Abstract
Purpose
Andrographolide is a diterpenoid lactone isolated from Andrographis paniculata (King of Bitters), an herbal medicine used in Asia. It has been reported to have anti-inflammatory, antihypertensive, anti-viral and immune-stimulant properties. Furthermore, it has been shown to inhibit cancer cell proliferation and induce apoptosis in leukemia and solid tumor cell lines.
Experimental Design
We studied the Burkitt p53 mutated Ramos cell line, the mantle-cell lymphoma (MCL) line Granta, the follicular lymphoma (FL) cell line HF-1 and the diffuse large B-cell lymphoma (DLBCL) cell line SUDHL4, as well as primary cells from patients with FL, DLBCL, and MCL.
Results
We found that andrographolide resulted in dose- and time-dependent cell death as measured by MTT. Andrographolide significantly increased reactive oxygen species (ROS) production in all cell lines. To determine mechanism of cell death, we measured apoptosis by Annexin-V/propidium iodide (PI) in the presence and absence of the antioxidant N-acetyl-L-cysteine (NAC), the glutathione-depleting agent buthionine sulfoxamine (BSO), or caspase inhibitors. We found that apoptosis was greatly enhanced by BSO, blocked by NAC, and accompanied by PARP cleavage and activation of caspases 3, 8 and 9. We measured BAX conformational change, and mitochondrial membrane potential, and using mouse embryonic fibroblast (MEF) Bax/Bak double knockouts (MEFBax−/−/Bak−/−), we found that apoptosis was mediated through mitochondrial pathways, but dependent on caspases in both cell lines and in patient samples.
Conclusions
Andrographolide caused ROS-dependent apoptosis in lymphoma cell lines and in primary tumor samples, which was enhanced by depletion of GSH and inhibited by NAC or the pan-caspase inhibitor Z-VAD-FMK. Further studies of diterpenoid lactones in lymphoma are warranted.
Keywords: non-Hodgkin lymphoma, andrographolide, apoptosis, oxidative stress, ROS, caspase
Introduction
Andrographolide is a diterpenoid lactone isolated from Andrographis paniculata (King of Bitters) (1–3), an important herbal medicine used in Asia to treat a range of diseases, such as respiratory infection, fever, bacterial dysentery and diarrhea (4–6). It also has been studied in patients with HIV (7). The major bioactive component extracted from Andrographis paniculata is andrographolide and the three hydroxyls at C-3, C-19 and C-14 are responsible for its biological activity (8).
Recently, the anti-cancer properties of andrographolide have been recognized, and some of its effects appear to proceed through redox-mediated pathways (9–12). We therefore hypothesized that andrographolide would lead to cell death in lymphoma cell lines and that the effect may be related to altered cellular redox state. We studied andrographolide in non-Hodgkin lymphoma cell lines as well as in primary malignant B-cells from patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), and follicular lymphoma (FL). We found that andrographolide induced ROS and caspase-dependent apoptosis in lymphoma cell lines and in primary tumor samples but not in normal lymphocytes, and that this was enhanced by depletion of glutathione (GSH) and inhibited by the antioxidant N-acetyl-L-cysteine (NAC) or the pan caspase inhibitor Z-VAD-FMK. Furthermore, these effects appeared to proceed through BAX/BAK pathways.
Materials and Methods
Reagents
Andrographolide (Andro) (Supplemental Figure 1), buthionine sulfoxamine (BSO), and NACwere purchased from Sigma Chemical co. (St. Louis, MO). Z-VAD-FMK, Ac-DEVD-CHO, Ac-IETD-CHO, Ac-LEHD-CHO were obtained from BioMol (Plymouth Meeting, PA). Antibodies to caspase 3, caspase 9, caspase 8, were purchased from Cell Signaling Technology (Beverly, MA) and GAPDH was purchased from Chemicon.
Cell culture
Ramos (Burkitt lymphoma) cell line was obtained from ATCC, HF-1 (FL) from Dr. Richard Miller, SUDHL4 (DLBCL) from Dr. Ron Gartenhaus, and Granta (MCL) was a kind gift from Dr Steven Bernstein. Malignant cells from patients with FL, DLBCL, and MCL were cultured in RPMI-1640 containing 10% fetal bovine serum, 1% sodium pyruvate (Granta only) and in the presence of penicillin/streptomycin/glutamine at 37°C in a humidified 5% CO2 incubator. Cell viability was measured using the trypan blue or propidium iodide exclusion method or MTT assay (see below). Cells were treated with andrographolide or andrographolide and BSO where indicated. Fifty mM in DMSO or 100mM in water stock solution was prepared for both substances. Before treatment, PBS was used to dilute andrographolide stock solution, and PBS/andrographolide solution added to medium to achieve the desired working concentration. The control groups used the same amount of DMSO and PBS in medium as the treatment groups.
Incubations
Cells were incubated with the following drugs: 0–100μM andrographolide(10), 100μM BSO(13), 10mM NAC(14), and 50μM caspase inhibitors (Z -VAD-FMK, Ac-DEVD-CHO, Ac-IETD-CHO, Ac-LEHD-CHO)(10).
Primary MCL, DLBCL and FL cells
Following written consent approved by the Northwestern University Institutional Review Board (IRB), peripheral blood was drawn from three patients with leukemic-phase FL, one with MCL, and two with transformed DLBCL. The 3 FL patients had bulky abdominal adenopathy (>12 cm) and a rapidly rising lymphocyte count (absolute lymphocyte count 238.5 K/μl) with FISH confirmation of t (14; 18) in 95% of nuclei. The patient with MCL had newly diagnosed MCL with a WBC of 28,000/μl, with 96% malignant cells and had t (11; 14) by FISH in the blood and bone marrow. The 2 patients with DLBCL had transformed lymphoma with >100,000/μl circulating large cells. The peripheral blood was diluted 1:1 with PBS (Ca2+ and Mg2+ free) and was layered over Ficoll-Paque Plus (Sigma). Samples werethen centrifuged at 150 × g for 20 minutes at room temperature;the buffy coat layer was removed and centrifuged again. Isolated peripheral blood mononuclear cells (all malignant cells in the 6 samples) (PBMC) were then re-suspended in RPMI + 10% fetal bovine serum to 1 × 106 cells/ml.
MTT assay
The effects of andrographolide on cell viability was measured by MTT assay in Ramos, Granta, HF-1, and SUDHL4 cells according to the instructions of an improved detection kit provided by the manufacturer (The CellTiter 96 AQueous One Solution Cell proliferation Assay, Promega). Briefly, 2.5×104 cells/90 μl were seeded in 96 well micro titer plates. After incubating with different concentrations of Andro (10μl) for the designated times, 20uL MTT solution was added to each well and the plates were incubated for an additional 1–4h at 37°C. The absorbance was read at 490nm using a micro plate reader (MRX Revelation) (DYNEX Technologies, Chantilly, VA). The OD values were expressed as % over the control group. Since reduction of MTT can only occur in metabolically active cells the level of activity is a measure of the viability of the cells.
ROS measurement
ROS accumulation in treated and untreated cells was measured by FACS (15, 16). Cells were incubated in 5μM 2’7’dichlorofluorescein diacetate (H2DCFDA) for 30 min at 37°C in the dark. After washing, cells were suspended in 1 ml cold Propidium Iodide (PI) (200ng/ml)/PBS for 5 minutes in order to test ROS in living cells. ROS were measured by oxidation of H2DCFDA to dichlorofluorescein (DCF). Fluorescence intensity was read by flow cytometry using the Beckman counter EPICS XL-MCL Cytometer on the FL1 channel. Results were analyzed and calculated by FCS Express V3 software (De Novo Software, Canada) and Excel (Microsoft).
Quantitation of apoptosis
After incubations and washing, 1×106 cells were labeled with Annexin V-FITC and PI reagent in the binding buffer according to the Annexin V-FITC apoptosis detection kit instruction provided by the manufacturer (Invitrogen). The Fluorescent signals of FITC and PI were detected respectively at 518nm and 620nm on a Beckman coulter FACS machine (Beckman). For each analysis 30,000 events were recorded. Results were analyzed and calculated by FCS Express V3 software (De Novo Software, Canada) and Excel (Microsoft). The % apoptosis was the sum of (Annexin V-FITC+/PI-) and (Annexin V-FITC+/PI+) cells.
Immunofluorescent staining
After washing and cytospin, cells were fixed 20 minutes by formaldehyde (4% in PBS). Then cells were blocked in blocking buffer (1% BSA with 0.02% Triton X-100) for 1 hour (h) and further incubated with anti-BAX 6A7 monoclonal antibody (Sigma) overnight at 4°C. After washing with PBS (+0.02% Triton X-100) and re-blocking with blocking buffer for 1h, cells were incubated with anti-mouse Alexa 633 or Cy3 secondary antibody for another 1h. After washing, cover slips were mounted onto slides using ProLong anti-fade mounting reagent (Invitrogen). Cells were visualized under Nikon C1Si confocal or UV LSM510 Meta confocal microscopes.
Western blotting
Following the various incubations, cells were washed with PBS, centrifuged, and cell pellets treated with lysis buffer containing protease and phosphatase inhibitors (Roche, Germany; Sigma, St. Louis, MO). Protein concentrations were determined with the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). An aliquot of each cell lysate was used for protein assay (17). 25–50μg total protein samples were subjected to 12% SDS-PAGE electrophoresis. Proteins were electrophoretically transferred to a nitrocellulose membrane. After incubating with blocking buffer containing 5% nonfat milk for 1h, the membranes were incubated with the primary antibody overnight at 4°C, washed with TBST thrice, incubated with secondary antibody for 1h at room temperature, and washed thrice with TBST. Immune complexes were visualized by ECL kit and film (Amersham Biosciences, Millipore, or Denville Scientific Inc).
Preparation of cytosolic and mitochondrial fractions
Cells were treated with andrographolide, or andrographolide and NAC, or andrographolide and Z-VAD-FMK for 0, 3, 18, and 24h. Mitochondrial and cytosolic fractions were prepared using a mitochondria isolation kit for cultured cells from Pierce (Pierce, Rockford, IL) according to the manufacturer’s instructions. The mitochondrial pellet was re-suspended in sample buffer for SDS-gel electrophoresis and analyzed by western blotting for BAX antibody (Cell Signaling). COX IV (Cell Signaling) is used as an internal control for the mitochondrial fraction, and GAPDH (Chemicon) for the cytosolic fraction. Cytosolic fractions were also subjected to western blotting for BAX.
MEFWt and MEFBax−/−/Bak−/− experiments
Mouse embryonic fibroblasts (MEFs) were a kind gift from Dr. Craig Thompson (University of Pennsylvania). Cells were cultured in DMEM containing 10% fetal bovine serum, and in the presence of penicillin/streptomycin/glutamine at 37°C in a humidified 5% CO2 incubator. Cell viability was measured using the trypan blue or propidium iodide exclusion method. Cells weretreated with andrographolide at 40μM and 60μM for 24h or 30h. The cell morphology was observed by microscope. Further, Annexin V-FITC/PI apoptosis assay was performed as described above.
Mitochondrial membrane potential (Δψm) measurement
Tetramethylrhodamine (TMRE) (Invitrogen, Carlsbad, CA) was added to the culture medium to a final concentration of 250nM just prior to the end of the incubation time. Cells were further incubated for 30 minutes at 37°C. Cells were washed in PBS twice and re-suspended in FACS buffer containing 20nM TMRE and 2% FBS. Fluorescence intensity was measured on the FL-2 channel of a flow cytometer. Results were analyzed and calculated by FCS Express V3 software (De Novo Software, Canada) and Excel (Microsoft).
Statistics
Data are expressed as means ± SD. Comparisons between two values were performed by unpaired Student’s t-test. For multiple comparisons among different groups of data, the significant differences were determined by the Bonferroni method. Significance was defined at p ≤ 0.05.
Results
Andrographolide inhibits cell viability
To evaluate the effect of andrographolide on cell viability, we treated Ramos, Granta, HF-1 and SUDHL4 cells with 0–100μM andrographolide for 24 or 48h. Andrographolide resulted in loss of cell viability (by MTT assay) in all four lymphoma cell lines in a dose and time-dependent manner (Figure 1). IC50 (defined herein as that concentration that achieved 50% inhibition of cell viability) at 48h was 20μM for Ramos, 40μM for Granta, 15μM for HF-1 and 30μM for SUDHL4. These data indicate that andrographolide inhibits cell viability in Ramos, Granta, HF-1, and SUDHL4 lymphoma cell lines in a dose and time-dependent manner.
Figure 1. MTT cell viability assay.
Cells were treated with andrographolide at the indicated concentration for the indicated period of time. The MTT reagent (Promega) as described in Materials and Methods was added and colored solution was quantified by measuring at 490nm wavelength. Andrographolide -induced inhibition of cell viability was dose and time-dependent in Ramos, Granta, HF-1 and SUDHL4 cell lines. IC50 at 48h was 20μM for Ramos, 40μM for Granta, 15μM for HF-1 and 30μM for SUDHL4. The data are expressed as % of control and are significant for each Andrographolide concentration at p>0.05 by students t-test as described in Materials and Methods.
Andrographolide induces ROS accumulation in lymphoma cell lines
To examine whether andrographolide affects the oxidative function of the cell, we quantified ROS at different time points by measuring the fluorescent signal of DCF using fluorescence activated cell sorting (FACS). Cells were treated with andrographolide at the indicated dose and time period (Figure 2). We demonstrated significant (p ≤ 0.05) ROS accumulation at 48h for Ramos, 1–6h for Granta, 0.5 to 3h for HF-1 and 4h for SUDHL4 (Figure 2A). A shift to the right (red) indicates ROS accumulation. Figure 2B shows quantification of ROS accumulation in all four cell lines. The data shown represent percentage increase compared to control and represents the mean +/− standard deviation. Taken together, these data demonstrate that andrographolide-induced ROS accumulation is dose and time-dependent in lymphoma cell lines.
Figure 2. Andrographolide -induced ROS accumulation and ψ change in lymphoma cell Δ m lines were dose dependent.
Cells were treated with andrographolide at the indicated dose and time period. (A) Representative histograms show that andrographolide induced ROS production in Ramos, Granta, HF-1 and SUDHL4 cell lines. A shift to the right (red) indicates ROS production. (B) Quantification of ROS accumulation in 4 cell lines. The data shown represent percentage increase compared to control and represents the mean +/− standard deviation of 3 experiments completed in triplicate. *= p<0.05 control vs. andrographolide (C) Quantification of Δψm in 3 cell lines. The data shown represent percentage decrease compared to control and represents the mean +/− standard deviation of 3 experiments completed in triplicate. (* =p ≤ 0.05, control vs. andrographolide.)
Andrographolide induces disruption of mitochondrial membrane potential (Δψm)
Disruption of the mitochondrial membrane potential is one of the earliest intracellular events that occur in apoptosis. It has been shown that andrographolide results in loss of MMP in a hepatocellular carcinoma cell line (12). To investigate whether andrographolide affects Δψm in lymphoma cell lines, we measured Δψm in Ramos (19h), HF-1 (16h) and SUDHL4 (16h) (Figure 2C). For Ramos, at lower concentrations of andrographolide (20–40 μM), we start to see the change of Δψm at 19 hours (Figure 2C). Similar results were seen in HF-1 and SUDHL4 cells. These data show that andrographolide results in dose-dependent loss of Δψm.
Andrographolide induces NAC-reversible, caspase-dependent apoptosis in Ramos, Granta, HF-1 and SUDHL4 cell lines
In order to determine whether andrographolide-induced loss of cell viability was related to apoptosis, we quantified apoptosis by FACS after staining with Annexin V-FITC and PI (Figure 3). We found that andrographolide resulted in significant (p <0.05) dose-dependent apoptosis in Ramos at 72h, and in Granta, HF-1 and SUDHL4 at 48h. The AC50 (defined herein as that concentration that achieved 50% apoptosis) was 40μM for Ramos at 72h, 40μM for Granta or HF-1 at 48h and 30μM for SUDHL4 at 48h.
Figure 3. Dependence of apoptosis on ROS and caspases.
Andrographolide-induced apoptosis was inhibited by the anti-oxidant NAC, or caspase inhibitors, but enhanced by BSO in Ramos, Granta, HF-1, and SUDHL4 cell lines. Cells were treated with andrographolide, andrographolide + BSO, andrographolide + NAC, or andrographolide + caspase inhibitors at the indicated dose and time period. Annexin V-FITC and PI reagent were added and analyzed by FACS. (A) Andrographolide induced apoptosis is dose-dependent. NAC completely inhibited andrographolide-induced apoptosis in all cell lines (p ≤ 0.05). (B) Cells were pre-treated with BSO (100μM) overnight, and then treated with andrographolide or andrographolide +NAC at the indicated dose and time period. BSO significantly increased andrographolide -induced apoptosis (p ≤ 0.05), and apoptosis was completely inhibited by NAC (p ≤ 0.05). Lower dose andrographolide (72h, 10μM in Ramos, 48h, 5μM in HF-1) with BSO dramatically increased apoptosis. (C) Cells were pre-treated with 50μM of the indicated caspase inhibitors (Ac-DEVD-CHO, Ac-IETD-CHO, Ac-LEHD-CHO or Z-VAD-FMK) for 1h, and then andrographolide was added at the indicated concentrations for 48h. All of the caspase inhibitors significantly inhibited andrographolide-induced apoptosis (p ≤ 0.05).
To determine if apoptosis was related to ROS, we co-incubated cells with the anti-oxidant NAC. In all cell lines, NAC completely abrogated andrographolide-induced apoptosis (Figure 3A) (p <0.05). These data suggest that apoptosis was related to the cellular redox state, perhaps as a consequence of depletion of the endogenous anti-oxidant GSH, since NAC restores intracellular GSH.
We further investigated the involvement of ROS in andrographolide-induced apoptosis by pre-treating cells with the reduced GSH depleting agent BSO (100μM), and then added andrographolide or andrographolide and NAC at the indicated dose and time periods (Figure 3B). We found that BSO greatly enhanced andrographolide-induced apoptosis, and apoptosis was again completely inhibited by the anti-oxidant NAC. With the addition of BSO, the AC50 was significantly lower in all cell lines (72h, 10μM in Ramos; 48h, 40uM in Granta; 48h, 5μM in HF-1, and 48h, 20uM in SUDHL4) compared with andrographolide alone. Further, BSO, when added to andrographolide in Ramos cells shows a striking increase in ROS production compared with andrographolide alone (data not shown).
Next, to determine if andrographolide-induced apoptosis was caspase-dependent, the caspase inhibitors Z-VAD-FMK, Ac-LEHD-CHO, Ac-IETD-CHO and Ac-DEVD-CHO, were pre-incubated with Ramos, Granta, HF-1 and SUDHL4 cell lines, then andrographolide added at the indicated doses and time periods (Figure 3C). In all four cell lines, the pan caspase inhibitor, Z-VAD-FMK resulted in complete inhibition, while the caspase 3 inhibitor, Ac-DEVD-CHO significantly (p <0.05) inhibited apoptosis in Ramos and Granta. Further, the caspase 8 inhibitor and the caspase 9 inhibitor (Ac-IETD-CHO and Ac-LEHD-CHO) also resulted in significant inhibition (p <0.05) in Ramos and Granta (Figure 3C). Together, these data demonstrate that andrographolide-induced apoptosis in lymphoma cell lines is ROS and caspase-dependent, and that both the intrinsic and extrinsic caspase pathways are relevant.
Andrographolide induces PARP and caspase cleavage
To further investigate the mechanism of apoptosis, we examined caspase activation and PARP cleavage by andrographolide using immunoblotting (Figure 4). After 24h of andrographolide exposure in Granta cells, we found that cleavage of PARP, caspase 3, and caspase 8 was dose-dependent and completely inhibited by NAC (Figure 4A). PARP cleavage was also time-dependent, as shown in Figure 4B. We begin to see PARP cleavage after 16h, and it is inhibited by NAC and Z-VAD-FMK. In Figure 4C and 4D, after 24h of andrographolide exposure, we observed dose-dependent, NAC-inhibitable cleavage of PARP, caspase 8, caspase 9 and caspase 3 in Ramos cells. In Figure 4E and 4F, we also demonstrate time-dependent cleavage of PARP, caspase 8, caspase 9, and caspase 3 at 40 and 30 μM respectively in HF-1 cells and SUDHL4 cells, at concentrations that have been shown to induce apoptosis (Figure 3). As in Granta cells, NAC and Z-VAD-FMK also inhibited caspase and PARP cleavage induced by andrographolide in HF-1 and SUDHL4. These data provide further evidence that andrographolide-induced apoptosis is ROS and caspase-dependent, and may proceed by both intrinsic and extrinsic pathways.
Figure 4. Mechanism of andrographolide -induced apoptosis.
Cells were treated with andrographolide in the presence or absence of NAC (10mM) or Z-VAD-FMK (50μM) at the indicated dose and time period, and Western Blot was performed. (A) Andrographolide resulted in cleavage of PARP and activation of caspase 3 and 8 in Granta. (B) Andrographolide-induced PARP cleavage started at 16h incubation. NAC and Z-VAD-FMK inhibited andrographolide-induced PARP cleavage in Granta. (C,D) Andrographolide resulted in cleavage of PARP and activation of caspase 3, 8 or caspase 9 in Ramos. (E,F) Andrographolide-induced cleavage of PARP, caspase 3, caspase 8, and caspase 9 was seen at 18h (HF-1) or 16h (SUDHL4), and was completely inhibited by NAC and by the pan-caspase inhibitor Z-VAD-FMK.
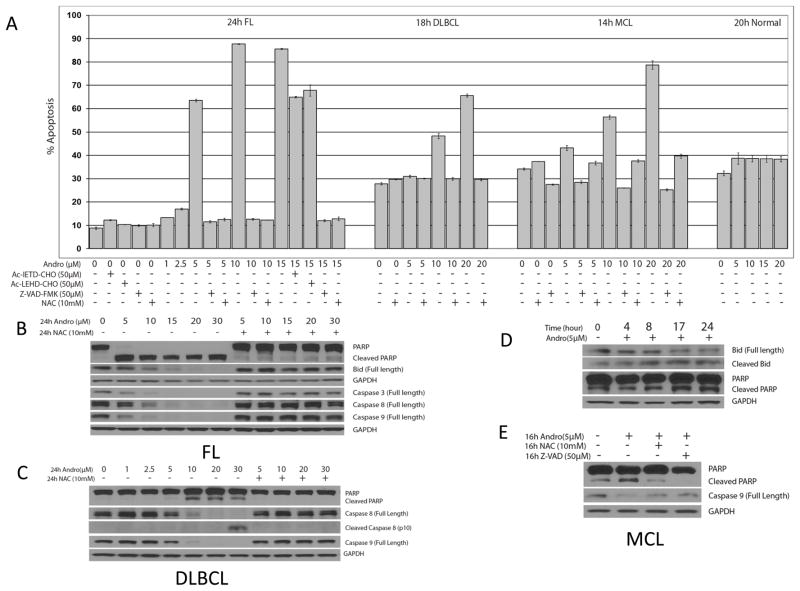
Andrographolide induces apoptosis in primary malignant cells from patients with FL, MCL, and DLBCL
Based on our observations in lymphoma cell lines, we examined whether andrographolide also induced apoptosis in primary lymphoma patient samples and normal human lymphocytes, and if so, by which cell death pathways. First, we treated fresh FL, MCL, and DLBCL malignant cells with andrographolide at the indicated doses and time periods (Figure 5 shows a representative sample. Similar results were seen in all patient samples). Interestingly, we found that primary malignant cells from patients were more sensitive to andrographolide than the cell lines. Andrographolide induced significant dose-dependent apoptosis (p <0.05) in primary malignant cells at a lower AC50 and earlier time points (5μM, 24h for FL; 10μM, 18h for DLBCL, 10μM, 14h for MCL). Similar to the cell lines, NAC completely prevented andrographolide-induced apoptosis (p <0.05) in FL, DLBCL, and MCL (Figure 5A). By contrast, andrographolide did not cause significant apoptosis in normal human lymphocytes compared with malignant cells at the same andrographolide concentrations (Figure 5A).
Figure 5. Effects of andrographolide on malignant cells from patients with FL, DLBCL and MCL.
Cells were pre-treated with caspase inhibitors for 1h, and then andrographolide was added at the indicated time period. These data are representative. Similar data were seen for all patient samples studied (3 FL, 1 MCL and 2 DLBCL) Annexin V-FITC and PI reagent were added and analyzed by FACS. Bar graphs represents apoptosis in (A) malignant cells from patients with FL, DLBCL, and MCL after the indicated time exposure to 1–20μM andrographolide with or without caspase inhibitors Z-VAD-FMK, Ac-IETD-CHO or Ac-LEHD-CHO, or anti-oxidant NAC, showing significant inhibition with Z-VAD-FMK or NAC (p ≤ 0.05). Andrographolide-induced apoptosis in patient samples was dose-dependent, and it was also significantly inhibited by Ac-IETD-CHO and Ac-LEHD-CHO in FL (p<0.05). However, at the same andrographolide concentrations, we saw no significant apoptosis in normal human lymphocytes compared with the control group (panel A right). (B) Andrographolide-induced cleavage of PARP, BID, and caspases 3, 8 and 9 was seen at concentrations as low as 5μM in malignant cells from a patient with FL, and all are inhibited by NAC. (C) In malignant cells from a patient with DLBCL, andrographolide-induced cleavage of PARP and caspase 8, 9 was dose-dependent, and completely inhibited by NAC. (D) Cleavage of PARP and BID was time-dependent in malignant cells from a patient MCL, with some baseline PARP cleavage increasing by 17h after exposure to low doses of andrographolide (5μM). (E) andrographolide-induced cleavage of PARP and caspase 9 was inhibited by NAC and Z-VAD-FMK in malignant cells from a patient with MCL.
Next, we pre-treated with the caspase inhibitors (50μM each) to determine if andrographolide-induced apoptosis was caspase-dependent. The inhibitors of caspase 8, caspase 9 and pan caspase inhibitor (Ac-IETD-CHO, Ac-LEHD-CHO and Z-VAD-FMK) significantly inhibited andrographolide-induced apoptosis (p <0.05) in primary FL cells. Z-VAD-FMK also significantly inhibited andrographolide-induced apoptosis (p <0.05) in MCL primary cells. The inhibition patterns were similar to the results we observed in the cell lines.
In Figure 5B, we show that in fresh FL cells NAC inhibited PARP cleavage starting at concentrations of andrographolide as low as 5μM, and that BID cleavage was seen at 10μM, and also inhibited by NAC. Similarly, caspase 3, 8 and 9 were cleaved at concentrations as low as 10μM, and this was inhibited by NAC. In Figure 5C, after 24h treatment of andrographolide in the presence or absence of NAC, we demonstrated dose-dependent cleavage of PARP, caspase 8 and caspase 9 in malignant cells from a patient with DLBCL. In Figure 5D and 5E, we demonstrate cleavage of PARP and caspase 9, starting at 16h of incubation with 5μM andrographolide in primary MCL cells. BID cleavage was also was time-dependent, and was first seen at 4–8h exposure. NAC and Z-VAD-FMK inhibited the activation of PARP and caspase 9 in these cells. Taken together, these data demonstrate that as in cell lines, andrographolide-induced apoptosis in primary lymphoma cells is dose, ROS and caspase dependent. It occurs at lower concentrations of andrographolide, and does not affect normal human lymphocytes.
Andrographolide-mediated apoptosis depends on mitochondrial pathways
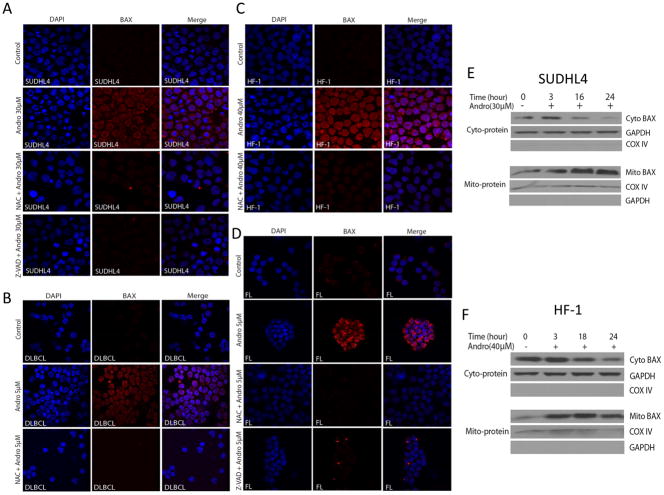
In order to investigate the involvement of mitochondrial pathways in lymphoma cell death, we determined the role of BAX/BAK in andrographolide-induced apoptosis. BAX is a “multi-domain” pro-apoptotic protein of the Bcl-2 family that is triggered by BID to undergo homo-oligomerization with BAK, resulting in release of cytochrome c from the mitochondria (18). To explore the role of Bcl-2 family proteins in andrographolide-induced apoptosis in lymphoma, we first performed whole cell protein Western immunoblots. We found that total BAX protein expression did not change after exposure to andrographolide (data not shown). However, we then investigated conformational change of BAX following mitochondrial translocation during apoptosis (10, 12, 19). Using the BAX (6A7) monoclonal antibody, which specifically binds the BAX protein with conformational change (10, 12), andrographolide induced BAX conformational change in the SUDHL4 (DLBCL cell line) and HF-1 (FL cell line) cells (Figure 6A and 6C respectively), and similarly in primary DLBCL and FL samples (Figure 6B and 6D respectively). These data demonstrate an increase of BAX fluorescent staining at 16–18h in andrographolide-treated cells, (Figure 6A, B, C and D). Furthermore, BAX conformational change was ROS and caspase-dependent, as treatment with NAC or pre-treatment with the pan caspase inhibitor Z-VAD-FMK eliminated the andrographolide-induced BAX conformational change in cell lines (Figure 6A and 6C) and primary patient samples (Figure 6B and 6D). These data indicate that andrographolide -induced apoptosis in both cell lines and primary lymphoma cells depends in part on Bcl-2 family proteins and is ROS- and caspase-dependent.
Figure 6. Andrographolide resulted in BAX conformational changes in cell lines (HF-1, SUDHL4) and patient samples (FL and DLBCL), and resulted in BAX mitochondrial translocation.
Andrographolide induced BAX conformational changes in (A) SUDHL4 cell line, (B) primary DLBCL cells (n=1 patient sample), (C) HF-1 cell line, and (D) primary FL cells (n=1 patient sample). All BAX conformational changes were blocked by NAC or Z-VAD-FMK. SUDHL4 (E) or HF-1 (F) cells were treated with andrographolide 30μM and 40μM respectively for the indicated period of time. Mitochondrial and cytosolic fractions were separated as described in Materials and Methods and analyzed by western blotting for pro-apoptotic BAX protein. COX IV and GAPDH were used as internal controls for mitochondrial and cytosolic extracts, respectively. Andrographolide-induced BAX translocation from cytoplasm to mitochondria was time-dependent. Abbreviations: Mito BAX, mitochondrial-related BAX; Cyto BAX, cytosolic-related BAX.
To further investigate mitochondrial events during andrographolide-induced apoptosis, we extracted cytosolic and mitochondrial fractions for immunoblotting. In the SUDHL4 and HF-1 cell lines, we found BAX accumulation in the mitochondrial fractions of both cell lines by 3h, while BAX in the cytoplasm was reduced (Figure 6E and 6F). Thus andrographolide induces BAX conformational change and mitochondrial translocation from the cytoplasm in lymphoma, leading to cellular apoptosis.
Andrographolide-mediated cell death is regulated by a BAX/BAK protein – dependent cell pathway
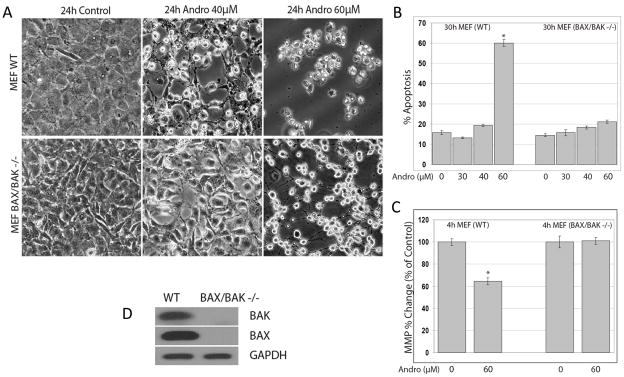
BAX and BAK and their complexes are known to play a central role in facilitating the release of mitochondrial intermembrane proteins during apoptosis (9). To further investigate the role of BAX and BAK in andrographolide-induced cell death, we examined apoptosis in wild type (WT) mouse embryonic fibroblasts (MEFs) (MEFwt) and in MEFs obtained from Bax/Bak double-knock-out (DKO) mice (MEFBax−/−/Bak−/−) after treatment with andrographolide. We found that andrographolide significantly increased cell death in MEFwt (p <0.05), but in MEFBax−/ −/Bak−/−, there was minimal cell death (Figure 7). Figure 7A demonstrates that progressive detachment and rounding up of cells after andrographolide exposure is dose-dependent in MEFwt, and not seen at 40μM and minimally at 60μM in the MEFBax− −/Bak−− (Figure 7A). In Figure 7B, apoptosis (Annexin V-FITC and PI) was performed after 30h andrographolide treatment. The bar graph shows that there was a significant difference in apoptosis between MEFwt and MEFBax−/−/Bak−/− at 60μM andrographolide. Moreover, after 4h, 60μM andrographolide caused significant loss of MMP in MEFwt (p <0.05), while in MEFBax−/−/Bak−/− this was not seen (Figure 7C). To further confirm that the MEFBax−/−/Bak−/− had no BAX and BAK proteins, we performed Western blots as shown in Figure 7D. These results suggest that BAX and/or BAK are necessary for andrographolide-mediated cell death, as their absence attenuated cell death in response to andrographolide.
Figure 7. Andrographolide-induced cell death was dependent on the BAX/BAK pathway.
Apoptosis in wild type (WT) mouse embryonic fibroblasts (MEFs) (MEFwt ) and in MEFs obtained from Bax/Bak double-knock-out (DKO) mice (MEFBax−/−/Bak−/−) after treatment with andrographolide was examined. Andrographolide significantly increased cell death in MEFwt (p ≤ 0.05), but in MEFBax−/−/Bak−/−, there was minimal cell death. (A) The progressive detachment and rounding up of cells after 24h andrographolide exposure was dose-dependent in MEFwt, and that was not seen in the MEF Bax−/−/Bak−/− under the microscope. (B) Apoptosis (Annexin V-FITC and PI) was performed after 30h andrographolide treatment. The bar graph shows that there was a significant difference (*= p<0.05) in apoptosis between MEFwt and MEF Bax−/−/Bak−/− at 60μM andrographolide, but no change from baseline in the MEF Bax−/−/Bak−/− cells compared with the MEFwt. (C) Similarly, there was a significant decrease in MMP after 4h andrographolide exposure in the MEFwt (*= p ≤ 0.05) but not in MEF Bax−/−/Bak−/− cells. (D) Western blots confirmed that the MEF Bax−/−/Bak−/− had no BAX and BAK proteins.
Discussion
We have shown that andrographolide, the active component derived from the plant Andrographis paniculata, causes redox-dependent apoptosis in several non-Hodgkin’s lymphoma cell lines and in primary lymphoma cells. It appears that the mechanism is redox dependent, mediated through caspase activation, depends on BAX conformational change, and is accompanied by translocation of BAX from the cytoplasm to the mitochondria.
The fundamental molecular mechanisms of the biological effects of lactone diterpenoids have been explored. Andrographolide targets nuclear transcription factor kappa B (NF-κB) for its anti-inflammatory activity (20, 21). The pharmacokinetics of andrographolide in human plasma also have been evaluated by oral administration of 200 mg andrographolide (22) and adducts of andrographolide in human urine have been documented after oral administration (23). The extract (> or =10% andrographolide) of Andrographis paniculata did not affect the reproductive and fertility ability in male Wistar rats at more than 1000 mg/kg per day (24). Recently, andrographolide has also been shown to inhibit cancer cell proliferation and induce apoptosis in cancer cell lines, including leukemia (HL-60), prostatic adenocarcinoma (PC-3), breast cancer (MDA-MB-231 and MCF 7), liver cancer (HepG2 and Hep3B), cervical cancer (HeLa) and colorectal cancer (HCT116 and HT-29) (9–11, 25–31), but not previously in lymphoma cell lines.
We hypothesized that andrographolide might inhibit cell proliferation and cause apoptosis in lymphoma cell lines and in primary lymphoma cells by mechanisms that involved cellular redox systems, caspase activation, and mitochondrial pathways. Indeed, we found that the effects of andrographolide were dose and time related, and were accompanied by ROS generation (Figure 2). That ROS played an important role in apoptosis of lymphoma cell lines Ramos, Granta, HF-1 and SUDHL4 was demonstrated by near complete abrogation of apoptosis by the antioxidant NAC and by enhancement of apoptosis by the GSH depleting agent BSO. These observations were not restricted to cell lines, as we also found that andrographolide, at lower concentrations, resulted in ROS and caspase-dependent inhibition in primary patient samples from patients with FL, MCL, and DLBCL.
We further postulated that andrographolide induced apoptosis in patient samples and cell lines through intrinsic caspase pathways, and that BCL-2 family proteins and mitochondrial regulation would be required. We found BID cleavage in patient samples (Figure 5B and 5D). We also found that andrographolide induced Δψm change in Ramos, HF-1 and SUDHL4 (Figure 2C). Further, we demonstrated that andrographolide induced conformational change of BAX in the SUDHL4 and the HF-1 cell lines and in malignant cells from patients with DLBCL and FL. This was inhibited by NAC, indicating that BAX conformational change depended on oxidant mechanisms (Figure 6). We also found that the andrographolide induces BAX mitochondrial translocation in HF-1 and SUDHL4 lymphoma cell lines (Figure 6), results similar to those in HepG2 cells as described byZhou et al (10). Additionally, BAX and BAK and their complexes are known to play a central role in facilitating the release of mitochondrial intermembrane proteins during apoptosis (9). To further characterize the mechanism, we used Bax/Bak MEF DKOs (MEFBax−/−/Bak−/−) (Figure 7), and found that MEFwt had reduced MMP and were killed by similar concentrations of andrographolide, but MEFBax−/−/Bak−/− were not, suggesting that apoptosis proceeds though the BAX/BAK pathway. We also found (Figure 6) that caspase inhibition blocked BAX conformational change, suggesting that caspases are required for this step in andrographolide-induced apoptosis.
The concentrations that achieved 50% growth inhibition (IC50) and 50% apoptosis (AC50) (Figure 1 and Figure 3), are similar or even lower than concentrations in other cancer cell lines (HL-60 cell line (29), a human carcinoma cell line SMMC-7721(12), and a human hepatoma cell line HepG2(10), and are clinically relevant. There are data that suggest that serum concentrations of between 1.9 and 3.8 μM can be achieved with doses of andrographolide commonly used in China (32), and that 20 fold higher doses can be given safely (7). This is well within the range that resulted in biological effects and apoptosis in patient samples in our studies. Further, based on our data (Figure 5A) andrographolide did not cause significant apoptosis in normal lymphocytes compared with patient samples or cell lines. Further, there was no hematologic toxicity seen in a Phase I trial in HIV and non-HIV patients (7).
The mechanism of ROS-dependent cell death related to andrographolide is not clear. Woo et al have reported that andrographolide up-regulates cellular-reduced glutathione in neonatal rat cardiomyocytes (NRCM) (33). However, andrographolide has been found to react with reduced thiols (33), so that initially there is a reduction in GSH, which is then followed by activation of glutamine cysteine ligase (GCL) and its modifier subunit (GCLM) as an endogenous antioxidant cellular defensive response to GSH reduction. However, Ji et al have reported that andrographolide initially increased intracellular GSH levels which then decreased later, while inhibition of cellular GSH synthesis by BSO augmented andrographolide-induced cytotoxicity and apoptosis in Hep3B cells (34). Our observation that NAC reverses andrographolide-induced apoptosis and ROS generation may be explained by the ability of NAC to restore GSH levels depleted by andrographolide. It is possible that the inherent cellular defense mechanism in NRCM to increase GSH after insult is not present in lymphoma cells or lymphoma cell lines, or that andrographolide may have a mechanism in lymphoma cell lines and patient samples similar to the liver cancer cell line (Hep3B). We have previously found that GSH levels in lymphoma cell lines are >5 fold higher in cell lines than in primary tumors (data not shown). This may explain our observations that the AC50 of andrographolide is much lower in patient samples than in cell lines.
We found that andrographolide induced BAX conformational change in lymphoma cell lines and in fresh patient samples from patients with FL and DLBCL (Figure 6). BAX conformational change is known to follow caspase 8 activation and is accompanied by pore formation in the outer mitochondrial membrane and precedes the release of cytochrome c from mitochondria, an important early step in mitochondrial mediated apoptosis (18, 19, 35). Zhou et al have shown that andrographolide induced BID cleavage and BAX conformational change in HepG2 cells without up-regulation of total BAX protein level (10). Similarly, we found no increase in total BAX (data not shown), but found NAC-inhibitable conformational change in lymphoma cell lines and fresh patient samples. These data suggest the potential clinical relevance to lymphoma biology and suggest that lactone diterpenoids may have anti-tumor activity in patients with lymphoma. Recently, radiation sensitizing effects of andrographolide have been published in an in vitro and in vivo model of Ras-transformed cells (36).
It is interesting that we see both caspase 8 and 9 activation with andrographolide, suggesting that both intrinsic and extrinsic caspase pathways are involved in andrographolide-induced apoptosis in lymphoma. Zhou et al (37) have found that andrographolide may enhance TRAIL-induced apoptosis through death receptor 4 up-regulation, and that this is mediated through p53. Our data suggesting that the pan-caspase inhibitor was required to block andrographolide-induced apoptosis are consistent with a process that may involve both caspase pathways and/or other pathways. Currently, studies of death receptor pathways in andrographolide-induced apoptosis of lymphoma cells are ongoing.
It is also intriguing that our data with andrographolide are extant for cell lines from B-cell lymphomas with very different biological and molecular signatures and indeed, hold true for samples from patients with the corresponding clinical-pathological subtypes of lymphoma. While there are marked differences in the clinical behavior of these three types of lymphoma that would argue against categorizing them as a single entity, it is possible that the activity of this natural diterpenoid lactone operates through biological pathways that are independent of the heretofore established biology of these lymphomas, and thereby will open new pathways to a common approach to treatment.
We have demonstrated that andrographolide, the active component of the plant Andrographis paniculata, causes cell death in lymphoma cell lines and in fresh malignant cells from lymphoma patients. The mechanism is related to the redox state of the cells, as it is blocked completely by NAC, and it is caspase-dependent. Furthermore, apoptosis is mediated through mitochondrial pathways in both cell lines and in primary patient samples. This novel, natural lactone diterpenoid deserves further pre-clinical and clinical investigation in lymphoma.
Supplementary Material
Supplemental Figure 1: Chemical structure for andrographolide (Andro).
Statement of Translational Relevance.
The anti-cancer properties of the diterpenoid lactone Andrographolide have recently been recognized, and the biological effects of this naturally occurring product derived from Andrographis paniculata are likely related to reactive oxygen species signaling. Based on these observations, we hypothesized that andrographolide would be cytotoxic to lymphoma cells. In these studies we examined lymphoma cell lines including Ramos, Granta and HF-1 as well as primary lymphoma cells derived from patients. We show that andrographolide results in apoptosis in lymphoma cell lines and in primary lymphoma cells, that this is mediated through reactive oxygen species(ROS)- mediated caspase activation, and that these effects proceed through BAX/BAK mitochondrial pathways. These studies will provide the pre-clinical rationale to bring this novel natural compound to clinical trials for the treatment of lymphoma.
Acknowledgments
Supported in part from grants from the National Cancer Institute (AME, K23 CA109613-A1).
References
- 1.Basak A, Cooper S, Roberge AG, Banik UK, Chretien M, Seidah NG. Inhibition of proprotein convertases-1, -7 and furin by diterpines of Andrographis paniculata and their succinoyl esters. Biochem J. 1999;338 ( Pt 1):107–13. [PMC free article] [PubMed] [Google Scholar]
- 2.Shariff A, Pk M, Klk P, MM Entrapment of andrographolide in cross-linked alginate pellets: II. Physicochemical characterization to study the pelletization of andrographolide. Pak J Pharm Sci. 2007;20:9–15. [PubMed] [Google Scholar]
- 3.Arnoult D. Apoptosis-associated mitochondrial outer membrane permeabilization assays. Methods. 2008;44:229–34. doi: 10.1016/j.ymeth.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Iruretagoyena MI, Tobar JA, Gonzalez PA, et al. Andrographolide interferes with T cell activation and reduces experimental autoimmune encephalomyelitis in the mouse. J Pharmacol Exp Ther. 2005;312:366–72. doi: 10.1124/jpet.104.072512. [DOI] [PubMed] [Google Scholar]
- 5.Poolsup N, Suthisisang C, Prathanturarug S, Asawamekin A, Chanchareon U. Andrographis paniculata in the symptomatic treatment of uncomplicated upper respiratory tract infection: systematic review of randomized controlled trials. J Clin Pharm Ther. 2004;29:37–45. doi: 10.1046/j.1365-2710.2003.00534.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang J, Zhang RM, Zhang Y, et al. Andrographolide drop-pill in treatment of acute upper respiratory tract infection with external wind-heat syndrome: a multicenter and randomized controlled trial. Zhong Xi Yi Jie He Xue Bao. 2008;6:1238–45. doi: 10.3736/jcim20081206. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese C, Berman SH, Babish JG, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–8. doi: 10.1002/1099-1573(200008)14:5<333::aid-ptr584>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 8.Nanduri S, Nyavanandi VK, Thunuguntla SS, et al. Synthesis and structure-activity relationships of andrographolide analogues as novel cytotoxic agents. Bioorg Med Chem Lett. 2004;14:4711–7. doi: 10.1016/j.bmcl.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 9.Zhou L, Chang DC. Dynamics and structure of the Bax-Bak complex responsible for releasing mitochondrial proteins during apoptosis. J Cell Sci. 2008;121:2186–96. doi: 10.1242/jcs.024703. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Zhang S, Ong CN, Shen HM. Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem Pharmacol. 2006;72:132–44. doi: 10.1016/j.bcp.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Manikam ST, Stanslas J. Andrographolide inhibits growth of acute promyelocytic leukaemia cells by inducing retinoic acid receptor-independent cell differentiation and apoptosis. J Pharm Pharmacol. 2009;61:69–78. doi: 10.1211/jpp/61.01.0010. [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Wu D, Luo K, Wu S, Wu P. Andrographolide enhances 5-fluorouracil-induced apoptosis via caspase-8-dependent mitochondrial pathway involving p53 participation in hepatocellular carcinoma (SMMC-7721) cells. Cancer Lett. 2009;276:180–8. doi: 10.1016/j.canlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Lewis-Wambi JS, Kim HR, Wambi C, et al. Buthionine sulfoximine sensitizes antihormone-resistant human breast cancer cells to estrogen-induced apoptosis. Breast Cancer Res. 2008;10:R104. doi: 10.1186/bcr2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornber K, Colomba A, Ceccato L, Delsol G, Payrastre B, Gaits-Iacovoni F. Reactive oxygen species and lipoxygenases regulate the oncogenicity of NPM-ALK-positive anaplastic large cell lymphomas. Oncogene. 2009;28:2690–6. doi: 10.1038/onc.2009.125. [DOI] [PubMed] [Google Scholar]
- 15.Evens AM, Lecane P, Magda D, et al. Motexafin gadolinium generates reactive oxygen species and induces apoptosis in sensitive and highly resistant multiple myeloma cells. Blood. 2005;105:1265–73. doi: 10.1182/blood-2004-03-0964. [DOI] [PubMed] [Google Scholar]
- 16.Chandra J, Tracy J, Loegering D, et al. Adaphostin-induced oxidative stress overcomes BCR/ABL mutation-dependent and -independent imatinib resistance. Blood. 2006;107:2501–6. doi: 10.1182/blood-2005-07-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 18.Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuwana T, Bouchier-Hayes L, Chipuk JE, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Xia YF, Ye BQ, Li YD, et al. Andrographolide attenuates inflammation by inhibition of NF-kappa B activation through covalent modification of reduced cysteine 62 of p50. J Immunol. 2004;173:4207–17. doi: 10.4049/jimmunol.173.6.4207. [DOI] [PubMed] [Google Scholar]
- 21.Hidalgo MA, Romero A, Figueroa J, et al. Andrographolide interferes with binding of nuclear factor-kappaB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144:680–6. doi: 10.1038/sj.bjp.0706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu L, Xiao DW, Lou S, et al. A simple and sensitive HPLC-ESI-MS/MS method for the determination of andrographolide in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:502–6. doi: 10.1016/j.jchromb.2008.12.065. [DOI] [PubMed] [Google Scholar]
- 23.Cui L, Chan W, Qiu F, Cai Z, Yao X. Identification of four urea adducts of andrographolide in humans. Drug Metab Lett. 2008;2:261–8. doi: 10.2174/187231208786734148. [DOI] [PubMed] [Google Scholar]
- 24.Allan JJ, Pore MP, Deepak M, Murali B, Mayachari AS, Agarwal A. Reproductive and fertility effects of an extract of Andrographis paniculata in male Wistar rats. Int J Toxicol. 2009;28:308–17. doi: 10.1177/1091581809339631. [DOI] [PubMed] [Google Scholar]
- 25.Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol. 2004;92:291–5. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Kim TG, Hwi KK, Hung CS. Morphological and biochemical changes of andrographolide-induced cell death in human prostatic adenocarcinoma PC-3 cells. In Vivo. 2005;19:551–7. [PubMed] [Google Scholar]
- 27.Zhao F, He EQ, Wang L, Liu K. Anti-tumor activities of andrographolide, a diterpene from Andrographis paniculata, by inducing apoptosis and inhibiting VEGF level. J Asian Nat Prod Res. 2008;10:467–73. doi: 10.1080/10286020801948334. [DOI] [PubMed] [Google Scholar]
- 28.Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol. 2003;3:147–58. doi: 10.1046/j.1359-4117.2003.01090.x. [DOI] [PubMed] [Google Scholar]
- 29.Cheung HY, Cheung SH, Li J, et al. Andrographolide isolated from Andrographis paniculata induces cell cycle arrest and mitochondrial-mediated apoptosis in human leukemic HL-60 cells. Planta Med. 2005;71:1106–11. doi: 10.1055/s-2005-873128. [DOI] [PubMed] [Google Scholar]
- 30.Satyanarayana C, Deevi DS, Rajagopalan R, Srinivas N, Rajagopal S. DRF 3188 a novel semi-synthetic analog of andrographolide: cellular response to MCF 7 breast cancer cells. BMC Cancer. 2004;4:26. doi: 10.1186/1471-2407-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen L, Zhu H, Wang R, Zhou K, Jing Y, Qiu F. ent-Labdane diterpenoid lactone stereoisomers from Andrographis paniculata. J Nat Prod. 2008;71:852–5. doi: 10.1021/np0704452. [DOI] [PubMed] [Google Scholar]
- 32.Panossian A, Hovhannisyan A, Mamikonyan G, et al. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine. 2000;7:351–64. doi: 10.1016/S0944-7113(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 33.Woo AY, Waye MM, Tsui SK, Yeung ST, Cheng CH. Andrographolide up-regulates cellular-reduced glutathione level and protects cardiomyocytes against hypoxia/reoxygenation injury. J Pharmacol Exp Ther. 2008;325:226–35. doi: 10.1124/jpet.107.133918. [DOI] [PubMed] [Google Scholar]
- 34.Ji L, Shen K, Liu J, Chen Y, Liu T, Wang Z. Intracellular glutathione regulates Andrographolide-induced cytotoxicity on hepatoma Hep3B cells. Redox Rep. 2009;14:176–84. doi: 10.1179/135100009X466122. [DOI] [PubMed] [Google Scholar]
- 35.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–56. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 36.Hung SK, Hung LC, Kuo CD, et al. Andrographolide sensitizes Ras-transformed cells to radiation in vitro and in vivo. Int J Radiat Oncol Biol Phys. 2010;77:1232–9. doi: 10.1016/j.ijrobp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 37.Zhou J, Lu GD, Ong CS, Ong CN, Shen HM. Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53-mediated death receptor 4 up-regulation. Mol Cancer Ther. 2008;7:2170–80. doi: 10.1158/1535-7163.MCT-08-0071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Chemical structure for andrographolide (Andro).