Abstract
Objectives
Secondary caries and restorative fracture are the two main reasons for restoration failures. Fluoride ion (F) release can help inhibit caries. Plaque pH after a sucrose rinse can decrease to a cariogenic pH of 4 – 4.5. The objective of this study was to investigate the effects of solution pH and immersion time on the mechanical properties and F release of restorative materials.
Methods
Three resin-modified glass ionomers (Viremer, Fuji II LC, Ketac Nano), one compomer (Dyract Flow), and one composite (Heliomolar), were tested. Flexural strength and elastic modulus were measured before and after 84 days of immersion in solutions of pH 4, 5.5, and 7. F release was measured as a function of pH and immersion time.
Results
Immersion and material type had significant effects on mechanical properties. Vitremer had a flexural strength (mean ± sd; n = 6) of (99 ± 25) MPa before immersion; it decreased to (42 ± 13) MPa after 84 d of immersion (p < 0.05). In comparison, Heliomolar had a smaller strength loss, decreasing from (99 ± 9) MPa to (65 ± 7) MPa (p < 0.05). Solution pH had little effect on mechanical properties. For example, Fuji II LC had a strength of (63 ± 15) MPa at pH 4, similar to (61 ± 30) MPa at pH 5.5, and (56 ± 22) MPa at pH 7 (p > 0.1). In contrast, solution pH had a significant effect on F release. F release at 84 d for Fuji was (609 ± 25) μg/cm2 at pH 4, much higher than (258 ± 36) μg/cm2 at pH 5.5, and (188 ± 9) μg/cm2 at pH 7.
Significance
The restoratives tested were able to greatly increase the F release at acidic, cariogenic pH, when these ions are most needed to inhibit caries. However, mechanical properties of these F-releasing restoratives degraded significantly in immersion. Efforts are needed to develop F-releasing restoratives with high levels of sustained F release, as well as improved durability of mechanical properties for large stress-bearing restorations.
Keywords: fluoride releasing restoratives, pH effects, immersion, mechanical properties, durability, caries inhibition
1. Introduction
Resin composites are increasingly used in tooth cavity restorations due to their direct-filling capability, esthetics, and improved performance [1–6]. Filler and matrix compositions have been optimized, mechanical properties have been enhanced, and polymerization shrinkage has been reduced [7–9]. However, secondary caries can develop at the tooth-restoration interfaces [10–13]. These caries, along with restoration fracture, are the most frequent causes for the failure of restorations [11,12,14–18]. Therefore, fluoride ion (F) releasing restoratives as well as calcium and phosphate ion releasing materials have been developed as they may reduce secondary caries at the restoration margins [19–25]. There are several mechanisms involved in the anticariogenic effects of fluoride, including the reduction of demineralization, the enhancement of remineralization, the interference with pellicle and plaque formation, and the inhibition of microbial growth and metabolism [25].
Commercial F-releasing restoratives fall into four categories: glass ionomer cements, resin-modified glass ionomer cements, polyacid-modified composites (compomers), and fluoride-releasing composites. The mechanical properties and fluoride release abilities vary between different materials. Compomers, glass ionomers, and resin-modified glass ionomers are generally weaker than composite resins [26]. Therefore, the clinical applications of F-releasing materials are usually limited to relatively small-sized restorations in moderate load-bearing areas [27].
The anticariogenic effect of F-releasing materials depends on the amount and sustainability of F release [28]. The F release from a restorative material is determined by the matrix of the restorative material, the mechanism by which it sets, and the amount of F-containing fillers [25]. The matrix of resin composites is much less hydrophilic, and F incorporated in the resin composite is only released in small amounts [29]. The pattern of F release is typically characterized by an initial rapid release, followed by a significant reduction in the rate of release after only a few days of immersion [30].
The mechanical properties and the F release rate of a material may also depend on the pH of the solution to which the material is exposed. The oral plaque pH after a sucrose rinse can decrease to 4 – 4.5 [31]. A plaque pH of above 6 is considered to be safe, a pH of 6 – 5.5 is potentially cariogenic, and a pH of 5.5 – 4 is cariogenic. However, the effects of solution pH and immersion time on the mechanical properties and F release of currently-available F-releasing restorative materials are yet to be investigated.
Accordingly, the objective of this study was to investigate the effects of solution pH and immersion on the mechanical properties and F release of restoratives. Five F-releasing materials were studied, including three resin-modified glass ionomers, one compomer, and one F-releasing composite. Three hypotheses were tested: (1) Solution pH will have a significant effect on F release, with higher release at acidic and cariogenic pH when the F ions are most needed; (2) Solution pH will have little effect on the mechanical properties of the immersed F-releasing specimens; (3) Materials with higher F release will in general have lower mechanical properties.
2. Materials and Methods
2.1. Fluoride Releasing Materials
Five materials were used (Table 1) to investigate the effect of pH on the mechanical properties and F release: Three resin-modified glass ionomers (Vitremer, Fuji II LC, and Ketac Nano), one flowable compomer (Dyract Flow), and one composite (Heliomolar). According to the manufacturers, Vitremer is indicated for Class III and Class V restorations, root caries lesion restorations, Class I and Class II restorations in primary teeth, and core buildup. Heliomolar is indicated for Class I and Class II restorations in the posterior region, Class III and Class IV anterior restorations, Class V restorations, and pit and fissure sealing in molar and premolar teeth. Dyract Flow is recommended for restoration of minimally invasive cavity preparations, pits and fissures, restoration of shallow Class V preparations, and as a base/liner under Class I and Class II restorations. Fuji II LC is indicated for Class III and Class V restorations, restoration of primary teeth, and core buildup. Ketac Nano is recommended for primary teeth restorations, small Class I restorations, and Class III and Class V restorations.
Table I.
Compositions of fluoride releasing restorative materials used in this study
| Material | Formulation | Filler Level | Manufacturer |
|---|---|---|---|
| Vitremer; shade B2; glass ionomer core buildup/restorative system; powder: lot 20081028, liquid: lot 20081111 | Powder: radiopaque, fluoroaluminosilicate glass with microencapsulated potassium persulfate and ascorbic acid; Liquid: aqueous solution of a polyalkenoic acid modified with methacrylate groups | 71.4 wt% | 3M ESPE (St. Paul, MN) |
| GC Fuji II LC (Improved); shade A2; radiopaque light curing reinforced glass ionomer restorative; lot 0810081 | Polyacrylic acid, 2- hydroxyethylmethacrylate, urethanedimethacrylate, camphorquinone | 76.2 wt% | GC Corporation (Alsip, IL) |
| Ketac Nano; shade A2; light curing nano- ionomer restorative; lot 20081023 | Polycarboxilic acid modified with methacrylate groups, fluoroaluminosilicate glass | 69 wt% | 3M ESPE (St. Paul, MN) |
| Dyract Flow; shade A2; compomer restorative material with flow characteristics; lot 090310 | Strontium-alumino-fluoro-silicate glass, highly dispersed silicon dioxide, ammonium salt of PENTA and N,N-dimethyl aminoethyl methacrylate, carboxylic acid modified macromonomers, diethylene glycol dimethacrylate, camphorquinone, ethyl-4- dimethylaminobenzoate, 2- hydroxymethylbenzophenone, butylated hydroxyl toluene, colorants, and titanium dioxide | 65 wt% | Dentsply DeTrey GmbH (Konstanz, Germany) |
| Heliomolar; shade A2; light curing resin-based microfilled composite; lot L43034 | Resin matrix: dimethacrylates; Fillers: highly dispersed silicon dioxide, ytterbium tri-fluoride and copolymers (particles sized 40–200 nm); Additional contents: catalysts, stabilizers, and pigments | 76.5 wt% | Ivoclar (Ontario, Canada) |
Vitremer and Fuji II LC are two part, powder/liquid systems. Specimens of both were fabricated using the manufacturer’s suggested powder/liquid ratio, which was 2.5/1.0 for Vitremer and 3.2/1.0 for Fuji II LC. Dyract Flow and Heliomolar are one component systems. Ketac Nano is a two part, paste/paste system and was dispensed using the Clicker Dispensing System. All materials were placed into 2x2x25 mm3 molds, photo-cured (Triad 2000, Dentsply, York, PA) for 1 min/side, and incubated for 24 hours at 37ºC before immersion.
2.2. Mechanical Testing
Specimens were tested in a three-point flexure using a crosshead speed of 1 mm/min with a 20 mm span on a Universal Testing Machine (5500R, MTS, Cary, NC). Flexural strength was calculated: S = 3PmaxL/(2bh2), where Pmax is the maximum load, L is the span, b is the specimen width, and h is the thickness [32]. Elastic modulus was calculated: E = (P/d)(L3/[4bh3]), where load P divided by displacement d is the slope of the load-displacement curve [33].
2.3. Fluoride Release
Following a previous study [34], a sodium chloride (NaCl) solution (133 mmol/L) was buffered to three different pHs: pH 4 with 50 mmol/L lactic acid, pH 5.5 with 50 mmol/L acetic acid, and pH 7 with 50 mmol/L HEPES. As in previous studies [35,36], three specimens of 2x2x12 mm3 were immersed in 50 mL of solution, yielding a specimen volume/solution of 2.9 mm3/mL, similar to the 3.0 mm3/mL in a previous study [37]. F concentration was measured at immersion times of 1 day (d), 2 d, 3 d, 7 d, 14 d, 21 d, 28 d, 35 d, 42 d, 49 d, 56 d, 63 d, 70 d, 77 d, and 84 d. At each time point, aliquots of 1 mL were removed and replaced by the same volume of fresh solution. F concentration was measured with a F ion selective electrode (Orion, Cambridge, MA) as described previously [38].
One-way and two-way ANOVA were performed to detect significant effects of the variables. Tukey’s multiple comparison test was used at p of 0.05.
3. Results
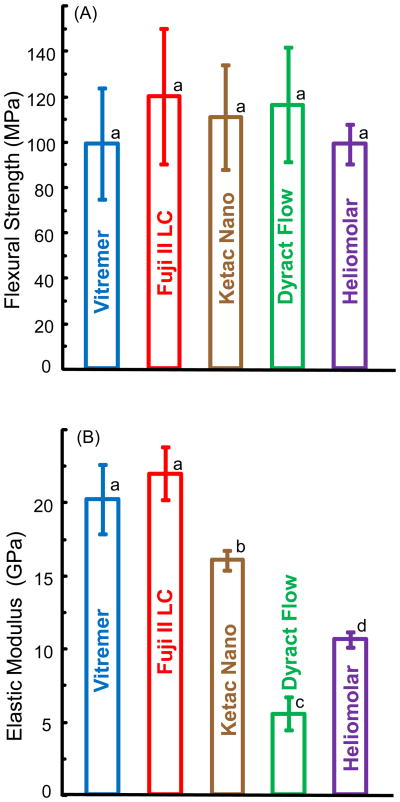
Fig. 1A and B plot the flexural strength and elastic modulus for the restorative materials before immersion (mean ± sd; n = 6). All five materials had strengths that were not significantly different (p > 0.1). They ranged from (99 ± 8.6) MPa for Heliomolar, (99 ± 25) MPa for Vitremer, to (120 ± 30) MPa for Fuji II LC. Vitremer and Fuji II LC had similar elastic moduli (p > 0.1). They were significantly higher than the moduli of the other materials (p < 0.05). Dyract Flow had the lowest elastic modulus as it is a flowable material with a low stiffness.
Figure 1.
(A) Flexural strength, and (B) elastic modulus of fluoride releasing materials before immersion. Each value is the mean of six measurements, with the error bar showing one standard deviation (mean ± sd; n = 6). In (A), bars with the same letter indicate values that are not significantly different (p > 0.1). In (B), bars with dissimilar letters indicate values that are significantly different from each other (p < 0.05).
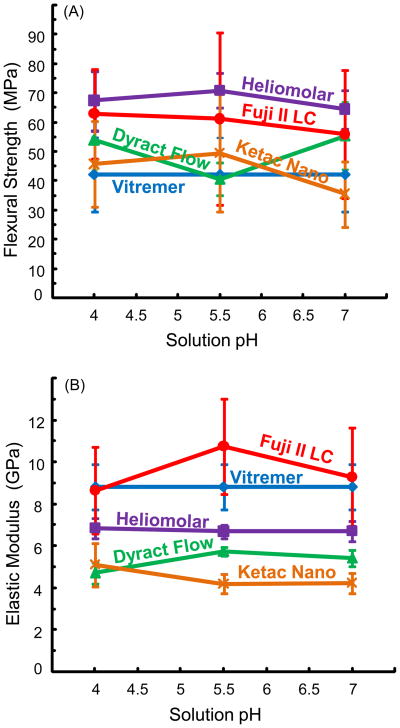
Fig. 2A and B plot the flexural strength and elastic modulus after 84 d of immersion in solutions of pH 4, pH 5.5, and pH 7 (mean ± sd; n = 6). In general, Heliomolar had the highest strength after immersion, followed by Fuji II LC and Dyract, while the strengths of Ketac and Vitremer were lower and similar to each other. For example, at pH 4, the strength was (67 ± 10) MPa for Heliomolar, (63 ± 15) MPa for Fuji, (54 ± 10) MPa for Dyract Flow, (46 ± 15) MPa for Ketac Nano, and (42 ± 13) MPa for Vitremer. The strength of Heliomolar was significantly higher than those of Vitremer and Ketac Nano (p < 0.05). None of the strengths was significantly different from those of Fuji and Dyract Flow (p > 0.1).
Figure 2.
(A) Flexural strength, and (B) elastic modulus of specimens immersed for 84 d in solutions of pH 4, pH 5.5, and pH 7. Each value is mean ± sd; n = 6.
Compared to the strengths before immersion (Fig. 1A), immersion for 84 d significantly decreased the strength for all five materials (p < 0.05). Ketac Nano had the largest strength loss of (61 ± 6.5)%, whereas Heliomolar had the smallest strength loss of (32 ± 3.2)%. However, as long as they were immersed, the solution pH had no significant effect on the strength (p > 0.1). For example, Heliomolar had a strength of (67 ± 10) MPa at pH 4, not significantly different from (71 ± 5.9) MPa at pH 5.5, and (65 ± 6.6) MPa at pH 7 (p > 0.1).
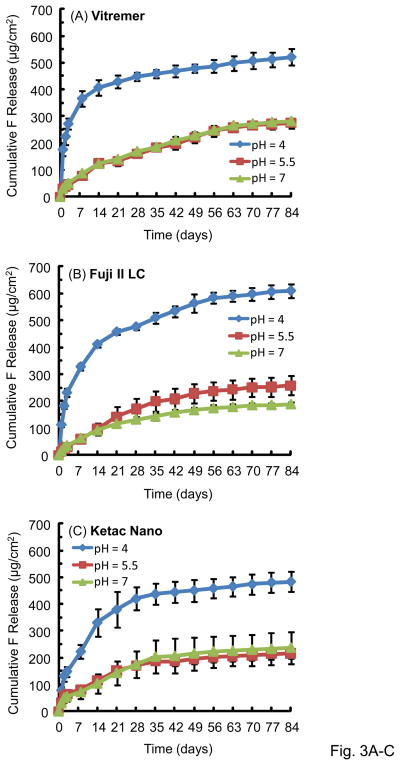
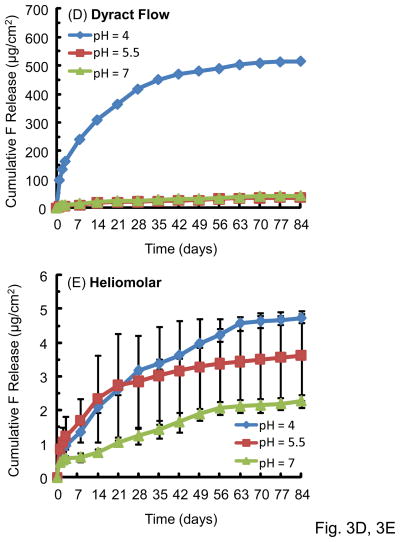
Fig. 3 plots the cumulative F release per specimen area (μg/cm2) (mean ± sd; n = 3). The F release increased rapidly in the first several weeks, then it continued to increase, but the rate of increase was slower. The F release was much higher at pH 4 than that at pH 5.5 or pH 7. For example, for Fuji at 84 d, the F release was (609 ± 25) μg/cm2 at pH 4, much higher than (258 ± 36) μg/cm2 at pH 5.5, and (188 ± 9) μg/cm2 at pH 7 (p < 0.05).
Figure 3.
Cumulative fluoride ion (F) release per specimen area (μg/cm2): (A) Vitremer, (B) Fuji II LC, (C) Ketac Nano, (D) Dyract Flow, and (E) Heliomolar. Each value is mean ± sd; n = 3. The F release was higher in pH 4 solution than in the pH 5.5 or pH 7 solution.
Comparing between different materials, the cumulative F release at 84 d and pH 4 was (609 ± 25) μg/cm2 for Fuji II LC, significantly higher than (521 ± 30) for Vitremer, (516 ± 6) for Dyract Flow, (484 ± 38) for Ketac Nano and (4.7 ± 0.1) for Heliomolar (p < 0.05).
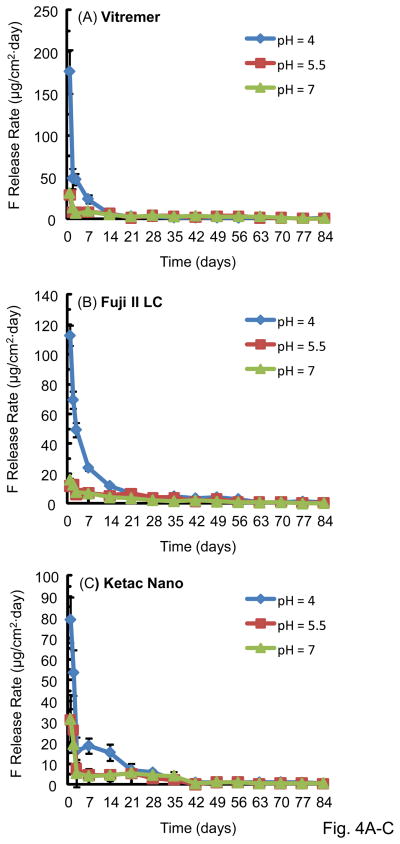
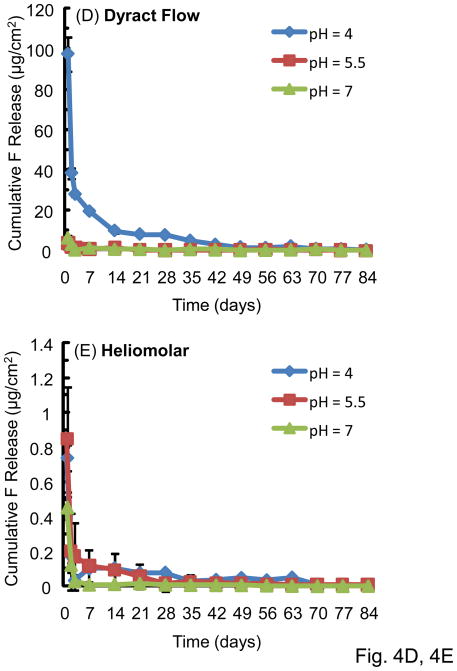
The F release rate (per specimen surface area per day, μg/[cm2·day]) is plotted in Fig. 4 (mean ± sd; n = 3). There was a high initial F release, followed by a much lower long-term release rate. This trend was the same for all the materials tested. Vitremer had the highest initial release rate at 1 d of (176 ± 25) (μg/[cm2·day]), significantly higher than (113 ± 7) for Fuji II LC, (97 ± 8) for Dyract Flow, (79 ± 11) for Ketac Nano, and (0.70 ± 0.08) for Heliomolar (p < 0.05). For all materials, the F release rate was higher at pH 4 than pH 5.5 and pH 7 for the first 2–3 weeks. After this time, there was no significant difference between the release rates at the three pHs. The F release rate then approached a plateau, and there was no significant difference in the release rate between 70 d – 84 d at the three different pHs (p > 0.1).
Figure 4.
F release rate, which is the F release per specimen surface area per day: (A) Vitremer, (B) Fuji II LC, (C) Ketac Nano, (D) Dyract Flow, and (E) Heliomolar. Each value is mean ± sd; n = 3.
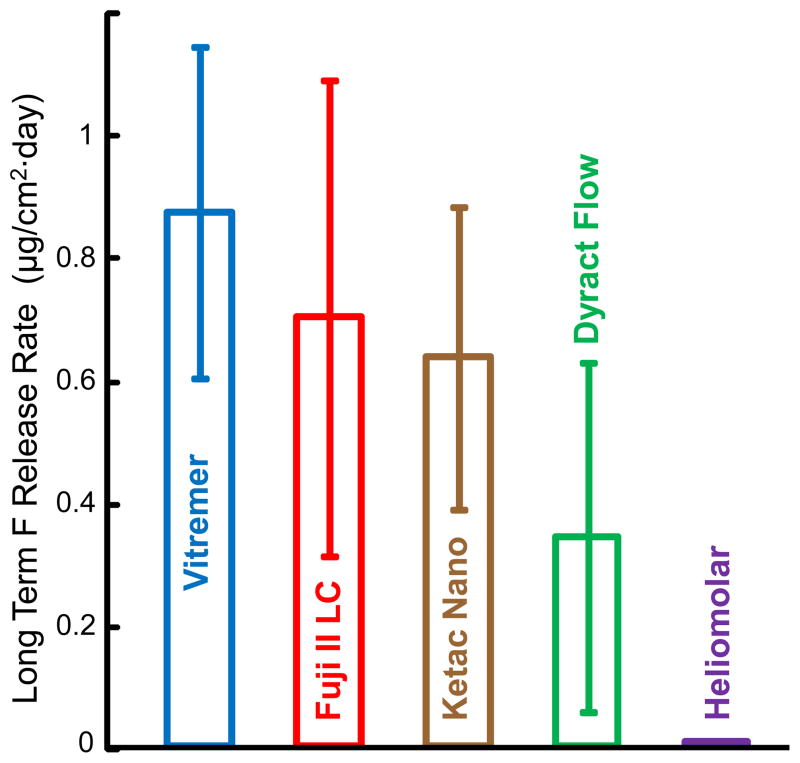
Since the F release rates at 70 d, 77 d, and 84 d and pH 4, 5.5, and 7 were similar for each material, these nine rates for each material were averaged to yield the long-term F release rate, which is plotted in Fig. 5. The long-term F release rate was (0.87 ± 0.27) (μg/[cm2·day]) for Vitremer, followed by (0.70 ± 0.39) for Fuji II LC, (0.63 ± 0.24) for Ketac Nano, and (0.34 ± 0.28) for Dyract Flow. Heliomolar had a much lower long-term release rate of (0.006 ± 0.004) (μg/[cm2·day]) (p < 0.05).
Figure 5.
Long term F release rate, which is the average of the nine release rates at days 70, 77, and 84 at pH 4, pH 5.5, and pH 7 for each material.
4. Discussion
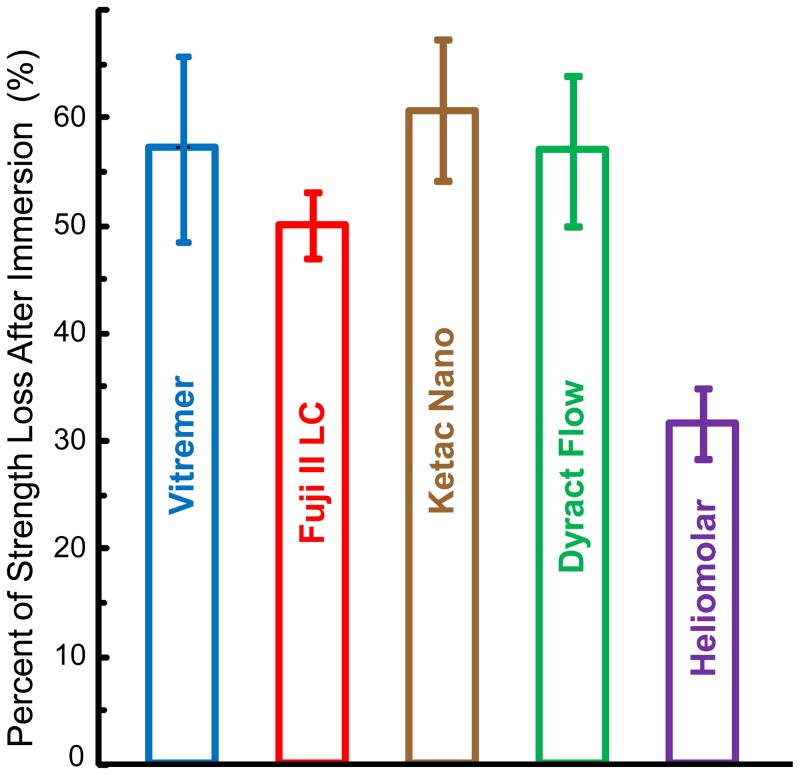
This study determined the effect of solution pH and immersion time on the mechanical properties and F release rates of commercial restorative materials. Immersion significantly decreased the mechanical properties for all materials. However, the pH of the solution from pH 4 - 7 had little effect on the mechanical properties of the restorative materials after 84 d of immersion. Prior to immersion (Fig. 1A), all five materials had statistically similar flexural strengths of 99–120 MPa. In order to gauge the extent of strength loss after 84 d of immersion, the percentage of strength loss was calculated as: Percentage of Strength Loss = [(Strength before immersion)–(Strength after immersion)] / (Strength before immersion). The results are plotted in Fig. 6. Vitremer lost 57% of its strength, Fuji II LC lost 50%, Ketac Nano lost 61%, Dyract Flow lost 57%. Heliomolar maintained its strength relatively well, losing only 32%. However, Heliomolar had the lowest F release.
Figure 6.
Percentage of strength loss after the specimens were immersed for 84 d calculated from the average strengths at the three pHs. Compared to specimens without immersion, Ketac Nano lost 61% of its strength after 84 d of immersion, Dyract Flow lost 57% of its strength, Fuji II LC lost 50%, and Heliomolar lost 32%.
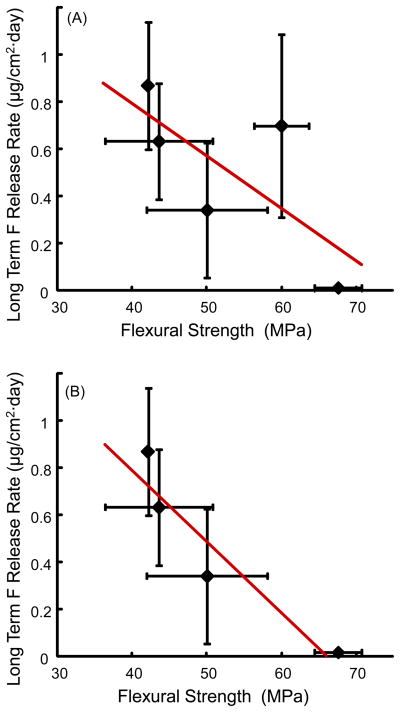
Therefore, it would be interesting to examine whether there was a correlation between the amount of F release and the mechanical properties of the restorative material. Fig. 7 plots the long-term F release vs. flexural strength of the materials, both after 84 d immersion. When all five materials were included in Fig. 7A, the R2 value was low at 0.51. However, when Fuji II LC, which exhibited both a high F release and a relatively high strength, was removed as in Fig. 7B, the R2 value was increased to 0.89. This indicates that, for most materials tested, there was a good correlation between the amount of F release and the strength of the material: The material with a higher F release tended to be weaker mechanically. It would be interesting to understand why the exception, Fuji II LC, had a relatively high strength and high F release. According to the manufacturer, the Fuji II LC powder consisted of 100% fluoroaluminosilicate glass. The Fuji II LC liquid consisted of 20–22% polyacrylic acid, 35–40% 2-hydroxyethyl methacrylate, 5–15% “proprietary ingredient”, 5–7% 2,2,4,Trimethyl hexamethylene dicarbonate, and 4–6% triethylene glycole dimethacrylate. The recommended Fuji II LC powder:liquid mass ratio is 3.2:1, which yielded a filler level mass fraction of 76.2%. One reason for its relatively high strength may be its relatively high filler level, compared to a filler level of approximately 71% mass fraction for Vitremer, and 69% for Ketac Nano. Another reason may be its matrix consisting of 2-hydroxyethyl methacrylate, which the other materials did not have. The high filler level of F-containing glass particles likely also contributed to its relatively high F releaser.
Figure 7.
Long-term F release rate versus flexural strength, after 84 d of immersion. (A) When all the five materials were included, the correlation coefficient R2 value was low at 0.51. (B) However, when Fuji II LC, which exhibited both a high release and a high strength, was removed, the R2 value was increased to 0.89. Hence, for most materials, F release and mechanical strength appeared to be inversely correlated.
The F-releasing restoratives initially displayed a high F release rate. This initial burst of release was followed by a lower, long-term, steady-state release rate. Vitremer had the highest initial release rate. One possible reason may be that the Vitremer matrix was slightly more hydrophilic, leading to a higher initial ion release. This may have also contributed to its relatively lower strength after immersion (Fig. 2). Fluoroaluminosilicate glass was the major component of the filler and the main source of fluoride in Vitremer, Fuji II LC, and Ketac Nano. Strontium was added to the fluoroaluminosilicate glass in Dyract Flow, and Heliomolar contained ytterbium fluoride for increased radiopacity. It would be interesting to examine why Heliomolar had a low F release. A previous study [42] found that Heliomolar had a F release of (0.30 ± 0.11) μg/cm2 after 28 d of immersion. This is slightly lower than the 1.1 μg/cm2 at 28 d and pH 7 of the present study, likely because experimental methods and material batch differences may have contributed to the measured F values. However, both values were much lower than the > 100 μg/cm2 for the other materials in Fig. 3, consistent with the fact that Heliomolar had relatively minimal F release. While the total filler level mass fraction for Heliomolar was 76%, only 10.6% were F-releasing ytterbium trifluoride fillers. This likely contributed to its low F release but relatively good strength durability in the immersion.
This study examined the pH effect on F release. The initial F release rate was higher at pH 4 than pH 7. This may advantageous because the restorative would “smartly” release more F when the pH is decreased to the cariogenic range, when these ions are most needed to inhibit caries. The fact that the restorative released less F at higher, safer pH is also beneficial because the F reserves are not quickly depleted. These results are consistent with another study [40] which showed that a glass ionomer (Ketac Fil) had a higher F release at pH 4 than at pH 7. Another study [41] found that two resin-modified glass ionomers (Vitremer and Fuji II LC), and a polyacid-modified composite (Freedom) had higher F releases at pH 4.6 than those at neutral pH. However, the present study found that, after 2 – 3 weeks, the F-release rates at all three pHs became similar. Two main factors had contributed to the F release. The first was that a lower, acidic pH would increase the fluoride release. The second factor was that a restorative material immersed in a solution with pH 4 would lose more F ions and decrease the amount of F ion reservoir near the specimen surface, compared to the same material immersed at pH 7. Initially, the first factor would dominate, leading to a higher F release at lower pH. However, after 2–3 weeks, the effect of the second factor gradually increased to offset the first factor, while the effect of the first factor decreased due to a decreased F reservoir. This led to a long-term effect where there was little effect of pH on the F release rate. A recent study on a novel nano-CaF2 filled composite also showed that while the short-term F release rate increased rapidly with decreasing pH, the long-term F release rate showed little difference at different pH [42].
Regarding future development of improved restoratives to combat secondary caries and restoration fracture, the relatively large strength loss after 84 d of immersion indicates that there is a strong need to design novel restoratives with improved longevity. Higher mechanical properties, durability over time, together with sustained F release, would help decrease the number of replacement restorations. The materials investigated in the present study are better F-releasing restoratives with enhanced properties compared to the first generation of F-releasing restoratives. Several of these materials had relatively high F releases to combat secondary caries. However, their mechanical properties and durability need to be significantly improved to extend their use to large-sized, load-bearing posterior restorations. Further study should improve the filler compositions, employ hybridization of F-releasing fillers with stable and strong reinforcing-fillers, and develop mechanically strong and durable matrix materials.
5. Conclusion
Effects of immersion. This study determined the effects of solution pH and immersion time on the mechanical properties and F release of restorative materials. Immersion for 84 d resulted in a significant decrease in the strength and elastic modulus of the restoratives.
Effects of pH on mechanical properties. As long as the materials were immersed in the solution, different solution pH values had little effect on the mechanical properties of the restoratives.
Effects of pH on F release. Solution pH had a dramatic effect on the initial F release, with much more F ions being released at pH 4 than at pH 7, in the first 2–3 weeks. However, after that time, the long-term F release rate became similar at different pH.
Effects of materials. Different materials showed different F release and strength loss, which were related to their microstructure and compositions. In general, materials with higher F release had lower mechanical properties. These F-releasing restoratives should be useful in releasing significant amounts of F to inhibit caries. It would be highly beneficial to further improve their mechanical properties and durability to extend their use to large stress-bearing restorations.
Acknowledgments
We are indebted to Dr. Limin Sun, Kathleen Hoffman, Anthony Giuseppetti, and Dr. Laurence C. Chow at the Paffenbarger Research Center, American Dental Association Foundation, National Institute of Standards and Technology, for discussions and experimental assistance. This study was supported by NIH grant R01 DE17974 (HX), the Maryland Nano-Biotechnology Award (HX), and the University of Maryland Dental School.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- 2.Ferracane JL, Berge HX. Fracture toughness of experimental dental composites aged in ethanol. J Dent Res. 1995;74:1418–1423. doi: 10.1177/00220345950740071501. [DOI] [PubMed] [Google Scholar]
- 3.Bayne SC, Thompson JY, Swift EJ, Jr, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. J Am Dent Assoc. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 4.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dent Mater. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 5.Watts DC, Marouf AS, Al-Hindi AM. Photo-polymerization shrinkage-stress kinetics in resin-composites: methods development. Dent Mater. 2003;19:1–11. doi: 10.1016/s0109-5641(02)00123-9. [DOI] [PubMed] [Google Scholar]
- 6.Lu H, Stansbury JW, Bowman CN. Impact of curing protocol on conversion and shrinkage stress. J Dent Res. 2005;84:822–826. doi: 10.1177/154405910508400908. [DOI] [PubMed] [Google Scholar]
- 7.Stansbury JW. Cyclopolymerizable monomers for use in dental resin composites. J Dent Res. 1990;69:844–848. doi: 10.1177/00220345900690030201. [DOI] [PubMed] [Google Scholar]
- 8.Ferracane JL, Berge HX, Condon JR. In vitro aging of dental composites in water – effect of degree of conversion, filler volume, and filler/matrix coupling. J Biomed Mater Res. 1998;42:465–472. doi: 10.1002/(sici)1097-4636(19981205)42:3<465::aid-jbm17>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Lim BS, Ferracane JL, Sakaguchi RL, Condon JR. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent Mater. 2002;18:436–444. doi: 10.1016/s0109-5641(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 10.Kielbassa AM, Schulte-Monting J, García-Godoy F, Meyer-Lueckel H. Initial in situ secondary caries formation: Effect of various fluoride-containing restorative materials. Oper Dent. 2003;28:765–772. [PubMed] [Google Scholar]
- 11.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dent Mater. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Cenci MS, Pereira-Cenci T, Cury JA, ten Cate JM. Relationship between gap size and dentine secondary caries formation assessed in a microcosm biofilm model. Caries Res. 2009;43:97–102. doi: 10.1159/000209341. [DOI] [PubMed] [Google Scholar]
- 14.Mjör IA, Moorhead JE, Dahl JE. Reasons for replacements of restorations in permanent teeth in general dental practice. International Dent J. 2000;50:361–366. doi: 10.1111/j.1875-595x.2000.tb00569.x. [DOI] [PubMed] [Google Scholar]
- 15.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Prim Dent Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 16.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. J Dent Res. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Prim Dent Care. 2009;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 18.Frencken JE. The ART approach using glass-ionomers in relation to global oral health care. Dent Mater. 2010;26:1–6. doi: 10.1016/j.dental.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Skrtic D, Antonucci JM, Eanes ED, Eichmiller FC, Schumacher GE. Physiological evaluation of bioactive polymeric composites based on hybrid amorphous calcium phosphates. J Biomed Mater Res. 2000;53B:381–391. doi: 10.1002/1097-4636(2000)53:4<381::aid-jbm12>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 20.Glasspoole EA, Erickson RL, Davidson CL. A fluoride-releasing composite for dental applications. Dent Mater. 2001;17:127–133. doi: 10.1016/s0109-5641(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 21.Dickens DH, Flaim GM, Takagi S. Mechanical properties and biochemical activity of remineralizing resin-based Ca-PO4 cements. Dent Mater. 2003;19:558–566. doi: 10.1016/s0109-5641(02)00105-7. [DOI] [PubMed] [Google Scholar]
- 22.Anusavice KJ, Zhang NZ, Shen C. Effect of CaF2 content on rate of fluoride release from filled resins. J Dent Res. 2005;84:440–444. doi: 10.1177/154405910508400508. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu SK. Clinical evaluations of resin-modified glass-ionomer restorations. Dent Mater. 2010;26:7–12. doi: 10.1016/j.dental.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 24.Ling L, Xu X, Choi G-Y, Billodeaux D, Guo G, Diwan RM. Novel F-releasing composite with improved mechanical properties. J Dent Res. 2009;88:83–88. doi: 10.1177/0022034508328254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weigand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials–fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007;23:343–362. doi: 10.1016/j.dental.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 26.Ellakuria J, Triana R, Minguez N, Soler I, Ibaseta G, García-Godoy F. Effect of one-year water storage on the surface microhardness of resin-modified versus conventional glass-ionomer cements. Dent Mater. 2003;19:286–290. doi: 10.1016/s0109-5641(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 27.Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003;24:2451–2461. doi: 10.1016/s0142-9612(02)00638-5. [DOI] [PubMed] [Google Scholar]
- 28.Tyas MJ. Clinical evaluation of glass-ionomer cement restorations. J Applied Oral Sci. 2006;14:10–13. doi: 10.1590/s1678-77572006000700003. [DOI] [PubMed] [Google Scholar]
- 29.Asmussen E, Peutzfeldt A. Long-term fluoride release from a glass ionomer cement, a compomer, and experimental resin composites. Acta Odontol Scand. 2002;60:93–97. doi: 10.1080/000163502753509482. [DOI] [PubMed] [Google Scholar]
- 30.Itota T, Carrick TE, Yoshiyama M, McCabe JF. Fluoride release and recharge in giomer, compomer and resin composite. Dent Mater. 2004;20:789–795. doi: 10.1016/j.dental.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Hefferren JJ, Koehler HM. Foods, nutrition and dental health. Park Forest South, IL: Pathotox Publishers; 1981. [Google Scholar]
- 32.ASTM D 790-03. Standard test methods for flexural properties of unreinforced and reinforced plastic and electrical insulating materials. West Conshohocken, PA: ASTM International; 2004. [Google Scholar]
- 33.ISO/FDIS 4049. Dentistry–polymer-based fillings, restorative and luting materials. Vol. 3. Geneva, Switzerland: International Organization for Standardization; 2000. [Google Scholar]
- 34.Xu HHK, Weir MD, Sun L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dental Mater. 2009;25:535–542. doi: 10.1016/j.dental.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu HHK, Sun L, Weir MD, Antonucci JM, Takagi S, Chow LC, Peltz M. Nano DCPA-whisker composites with high strength and Ca and PO4 release. J Dent Res. 2006;85:722–727. doi: 10.1177/154405910608500807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HHK, Weir MD, Sun L. Dental nanocomposites with Ca-PO4 release: Effects of reinforcement, dicalcium phosphate particle size and silanization. Dent Mater. 2007;23:1482–1491. doi: 10.1016/j.dental.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skrtic D, Antonucci JM, Eanes ED. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent Mater. 1996;12:295–301. doi: 10.1016/s0109-5641(96)80037-6. [DOI] [PubMed] [Google Scholar]
- 38.Xu HHK, Moreau JL, Sun L, Chow LC. Strength and fluoride release characteristics of a calcium fluoride based dental nanocomposite. Biomaterials. 2008;29:4261–4267. doi: 10.1016/j.biomaterials.2008.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itota T, Carrick TE, Rusby S, Al-Naimi OT, Yoshiyama M, McCabe JF. Determination of fluoride ions released from resin-based dental materials using ion-selective electrode and ion chromatograph. J Dent. 2004;32:117–122. doi: 10.1016/j.jdent.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 40.Carey CM, Spencer M, Gove RJ, Eichmiller FC. Fluoride release from a resin-modified glass-ionomer cement in a continuous-flow system: Effect of pH. J Dent Res. 2003;82:829–832. doi: 10.1177/154405910308201013. [DOI] [PubMed] [Google Scholar]
- 41.Silva KG, Pedrini D, Delbem ACB, Cannon M. Microhardness and fluoride release of restorative materials in different storage media. Braz Dent J. 2007;18:309–313. doi: 10.1590/s0103-64402007000400007. [DOI] [PubMed] [Google Scholar]
- 42.Xu HHK, Moreau JL, Sun L, Chow LC. Novel CaF2 nanocomposite with high strength and F ion release. J Dent Res. 2009 doi: 10.1177/0022034510364490. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]