Abstract
Background/Aims
To assess (1) the duration and symptoms present in participants with mild cognitive impairment (MCI) and (2) the impact of these variables on predicting conversion to Alzheimer's disease (AD).
Methods
Participants with MCI (n = 148) were assessed and followed systematically.
Results
Decline in memory was reported as the first symptom in 118 of the cases. Converters had more symptoms (e.g. language decline, depression), and the combination of decline in memory and in performance of high-order social/cognitive activities as well as disorientation more often than nonconverters (p = 0.036). In an age-stratified Cox model, predictors of conversion to AD were shorter time since onset of memory decline and lower baseline MMSE score.
Conclusions
Recent onset of memory decline with older age, decreased MMSE score, change in performance and disorientation indicate a greater likelihood of short-term conversion to AD.
Copyright © 2010 S. Karger AG, Basel
Key Words: Age at onset, Alzheimer's disease, Behavioral symptoms, Mild cognitive impairment, Neuropsychological assessment
Introduction
Mild cognitive impairment (MCI) is often a transitional stage between age-related memory decline and Alzheimer's disease (AD) [1,2], though many individuals with MCI remain cognitively stable. Identifying the earliest symptoms and their relative temporal onset may help to improve early diagnosis, estimate prognosis and eventually impact treatment. Efforts have been made to standardize methods of determining the date of onset and the types of symptoms present [3,4,5,6,7]. Symptoms appear well before one seeks treatment, and clarifying that time course by the employment of thorough interview techniques yields reliable findings [7]. Although memory decline is often the first symptom identified, other symptoms may also be the earliest to appear. Oppenheim [3] found in a retrospective review of patients diagnosed with AD that about half of them reported early changes in psychiatric, neurological and functional modalities. Kang et al. [8] interviewed caregivers of persons with AD and found that, in addition to memory decline, changes in orientation, judgment, depression and language preceded diagnosis.
In the development of the ‘Onset of Illness Interview’, our group used a standardized semistructured interview to assess time of onset of specific domains of symptoms, and tested it with informants of 36 individuals with dementia [4]. Open-ended questions about the first identified symptom referenced calendar dates and assisted informants to recall salient personal events to pinpoint specific occurrences. The interview format also helped informants identify the date when that symptom was not present [4]. This semistructured interview process showed good interrater and interinformant reliability [4] and has been used effectively in other studies [8,9,10,11,12]. In addition to the reliability of the semistructured interview, there is a considerable literature to suggest that eliciting information from informants is more reliable than self-report in patients with MCI or AD [13,14,15,16,17,18].
In this study that examined several predictors of conversion from MCI to AD, the Onset of Illness Interview [4] was administered to the informants of patients who presented with cognitive impairment, but not dementia. The type of informant-reported symptoms present at illness onset and the duration of these symptoms from onset to study entry were also examined as predictors of conversion to AD. We hypothesized that a greater number of early symptoms and a shorter time course of these symptoms would be associated with a quicker conversion to AD. In addition, we planned to explore the relationship between early informant-reported symptoms and objectively measured baseline cognitive performance.
Materials and Methods
Participants
Patients presented with memory complaints to either the Memory Disorders Clinic or the Center for Memory and Behavioral Disorders at the New York State Psychiatric Institute/Columbia University. All participants met study criteria for cognitive impairment without dementia and provided informed consent in this New York State Psychiatric Institute/Columbia University institutional-review-board-approved protocol.
Eligibility Criteria
Inclusion criteria identified a wide group of cognitively impaired individuals, aged 41−85 years, with memory complaints and without dementia with onset of cognitive impairment between 6 months and 10 years prior to enrollment, and Folstein MMSE scores of ≥22/30 [19]. Cognitive screening inclusion guidelines were described previously [20] and included Folstein MMSE recall of ≤2/3 objects at 5 min, or a Buschke Selective Reminding Test delayed recall score >1 SD below norms, or a Wechsler Adult Intelligence Scale-Revised performance IQ score ≥10 points below the respective verbal IQ score (a common neuropsychological pattern associated with cognitive decline). Final determination of inclusion was based on a consensus diagnosis between 2 expert raters (D.P.D. and Y.S.) after review of the baseline cognitive, functional and clinical information, neuropsychological test performance, laboratory test results and MRI radiological readings.
This study began prior to the publication by Petersen et al. [1] of the criteria to define MCI. All participants had memory complaints (a diagnostic criterion for MCI) and completed an extensive neuropsychological test battery, making it possible to determine the baseline MCI subtype, as previously described [20]. Using this classification method, 73% of the patients met criteria for amnestic MCI with or without other cognitive domain deficits, 13.5% had nonamnestic MCI, and 13.5% had cognitive scores <1.5 SD below norms, i.e. insufficient scores to meet the MCI criteria [20].
A total of 150 participants who met the study eligibility criteria, but without dementia, were enrolled. Within 6 months of presentation, 2 participants were excluded following clinical diagnoses of other neurologic disorders. The mean age of the remaining 148 participants was 67.1 ± 9.9 years, 55% of them were female, the mean level of education was 15.1 ± 4.3 years, and the mean score on the baseline MMSE was 27.5 ± 2.2.
Procedures
The study physician completed a history and physical, psychiatric and neurological examination, laboratory tests and an MRI brain scan. All participants received a neuropsychological test battery at baseline, and again annually, that included measures of learning and memory, orientation, abstract reasoning, language, attention and visuospatial ability, as described elsewhere [20]. A neuropsychologist (Y.S.) reviewed the results and 2 expert raters made a consensus diagnosis at each follow-up visit while remaining blind to the data from prior visits. The diagnosis of dementia was based on DSM-IV criteria and possible or probable AD based on NINCDS-ADRDA (National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer's Disease and Related Disorders Association) criteria [21]. The endpoint of conversion to AD required this diagnosis at any 2 consecutive annual assessments during the entire follow-up period.
Baseline Assessment
The study participants identified a reliable informant, someone who knew them well and had frequent contact. Data were gathered regarding the onset of illness from the informant related to the types of symptoms exhibited by the study participant and the duration of each symptom [4]. In the Onset of Illness interview, the informants reported on the baseline functional abilities of the patients and identified the time of onset for the first and all subsequent symptoms present from a list of 7 categories: change in memory, performance of higher-order social/cognitive activities, language, disorientation, personality, development of psychosis or depressed mood.
Subjective memory was assessed by the Memory Function Questionnaire (MFQ) [22], which included a single question requesting a global rating of memory problems.
Statistical Analyses
One-way analysis of variance and χ2 tests were used to compare continuous and categorical baseline measures as appropriate between patients who converted to AD on follow-up and patients who did not convert (hereafter referred to as ‘converters’ and ‘nonconverters’, respectively).
In a Cox proportional hazards model survival analysis conducted for the entire length of the follow-up period (1−9 years), the outcome variable was the time from baseline to first time point of AD diagnosis (follow-up duration varied across participants), with censoring by the time point of the last available evaluation. Age-stratified Cox discrete time-regression models were used as the hazard of AD conversion increased with age: in the ≥75-year age group, 53% (20/38) converted; from the ages of 68 to 74 years, 31% (11/36) converted; between the ages of 60 and 67 years, 15% (6/39) converted, and at ages of <60 years, 6% (2/35) converted.
Informant-reported memory deficits were the first symptom noted in the vast majority of patients and symptoms in other domains were rarely reported as the first symptom. Therefore, the final age-stratified Cox analyses included 4 models with the following independent variables: demographic variables known to affect cognitive performance (sex and education); objective measurement of baseline cognitive performance (MMSE), and those informant-reported symptoms that differed significantly between the groups. Model 1 included the time from onset of memory decline to study entry (our first hypothesized predictor), sex, education and baseline MMSE score. Model 2 included the number of informant-reported symptoms (our second hypothesized predictor), sex, education and MMSE score. Model 3 included informant-reported symptoms that differed in frequency between converters and nonconverters: the combined symptoms of memory decline, change in performance of higher-order social/cognitive activities and disorientation plus sex, education and MMSE score. Finally, model 4 (the full model) included the time from onset of memory decline to study entry, the number of informant-reported symptoms, change in performance of higher-order social/cognitive activities and disorientation, sex, education and MMSE score. All analyses were conducted by SPSS Statistics 17.0 for PC.
Results
Of the 148 participants, 39 (26%) converted to AD. All converters were at least 55 years old at baseline; 31 had probable AD and 8 had possible AD, i.e. AD with concomitant conditions that developed during follow-up (depression: n = 6; prescription drug abuse: n = 1; parkinsonian features without meeting criteria for Lewy body dementia: n = 1). At baseline, the converters were older and had lower MMSE scores compared to the nonconverters (table 1). The groups did not differ in severity of subjective memory complaint (MFQ), education or sex distribution.
Table 1.
Demographic and clinical features of converters and nonconverters
| Converters (n = 39) | Nonconverters (n = 109) | Significance | |
|---|---|---|---|
| Age, years | 73.2 ±7.1 | 64.9 ±9.9 | p< 0.001 |
| Sex, n | 22 F (56%); 17 M | 60 F (55%); 49 M | ns |
| Education, years | 14.0 ±4.7 | 15.4±4.1 | ns |
| Baseline MMSE score | 26.3 ±2.2 | 27.9 ±2.0 | p< 0.001 |
| Memory as 1st symptom, n | 28 (71.8%) | 90 (82.6%) | ns |
| Time since onset of memory symptoms, months | 29.6 ±15.6 | 41.7±30.1 | p = 0.002 |
| Subjective memory complaint MFQ score (range 1–7) | 3.8 ±1.5 | 4.0 ±1.4 | ns |
Values denote means ± SD unless specified otherwise, ns = Not significant.
The mean (SD) duration of follow-up was 24.1 (18.8) months for converters and 61.2 (29.4) months for nonconverters (follow-up was shorter with converters because they exited the protocol after 2 consecutive annual diagnoses of AD). Of the 148 patients enrolled in the longitudinal study, 8 died. Of the remaining 140, 10 dropped out by the third follow-up visit, 5 refused further follow-up, 2 discontinued owing to serious medical illness, and 3 lost contact.
Type of Informant-Reported Symptoms
Decline in memory was reported by informants as the first symptom in 118 (80%) of the cases. Depressed mood was reported as the first symptom in 13 (9%), change in language in 6 (4%), change in performance of higher-order social/cognitive activities in 4 (3%), disorientation in 3 (2%), personality change in 2 (1%) and behavior change in 2 (1%). There were no group differences with regard to the distribution of first symptoms reported (table 2). In addition to the first symptom identified, informants often reported that more than 1 of the above symptoms was present prior to baseline evaluation. The total number of symptoms reported (first identified symptom plus subsequent informant-reported symptoms) differed between converters and nonconverters (3.53 ± 1.81 and 2.70 ± 1.58, respectively; p < 0.01). In addition, converters had the combination of decline in memory and in performance of higher-order social/cognitive activities and disorientation more often than nonconverters (p < 0.05).
Table 2.
Incidence of (1) first symptom experienced by each participant and (2) all informant-reported symptoms (includes first and any subsequent symptoms experienced) (n)
| First symptoms endorsed |
All symptoms endorsed |
|||
|---|---|---|---|---|
| converters (n = 39) | nonconverters (n = 109) | converters (n = 39) | nonconverters (n = 109) | |
| Memory | 28 (72) | 90 (83) | 39 (100) | 109(100) |
| Depressed | 5(13) | 8(7) | 20(51) | 47 (43) |
| Change in language | 1(2) | 5(5) | 19 (49) | 37 (34) |
| Change in performance | 2(5) | 2(<2) | 24 (62)* | 44 (40)* |
| Disorientation | 1(2) | 2(<2) | 15 (39)* | 16(15)* |
| Change in personality | 1(2) | 1(<1) | 13 (33) | 23(21) |
| Change in behavior | 1(2) | 1(<1) | 5(13) | 15(14) |
| Psychosis | 1(2) | 1(<1) | 2(5) | 1 (1) |
Values in parentheses denote percentages. An independent t test revealed that a higher proportion of converters endorsed change in performance of higher-order social/cognitive activities and disorientation compared to nonconverters.
p < 0.05.
Time since Onset of Informant-Reported Symptoms
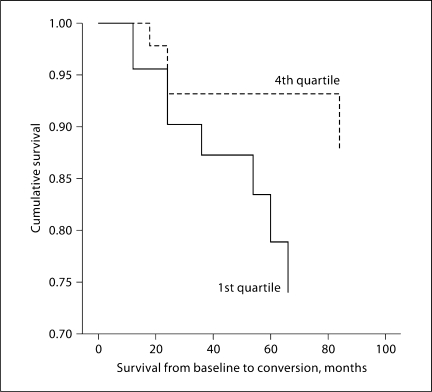
Converters had a significantly shorter time from onset of informant-reported memory problems to study entry compared to nonconverters (29.6 ± 15.6 vs. 41.7 ± 30.1 months; p < 0.01). Twenty percent of the converters had had memory symptoms for <18 months (1st quartile) prior to study enrollment, while only 10% had had those symptoms for >51 months (4th quartile). Twenty-four percent of the nonconverters had had symptoms for <18 months, while the greatest number (31%) had had symptoms for >51 months (fig. 1).
Fig. 1.
Cumulative survival as a function of time since the onset of memory problems. The data display the 1st (0–18 months) and 4th (>51 months) quartiles, excluding the middle quartiles to illustrate the difference in survival between the short- and longterm ends of the data spectrum.
There were no significant correlations between the time since onset of memory problems and age, education, baseline MMSE score or informant report of functional abilities. However, the higher the number of informant-reported symptoms present prior to study enrollment, the worse the cognitive status (MMSE: r = −0.24; p < 0.01) and informant report of functional abilities (r = 0.32; p < 0.001).
Cox Analyses with Outcome of Conversion to AD
In an age-stratified Cox analysis using model 1, shorter time since onset of informant-reported memory problems was a significant predictor of conversion to AD alone as well as with sex, education and baseline MMSE score included in the model (risk ratio: 0.98; 95% CI: 0.97–0.99; p < 0.05). MMSE score was also a significant predictor of conversion (risk ratio: 0.76; 95% CI: 0.65–0.89; p < 0.01). In model 2, a higher number of informant-reported symptoms present prior to study enrollment was a significant predictor of conversion to AD alone as well as with sex and education in the model (risk ratio: 1.25; 95% CI: 1.06–1.48; p ≤ 0.01), but did not remain significant with MMSE score included (risk ratio: 1.14; 95% CI: 0.95–1.36; p = 0.17). In model 3, endorsement of the combination of the symptoms of decline in memory and performance of higher-order social/cognitive activities plus disorientation present prior to study enrollment was a significant predictor of conversion to AD alone (risk ratio: 2.09; 95% CI: 1.01–4.36; p < 0.05), but did not remain significant with sex, education and MMSE score included (risk ratio: 1.08; 95% CI: 0.46–2.52; p = 0.86). In model 4, shorter time since onset of memory decline (risk ratio: 0.98; 95% CI: 0.97–0.99; p < 0.05) and lower MMSE score (risk ratio: 0.77; 95% CI: 0.65–0.92; p < 0.01) were significant predictors.
The same analyses were conducted in only the 108 participants who met criteria for amnestic MCI (n = 21) or amnestic MCI with deficits in multiple cognitive domains (n = 87). The results were similar to those obtained from the entire sample of 148 participants, with shorter elapsed time since onset of memory problems, greater number of informant-reported symptoms and MMSE score predicting conversion.
Discussion
MCI participants who converted to AD had a shorter time since the onset of memory problems and had a greater number of informant-reported symptoms than participants who did not develop AD. The shorter duration of memory problems in the converters along with a greater number of informant-reported symptoms, especially the constellation of memory decline, change in performance of higher-order social/cognitive activities and disorientation, clearly indicated a more malignant process than was present in the nonconverters. This difference could not be solely explained by the degree of cognitive decline present at baseline because the effect of the shorter duration of memory problems remained after controlling for baseline MMSE scores. The converters did have lower baseline MMSE scores though and were older than the nonconverters. Taking these factors into account, one may infer a greater risk of conversion to AD when an older patient experiences a relatively recent onset of cognitive deficits and other nonmemory symptoms such as change in performance of higher-order social/cognitive activities and increased disorientation. Conversely, there is less risk for the development of AD in someone with only subjective memory complaints that have persisted for a long time without resulting in objective evidence of cognitive impairment. A common limitation in the clinical setting is the lack of resources for formal neuropsychological testing. We have shown that the clinical interview can increase the value of the prediction of progression by extracting accurate information on the onset and type of symptoms.
These findings are consistent with those published in a recent review article [23]. The authors showed that studies enrolling participants with a short duration of follow-up (1–2 years) reported a high annual conversion rate to dementia or AD (10–15%) [24,25,26,27]. However, if this annual rate of conversion remained that high over time, within 10 years nearly all persons with MCI would convert to dementia. Increasing the length of follow-up in studies (to more than 5 years) has proven that the high rate of conversion initially recorded decreases with the passage of time [28,29,30,31]. The authors hypothesize that those with an aggressive condition convert early and those that do not convert early remain relatively resilient to conversion. Our results support this view: patients who presented to our study with a relatively short time course since the onset of symptoms had worse global cognitive performance and were more likely to convert to AD than those who presented after having had symptoms for a longer period of time.
Participants with a greater number of informant-reported symptoms also had poorer baseline MMSE scores, and both these variables predicted conversion to AD. Details about the duration of symptoms, number and type of informant-reported symptoms, and objectively measured cognitive performance provide useful information for counseling a patient and family. Administration of the Onset of Illness Interview provides accurate information about the time course of symptoms. Early recognition of symptoms and diagnosis is important in addressing factors such as safety, financial planning, advance directives and other salient topics of discussion between a clinician and patient/family [32].
One limitation of the study relates to the composition of the study sample. Enrollment in this study began prior to the development of the term ‘mild cognitive impairment’ and its resultant definition; consequently, the study sample was somewhat heterogeneous, limiting the ability to generalize the results. In all, 128 of the participants met the criteria for MCI and, of those, 108 met the criteria for amnestic MCI. However, 20 participants did not fit the definition of MCI. In addition, a small percentage of study participants were fairly young. Everyone who converted to AD was at least 55 years old at enrollment. Repeating the main analyses with only the 108 participants with amnestic MCI, or excluding the 19 participants under the age of 55 years, did not alter the main findings of the study (data not shown).
In a clinical setting, it is difficult to discern which patients presenting with similar subjective memory complaints are following a rather benign course versus those at greater risk for conversion to AD. Subjective memory complaint, a key feature of MCI, has not proven to be a reliable means of determining risk [33,34,35,36] and did not differ between converters and nonconverters in this study. One reason for the poor reliability of self-report is related to the more recent recognition that patients with MCI often have anosognosia, [37] defined in this case as attenuated awareness of deficits in higher cognitive function [38]. It has been shown that MCI patients who lack awareness of functional decline are at greater risk of conversion to AD than MCI patients who acknowledge their deficits [39]. In fact, researchers have documented that the inclusion of an informant report of cognitive or functional decline, rather than the subjective complaint of either, improves the ability to make a diagnosis and predict the future trajectory [13,14,15,16,17,18,39]. This study provides new information about the relationship of early symptoms in persons presenting with cognitive decline via the administration of the Onset of Illness Interview. The findings suggest that informant report of recent onset of memory decline together with other symptoms, particularly worsening in performance of higher-order social/cognitive activities and disorientation, may indicate a greater likelihood of short-term conversion to a diagnosis of AD.
Disclosure Statement
The authors have no conflicts of interest to disclose relevant to this paper or this study.
Acknowledgement
This study was supported in part by grant No. R01AG17761 from the National Institute on Aging.
References
- 1.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 2.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 3.Oppenheim G. The earliest signs of Alzheimer's disease. J Geriatr Psychiatry Neurol. 1994;7:116–120. doi: 10.1177/089198879400700207. [DOI] [PubMed] [Google Scholar]
- 4.Sano M, Devanand DP, Richards M, Miller LW, Marder K, Bell K, Dooneief G, Bylsma FW, Lafleche G, Albert M, et al. A standardized technique for establishing onset and duration of symptoms of Alzheimer's disease. Arch Neurol. 1995;52:961–966. doi: 10.1001/archneur.1995.00540340045012. [DOI] [PubMed] [Google Scholar]
- 5.Doody RS, Dunn JK, Huang E, Azher S, Kataki M. A method for estimating duration of illness in Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:1–4. doi: 10.1159/000074078. [DOI] [PubMed] [Google Scholar]
- 6.Rouah F, Wolfson C. A recommended method for obtaining the age at onset of dementia from the CSHA database. Int Psychogeriatr. 2001;13(suppl 1):57–70. doi: 10.1017/s1041610202007998. [DOI] [PubMed] [Google Scholar]
- 7.Fiske A, Gatz M, Aadnoy B, Pedersen NL. Assessing age of dementia onset: validity of informant reports. Alzheimer Dis Assoc Disord. 2005;19:128–134. doi: 10.1097/01.wad.0000174947.76968.74. [DOI] [PubMed] [Google Scholar]
- 8.Kang SJ, Jeong Y, Lee BH, Baek MJ, Kwon JC, Chin J, Na DL. How early are initial symptoms recognized in Korean patients with Alzheimer's disease? Int J Geriatr Psychiatry. 2004;19:699–700. doi: 10.1002/gps.1121. [DOI] [PubMed] [Google Scholar]
- 9.Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, Scarmeas N, Braun I, Stern Y, Mayeux R. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62:975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 10.Cattel C, Gambassi G, Sgadari A, Zuccala G, Carbonin P, Bernabei R. Correlates of delayed referral for the diagnosis of dementia in an outpatient population. J Gerontol A Biol Sci Med Sci. 2000;55:M98–M102. doi: 10.1093/gerona/55.2.m98. [DOI] [PubMed] [Google Scholar]
- 11.Hirono N, Hashimoto M, Yasuda M, Ishii K, Sakamoto S, Kazui H, Mori E. The effect of APOE ∊4 allele on cerebral glucose metabolism in AD is a function of age at onset. Neurology. 2002;58:743–750. doi: 10.1212/wnl.58.5.743. [DOI] [PubMed] [Google Scholar]
- 12.Clark CM, DeCarli C, Mungas D, Chui HI, Higdon R, Nuñez J, Fernandez H, Negrón M, Manly J, Ferris S, Perez A, Torres M, Ewbank D, Glosser G, van Belle G. Earlier onset of Alzheimer disease symptoms in Latino individuals compared with Anglo individuals. Arch Neurol. 2005;62:774–778. doi: 10.1001/archneur.62.5.774. [DOI] [PubMed] [Google Scholar]
- 13.Chung JC, Man DW. Self-appraised, informant-reported, and objective memory and cognitive function in mild cognitive impairment. Dement Geriatr Cogn Disord. 2009;27:187–193. doi: 10.1159/000200467. [DOI] [PubMed] [Google Scholar]
- 14.Tierney MC, Szalai JP, Snow WG, Fisher RH. The prediction of Alzheimer disease: the role of patient and informant perceptions of cognitive deficits. Arch Neurol. 1996;53:423–427. doi: 10.1001/archneur.1996.00550050053023. [DOI] [PubMed] [Google Scholar]
- 15.Frerichs RJ, Tuokko HA. Reliable change scores and their relation to perceived change in memory: implications for the diagnosis of mild cognitive impairment. Arch Clin Neuropsychol. 2006;21:109–115. doi: 10.1016/j.acn.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Jorm AF, Christensen H, Henderson AS, Korten AE, Mackinnon AJ, Scott R. Complaints of cognitive decline in the elderly: a comparison of reports by subjects and informants in a community survey. Psychol Med. 1994;24:365–374. doi: 10.1017/s0033291700027343. [DOI] [PubMed] [Google Scholar]
- 17.Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Mackinnon A. Informant ratings of cognitive decline of elderly people: relationship to longitudinal change on cognitive tests. Age Ageing. 1996;25:125–129. [PubMed] [Google Scholar]
- 18.Isella V, Villa L, Russo A, Regazzoni R, Ferrarese C, Appollonio IM. Discriminative and predictive power of an informant report in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2006;77:166–171. doi: 10.1136/jnnp.2005.069765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 20.Tabert MH, Manly JJ, Liu X, Pelton GH, Rosenblum S, Jacobs M, Zamora D, Goodkind M, Bell K, Stern Y, Devanand DP. Neuropsychological prediction of conversion to Alzheimer disease in patients with mild cognitive impairment. Arch Gen Psychiatry. 2006;63:916–924. doi: 10.1001/archpsyc.63.8.916. [DOI] [PubMed] [Google Scholar]
- 21.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 22.Gilewski MJ, Zelinski EM, Schaie KW. The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol Aging. 1990;5:482–490. doi: 10.1037//0882-7974.5.4.482. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell AJ, Shiri-Feshki M. Temporal trends in the long-term risk of progression of mild cognitive impairment: a pooled analysis. J Neurol Neurosurg Psychiatry. 2008;79:1386–1391. doi: 10.1136/jnnp.2007.142679. [DOI] [PubMed] [Google Scholar]
- 24.Luis CA, Barker WW, Loewenstein DA, Crum TA, Rogaeva E, Kawarai T, St George-Hyslop P, Duara R. Conversion to dementia among two groups with cognitive impairment: a preliminary report. Dement Geriatr Cogn Disord. 2004;18:307–313. doi: 10.1159/000080124. [DOI] [PubMed] [Google Scholar]
- 25.Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer's disease. Dement Geriatr Cogn Disord. 2005;19:383–389. doi: 10.1159/000084709. [DOI] [PubMed] [Google Scholar]
- 26.Schmidtke K, Hermeneit S. High rate of conversion to Alzheimer's disease in a cohort of amnestic MCI patients. Int Psychogeriatr. 2008;20:96–108. doi: 10.1017/S1041610207005509. [DOI] [PubMed] [Google Scholar]
- 27.Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 28.Visser PJ, Kester A, Jolles J, Verhey F. Ten-year risk of dementia in subjects with mild cognitive impairment. Neurology. 2006;67:1201–1207. doi: 10.1212/01.wnl.0000238517.59286.c5. [DOI] [PubMed] [Google Scholar]
- 29.Tyas SL, Salazar JC, Snowdon DA, Desrosiers MF, Riley KP, Mendiondo MS, Kryscio RJ. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol. 2007;165:1231–1238. doi: 10.1093/aje/kwm085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Devanand DP, Pradhaban G, Liu X, Khandji A, de Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 31.Palmer K, Wang HX, Bäckman L, Winblad B, Fratiglioni L. Differential evolution of cognitive impairment in nondemented older persons: results from the Kungsholmen Project. Am J Psychiatry. 2002;159:436–442. doi: 10.1176/appi.ajp.159.3.436. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- 33.Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 34.Jorm AF, Butterworth P, Anstey KJ, Christensen H, Easteal S, Maller J, Mather KA, Turakulov RI, Wen W, Sachdev P. Memory complaints in a community sample aged 60–64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 35.Jorm AF, Masaki KH, Davis DG, Hardman J, Nelson J, Markesbery WR, Petrovitch H, Ross GW, White LR. Memory complaints in nondemented men predict future pathologic diagnosis of Alzheimer disease. Neurology. 2004;63:1960–1961. doi: 10.1212/01.wnl.0000144348.70643.f2. [DOI] [PubMed] [Google Scholar]
- 36.Jorm AF, Christensen H, Korten AE, Jacomb PA, Henderson AS. Memory complaints as a precursor of memory impairment in older people: a longitudinal analysis over 7–8 years. Psychol Med. 2001;31:441–449. [PubMed] [Google Scholar]
- 37.Ries ML, Jabbar BM, Schmitz TW, Trivedi MA, Gleason CE, Carlsson CM, Rowley HA, Asthana S, Johnson SC. Anosognosia in mild cognitive impairment: relationship to activation of cortical midline structures involved in self-appraisal. J Int Neuropsychol Soc. 2007;13:450–461. doi: 10.1017/S1355617707070488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heilman KM, Barrett AM, Adair JC. Possible mechanisms of anosognosia: a defect in self-awareness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1903–1909. doi: 10.1098/rstb.1998.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tabert MH, Albert SM, Borukhova-Milov L, Camacho Y, Pelton G, Liu X, Stern Y, Devanand DP. Functional deficits in patients with mild cognitive impairment: prediction of AD. Neurology. 2002;58:758–764. doi: 10.1212/wnl.58.5.758. [DOI] [PubMed] [Google Scholar]