and this accumulation of nitrite was blocked by L-NIL. (C) No IDO mRNA was detected in microglia incubated for 4 h in medium (>40 amplification cycles). However, IFNγ + LPS increased expression of steady-state IDO transcripts by over a 1,000-fold, and this increase in IDO mRNA was not affected by L-NIL (Ct cycles for IFNγ + LPS treatment were 29 ± 0.7 in the absence of L-NIL and 29 ± 0.3 in the presence of L-NIL). (D) IDO enzymatic activity in microglia was increased by the combination of IFNγ + LPS, and this increase was not affected by inhibition of iNOS. ** p < 0.01 compared to control medium. Each bar represents the mean ± SEM of results from 3 separate experiments.
L-NIL Does Not Inhibit IFNγ + LPS-Induced Upregulation of Either IDO Expression and Enzymatic Activity
As expected, IFNγ + LPS increased both IDO mRNA that was measured at 4 h (Fig. 1C) and IDO enzymatic activity measured after 24 h of treatment (Fig. 1D). L-NIL had no effect on either measure of IDO activation.
Discussion
Results of the present experiments confirm that primary murine microglial cells respond to IFNγ + LPS by activation of both iNOS and IDO. The hypothesis that we tested was whether biologically-significant crosstalk occurs between iNOS and IDO in primary microglia, as is very well documented in murine macrophages. These data clearly establish that inhibition of iNOS by L-NIL does not down regulate IDO in primary murine microglia. It is therefore likely that the unrestrained activation of IDO in these cells permits more continuous production of kynurenine and its downstream metabolites in the CNS than in peripheral tissues.
Various forms of reciprocal interactions between IDO and iNOS have already been described in many different cell types. IDO activation can down-regulate the expression and activity of iNOS. Conversely, induction of NO by activation of iNOS down-regulates IDO activity in cell types as diverse as human uroepithelial transformed cells (Daubener et al., 1999), murine bone marrow-derived myeloid dendritic cells (Hara et al., 2008), human transformed and primary macrophages (Thomas et al., 1994) and mouse peritoneal cells (Ohtaki et al., 2009).
The large amount of data on the ability of NO to down regulate IDO activity in macrophages and other peripheral cell types has attracted little attention to the possibility that there might be exceptions to this well-accepted crosstalk. During the course of a study on IDO expression in transformed and immortalized murine macrophages and microglia, Alberati-Giani et al. observed that IFNγ-induced NO production led to a reduction in IDO activity in macrophages but not in a microglial cell line (Alberati-Giani et al., 1997). In these experiments, they measured IDO activity by enzymatic activity in cell pellets. Several iNOS inhibitors that were tested increased IFNγ-induced IDO enzymatic activity in MT2 macrophages. In contrast, the same treatment had no effect on IDO enzymatic activity in IFNγ-stimulated N11 immortalized microglial cells. Addition of NO donors in the culture medium decreased IFNγ-induced IDO enzymatic activity in MT2 macrophages but not in the transformed N11 microglia. IFNγ was about 5 times less active in stimulating IDO and iNOS enzymatic activity in microglia than in macrophages. Interestingly, addition of LPS augmented IFNγ-induced IDO and iNOS enzymatic activity in N11 macrophages but it decreased IDO enzymatic activity in MT2 macrophages probably because of their higher sensitivity to LPS-induced triggering of iNOS activation by IFNγ. The unresponsiveness of transformed microglial cells to NO was claimed to be also present in primary microglia, but no supporting data for this statement were included in the original research paper.
In the present study, we made use of a previously described reliable technique for inducing IDO in LPS-stimulated primary cultures of microglia derived from neonatal C57BL/6J mice (Wang et al., 2010). We confirmed that co-stimulation of primary murine microglia by IFNγ + LPS activates both IDO and iNOS enzymatic activity. As expected, blockade of iNOS enzymatic activity by L-NIL blocked the release of nitrites caused by released into the culture medium caused by IFNγ + LPS. However, despite complete inhibition of nitrite accumulation, L-NIL had no effect on either IDO steady state mRNA or IDO enzymatic activity.
Despite the evidence in this report, it remains possible that microglia differ quantitatively rather than qualitatively from macrophages in the ability of NO to negatively regulate IDO expression and activity. In microglia as in other cell types, induction of iNOS in response to inflammatory stimuli is dependent on the production of superoxide and other NADPH oxidase-derived oxygen species, including peroxynitrite (Li et al., 2005; Pawate et al., 2004). However, the superoxide respiratory burst is probably of decreased magnitude in microglia compared to macrophages (Turchan-Cholewo et al., 2009). In addition, direct binding of NO to heme iron in IDO, which is one of the mechanisms by which NO inhibits IDO at the post-translational level, is dependent on a number of cellular factors, including NO abundance, pH, redox environment and tryptophan availability (Samelson-Jones and Yeh, 2006). All these issues are likely to account for the unresponsiveness of IDO to NO inhibition in primary murine microglia.
In conclusion, results of the present series of experiments indicate that IDO in primary murine microglia costimulated with IFNγ + LPS is not impaired by the production of NO, as is known to occur in murine macrophages. Since activation of the kynurenine pathway in microglia leads to formation of potentially neurotoxic kynurenine metabolites, including 3-hydroxy kynurenine and quinolinic acid, this lack of down-regulation of IDO activation by NO could be one of the factors that explain the exquisite sensitivity of the brain to depressogenic activity of proinflammatory cytokines. These aspects clearly deserve further investigation.
Acknowledgments
The authors have no conflicting financial interests. Supported by NIH to KWK (AG029573) and RD (MH079829; MH71349)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberati-Giani D, Malherbe P, Ricciardi-Castagnoli P, Kohler C, Denis-Donini S, Cesura AM. Differential regulation of indoleamine 2,3-dioxygenase expression by nitric oxide and inflammatory mediators in IFN-gamma-activated murine macrophages and microglial cells. J Immunol. 1997;159:419–426. [PubMed] [Google Scholar]
- Alberati-Giani D, Ricciardi-Castagnoli P, Kohler C, Cesura AM. Regulation of the kynurenine pathway by IFN-gamma in murine cloned macrophages and microglial cells. Adv Exp Med Biol. 1996;398:171–175. doi: 10.1007/978-1-4613-0381-7_28. [DOI] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, Mallet J, Cumano A, Krause KH, Mallat M. Neurotoxic activation of microglia is promoted by a nox1-dependent NADPH oxidase. J Neurosci. 2008;28:12039–12051. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor TJ, Starr N, O’Sullivan JB, Harkin A. Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-gamma? Neurosci Lett. 2008;441:29–34. doi: 10.1016/j.neulet.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubener W, Posdziech V, Hadding U, MacKenzie CR. Inducible anti-parasitic effector mechanisms in human uroepithelial cells: tryptophan degradation vs. NO production. Med Microbiol Immunol. 1999;187:143–147. doi: 10.1007/s004300050086. [DOI] [PubMed] [Google Scholar]
- Davila DR, Edwards CK, 3rd, Arkins S, Simon J, Kelley KW. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. Faseb J. 1990;4:2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- Fujigaki H, Saito K, Lin F, Fujigaki S, Takahashi K, Martin BM, Chen CY, Masuda J, Kowalak J, Takikawa O, Seishima M, Markey SP. Nitration and inactivation of IDO by peroxynitrite. J Immunol. 2006;176:372–379. doi: 10.4049/jimmunol.176.1.372. [DOI] [PubMed] [Google Scholar]
- Fujigaki S, Saito K, Takemura M, Maekawa N, Yamada Y, Wada H, Seishima M. L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase. Infect Immun. 2002;70:779–786. doi: 10.1128/iai.70.2.779-786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin GJ, Smythe G, Takikawa O, Brew BJ. Expression of indoleamine 2,3-dioxygenase and production of quinolinic acid by human microglia, astrocytes, and neurons. Glia. 2005;49:15–23. doi: 10.1002/glia.20090. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hara T, Ogasawara N, Akimoto H, Takikawa O, Hiramatsu R, Kawabe T, Isobe K, Nagase F. High-affinity uptake of kynurenine and nitric oxide-mediated inhibition of indoleamine 2,3-dioxygenase in bone marrow-derived myeloid dendritic cells. Immunol Lett. 2008;116:95–102. doi: 10.1016/j.imlet.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Hucke C, MacKenzie CR, Adjogble KD, Takikawa O, Daubener W. Nitric oxide-mediated regulation of gamma interferon-induced bacteriostasis: inhibition and degradation of human indoleamine 2,3-dioxygenase. Infect Immun. 2004;72:2723–2730. doi: 10.1128/IAI.72.5.2723-2730.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ID, Lee CM, Jeong YI, Lee JS, Park WS, Han J, Park YM. Differential regulation of indoleamine 2,3-dioxygenase by lipopolysaccharide and interferon gamma in murine bone marrow derived dendritic cells. FEBS Lett. 2007;581:1449–1456. doi: 10.1016/j.febslet.2007.02.073. [DOI] [PubMed] [Google Scholar]
- Kelm M. Nitric oxide metabolism and breakdown. Biochim Biophys Acta. 1999;1411:273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, Nitsch R, Bechmann I. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- Lestage J, Vernier D, Palin K, Dantzer R. The enzyme indoleamine 2,3-dioxygenase is induced in the mouse brain in response to peripheral administration of lipopolysaccharide and superantigen. Brain Behav Immun. 2002;16:596–601. doi: 10.1016/s0889-1591(02)00014-4. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci U S A. 2005;102:9936–9941. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mir M, Tolosa L, Asensio VJ, Llado J, Olmos G. Complementary roles of tumor necrosis factor alpha and interferon gamma in inducible microglial nitric oxide generation. J Neuroimmunol. 2008;204:101–109. doi: 10.1016/j.jneuroim.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Oh GS, Pae HO, Choi BM, Chae SC, Lee HS, Ryu DG, Chung HT. 3-Hydroxyanthranilic acid, one of metabolites of tryptophan via indoleamine 2,3-dioxygenase pathway, suppresses inducible nitric oxide synthase expression by enhancing heme oxygenase-1 expression. Biochem Biophys Res Commun. 2004;320:1156–1162. doi: 10.1016/j.bbrc.2004.06.061. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ito H, Ando K, Ishikawa T, Hoshi M, Tanaka R, Osawa Y, Yokochi T, Moriwaki H, Saito K, Seishima M. Interaction between LPS-induced NO production and IDO activity in mouse peritoneal cells in the presence of activated Valpha14 NKT cells. Biochem Biophys Res Commun. 2009;389:229–234. doi: 10.1016/j.bbrc.2009.08.120. [DOI] [PubMed] [Google Scholar]
- Oxenkrug GF. Genetic and hormonal regulation of tryptophan kynurenine metabolism: implications for vascular cognitive impairment, major depressive disorder, and aging. Ann N Y Acad Sci. 2007;1122:35–49. doi: 10.1196/annals.1403.003. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Perez-De La Cruz V, Konigsberg M, Santamaria A. Kynurenine pathway and disease: an overview. CNS Neurol Disord Drug Targets. 2007;6:398–410. doi: 10.2174/187152707783399229. [DOI] [PubMed] [Google Scholar]
- Rafice SA, Chauhan N, Efimov I, Basran J, Raven EL. Oxidation of L-tryptophan in biology: a comparison between tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase. Biochem Soc Trans. 2009;37:408–412. doi: 10.1042/BST0370408. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- Rivest S. Regulation of innate immune responses in the brain. Nat Rev Immunol. 2009;9:429–439. doi: 10.1038/nri2565. [DOI] [PubMed] [Google Scholar]
- Samelson-Jones BJ, Yeh SR. Interactions between nitric oxide and indoleamine 2,3-dioxygenase. Biochemistry. 2006;45:8527–8538. doi: 10.1021/bi060143j. [DOI] [PubMed] [Google Scholar]
- Schmid CD, Melchior B, Masek K, Puntambekar SS, Danielson PE, Lo DD, Sutcliffe JG, Carson MJ. Differential gene expression in LPS/IFNgamma activated microglia and macrophages: in vitro versus in vivo. Journal of neurochemistry. 2009;109(Suppl 1):117–125. doi: 10.1111/j.1471-4159.2009.05984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S, Dikshit M. Modulation of polymorphonuclear leukocytes function by nitric oxide. Thromb Res. 2000;100:223–247. doi: 10.1016/s0049-3848(00)00320-0. [DOI] [PubMed] [Google Scholar]
- Soulet D, Rivest S. Microglia. Curr Biol. 2008;18:R506–508. doi: 10.1016/j.cub.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Taylor MW, Feng GS. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- Thomas SR, Mohr D, Stocker R. Nitric oxide inhibits indoleamine 2,3-dioxygenase activity in interferon-gamma primed mononuclear phagocytes. J Biol Chem. 1994;269:14457–14464. [PubMed] [Google Scholar]
- Thomas SR, Stocker R. Redox reactions related to indoleamine 2,3-dioxygenase and tryptophan metabolism along the kynurenine pathway. Redox Rep. 1999;4:199–220. doi: 10.1179/135100099101534927. [DOI] [PubMed] [Google Scholar]
- Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lawson MA, Dantzer R, Kelley KW. LPS-induced indoleamine 2,3-dioxygenase is regulated in an interferon-gamma-independent manner by a JNK signaling pathway in primary murine microglia. Brain Behav Immun. 2010;24:201–209. doi: 10.1016/j.bbi.2009.06.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression--what is the link? Brain Behav Immun. 2002;16:590–595. doi: 10.1016/s0889-1591(02)00006-5. [DOI] [PubMed] [Google Scholar]