Abstract
Purpose
Outcomes of treatment for young men compared with older men with prostate cancer are poorly defined outside of limited institutional series. This study examines the association between age at diagnosis and grade, stage, treatment, and survival outcomes in men diagnosed during the era of prostate-specific antigen testing.
Patients and Methods
The NCI SEER database was used to identify men diagnosed with prostate cancer between 1988 and 2003. Men aged 35-74 were stratified by age at diagnosis to examine differences in tumor characteristics, treatment, and survival within each age group.
Results
We identified 318,774 men ages 35 to 74 diagnosed with adenocarcinoma of the prostate between 1988 and 2003. The proportion of men age 55 and younger at diagnosis increased over the study period, from 2.3% between the years 1988-1991 to 9.0% between the years 2000-2003, and median age at diagnosis decreased from 72 in 1988 to 68 in 2003. Younger men were less frequently diagnosed with organ confined tumors (p<0.001), but less likely to be diagnosed with high grade cancer (p<0.001). Older men were more likely to receive no local therapy or external beam radiation than young men (p< 0.001 for trends). Among men with Gleason 5-7 tumors, overall survival was worse with advancing age. However, among all age groups with high grade and stage, the youngest men (35-44) were at the highest risk of all cause and cancer specific death.
Conclusions
Age at diagnosis among men with prostate cancer continues to decline. Younger men are more likely to be treated with prostatectomy, have lower grade cancer, and as a group have better overall, and equivalent cancer specific survival at 10 years compared to older men. Among men with high grade and locally advanced prostate cancer, the youngest men have a particularly poor prognosis compared to older men.
Keywords: prostate cancer, young, survival, prostatectomy
Background
Age at diagnosis of cancer is a well recognized prognostic factor for patients with malignancy, and younger patients have better outcomes independent of comorbidity or performance status 1. A definition of “younger” men with prostate cancer has been poorly characterized and ranges from “< 50” to “< 60” years of age in previous reports 2-5. Although these divisions of younger and older men with prostate cancer have varied, previous reports have typically found that “younger” men have better biochemical progression-free survival after prostatectomy 6,7 and less advanced disease at prostatectomy. 3 In contrast, Rosser et al found that men under the age of 60 treated with external beam radiotherapy suffered higher rates of biochemical progression4.
No studies have assessed the more significant endpoints of overall survival (OS) or disease-specific survival (DSS) in young men with local or regional prostate cancer, primarily due to limited follow-up and small numbers of patients in single-institution series. In clinical practice, patients are usually considered for definitive local therapy if their life expectancy is greater than 10 years, a threshold that is crossed when men reach the age of 75 8. The purpose of this study was to describe trends in age at diagnosis and characterize the relationship between age at diagnosis and survival outcomes in men diagnosed with prostate cancer and who were considered candidates for definitive therapy based on their age. Specifically, we examined the association between age at diagnosis, tumor characteristics, and survival among men with prostate cancer in the United States.
Material and Methods
Data source
The Surveillance, Epidemiology, and End Results (SEER) Program database was used to identify the cohort of patients for this study. SEER collects cancer incidence and survival data from seventeen population-based cancer registries accounting for approximately 26% of the United States population. Data from 1988-2003 from 17 SEER registries were used (San Francisco-Oakland SMSA, Connecticut, Metropolitan Detroit, Hawaii, Iowa, New Mexico, Seattle (Puget Sound), rural Georgia, Utah, Metropolitan Atlanta, Alaska, San Jose-Monterey, Los-Angeles, Kentucky, Louisiana, New Jersey, and Greater California). Cases are available only for years 1992-2003 from Alaska, San Jose-Monterey, Los Angeles, rural Georgia and for years 2000-2003 from Kentucky, Louisiana, New Jersey, and Greater California.
Study population
Cases were initially identified using International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) site codes for the prostate (C619) and histology classification codes for adenocarcinoma (8140) and acinar carcinoma (8550). Men younger than 35 years of age were excluded. For the survival analyses, we only included men who were ages 35-74 at time of diagnosis, as these men are most likely to be candidates for local therapy with curative intent, as their actuarial life expectancy exceeds 10 years.
Data collection and coding
Demographic data included subject age, race, and year of treatment. Age was categorized into 10-year categories. Race was categorized as Caucasian, African-American, or other based on SEER coding. Initial treatment type (prostatectomy, external beam radiotherapy, brachytherapy, external beam + brachytherapy, or no local therapy) was also determined. Pathologic data included SEER classification of tumor grade (low=Gleason score 2-4, intermediate=Gleason score 5-7, high=Gleason score 8-10) and SEER modified AJCC stage (stages I and II=T1/T2N0M0, stage III=T3N0M0, and stage IV=T4 or N+ or M+). SEER reports pathologic stage when available, while patients who did not undergo prostatectomy have only clinical stage data recorded. Chemotherapy and co-morbidity data are not available in SEER. Patients without complete stage and grade data were excluded from the survival analyses. Survival time was calculated starting at the date of diagnosis to the date of death. If death was not observed, patients were censored at the date of last follow-up. Prostate cancer-specific cause of death was determined using SEER site-specific death codes.
Statistical Analysis
Demographic and pathologic data are reported for the entire cohort. Multivariate Cox regression was performed to evaluate differences in risk of death based on age, stratified by grade and stage of diagnosis. All multivariate analyses were adjusted for race, year of diagnosis, and initial treatment type. In order to minimize bias that may occur from inaccurate clinical staging data, a sub-analysis of patients who underwent prostatectomy with associated pathologic staging data was also performed. The proportional hazards assumption for the Cox regression model was evaluated with Schoenfeld residuals. Variables that did not meet proportional hazards assumptions were stratified in the Cox model. Hazard ratios (HR) are presented along with their 95% confidence intervals (95% CI). All statistical analyses were conducted using Stata software, Version 9 (Stata, Inc., College Station, TX).
Results
We identified 453,195 men aged 35 and older diagnosed with adenocarcinoma of the prostate between 1988 and 2003 in the SEER tumor registry. 318,774 of the men were between the ages of 35 and 74, the age range in which active treatment is typically considered. The proportion of men age 55 and younger at diagnosis increased over the study period, from 3.9% between the years 1988-1991 to 10.9% between the years 2000-2003 (p<0.001), while the median age at diagnosis decreased from 72 in 1988 to 68 in 2003. Table 1 gives demographic and tumor characteristics stratified by age for those men 35-74 years old at time of diagnosis. The younger men were more commonly African American, were less likely to have a high grade tumor (p<0.001), less likely to be diagnosed with organ confined disease (p<0.001), and more likely to undergo radical prostatectomy (p<0.001). Conversely, the use of radiotherapy or no local therapy was more common in older men.
TABLE 1. Baseline characteristics of all men ages 35-74 with prostate cancer.
| Patient Characteristics | Age Groups | ||||
|---|---|---|---|---|---|
| 35-44 yrs (n=1,673) |
45-54 yrs (n=28,665) |
55-64 yrs (n=107,337) |
65-74 yrs (n=181,099) |
||
| Grade (%) | Low | 5.0 | 5.7 | 7.4 | 10.2 |
| Intermediate | 74.4 | 73.5 | 70.0 | 64.4 | |
| High | 17.1 | 17.8 | 19.1 | 21.1 | |
| Unknown | 3.5 | 3.0 | 3.5 | 4.3 | |
| χ2 p<0.001 | |||||
| AJCC Stage (%) | I or II | 32.1 | 35.9 | 38.4 | 42.5 |
| III | 13.5 | 15.3 | 15.0 | 11.6 | |
| IV | 10.9 | 8.8 | 8.4 | 8.0 | |
| Unknown | 43.5 | 40.0 | 38.2 | 37.9 | |
| χ2 p<0.001 | |||||
| Race (%) | Caucasian | 70.1 | 76.6 | 79.9 | 82.0 |
| African American | 24.7 | 18.3 | 14.5 | 11.0 | |
| Other | 3.4 | 3.1 | 3.7 | 5.1 | |
| Unknown | 1.9 | 2.0 | 1.9 | 1.9 | |
| χ2 p<0.001 | |||||
| Local therapy (%) | None | 16.1 | 15.1 | 18.4 | 28.3 |
| RP | 52.7 | 50.9 | 43.0 | 25.0 | |
| EBRT | 10.8 | 12.7 | 17.6 | 28.9 | |
| Brachytherapy | 3.0 | 5.3 | 6.8 | 6.7 | |
| Brachy +EBRT | 3.0 | 4.1 | 4.8 | 5.2 | |
| Unknown | 14.4 | 12.0 | 9.4 | 5.9 | |
| χ2 p<0.001 | |||||
| Diagnosis year (%) | 1988-1991 | 4.0 | 4.1 | 8.3 | 13.1 |
| 1992-1995 | 10.9 | 13.8 | 18.9 | 24.6 | |
| 1996-1999 | 22.5 | 23.2 | 21.7 | 21.0 | |
| 2000-2003 | 62.6 | 58.9 | 51.1 | 41.3 | |
| χ2 p<0.001 | |||||
Abbreviations: RP=radical prostatectomy, EBRT=external beam radiotherapy
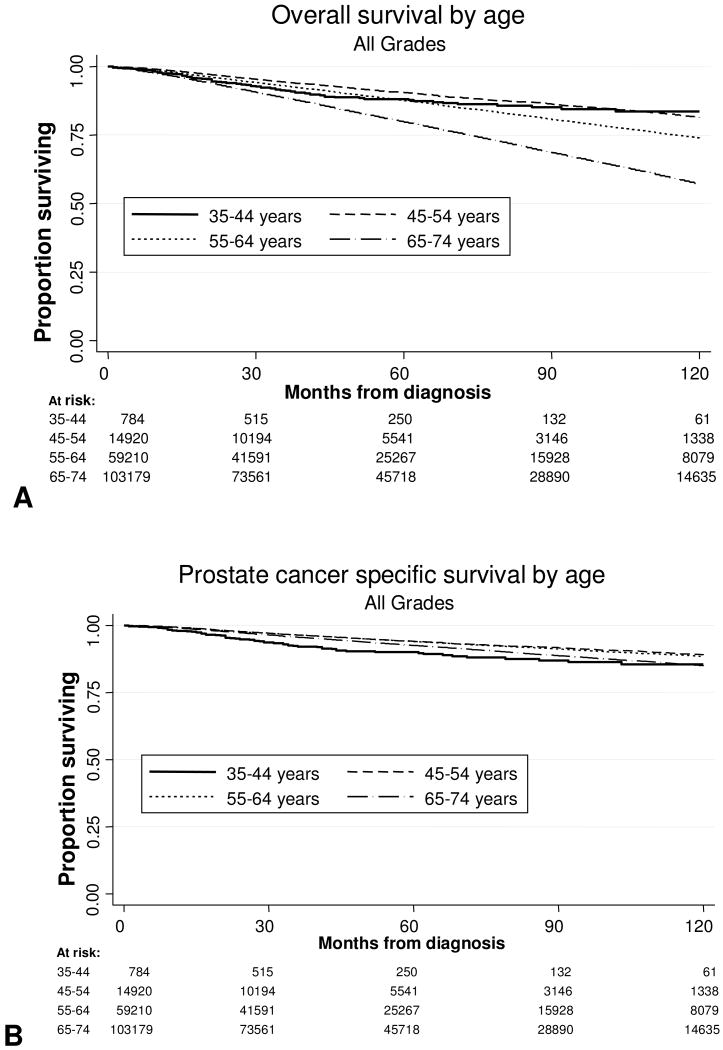
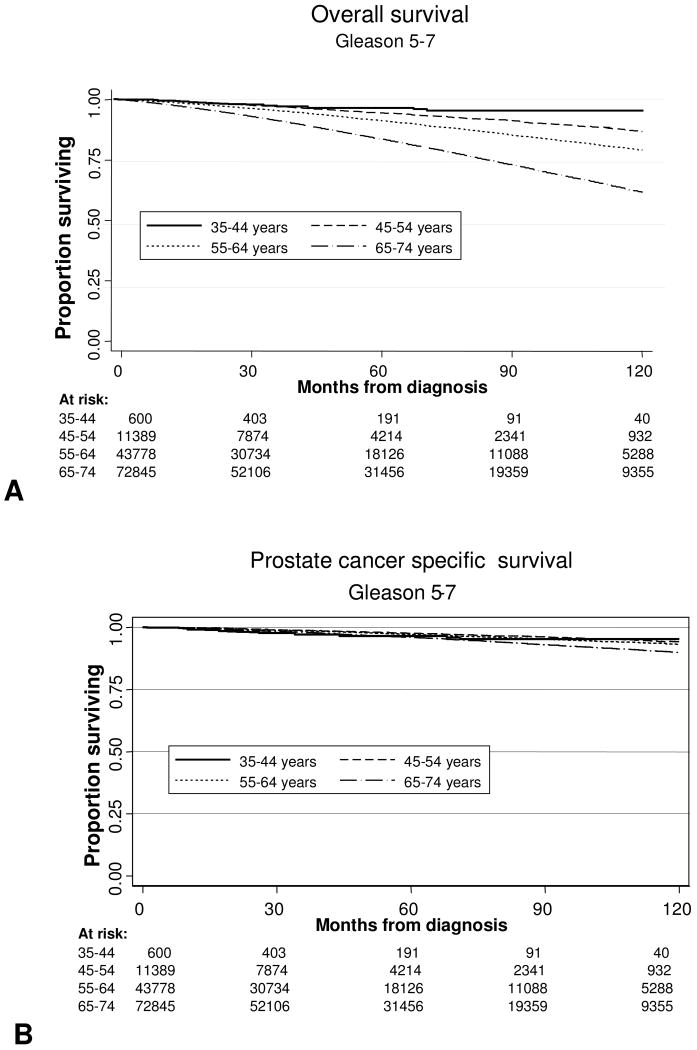
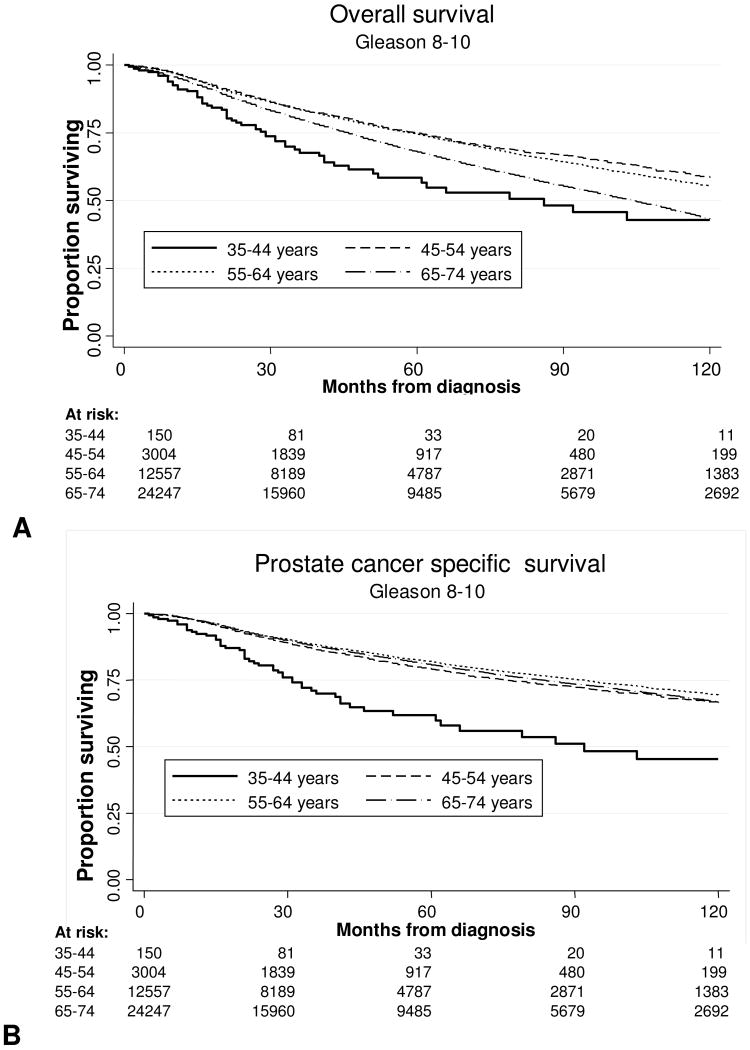
We examined both OS and DSS, stratified by age, grade, and stage. Median follow-up for the entire cohort was 45 months. The Kaplan-Meier survival curves for the entire cohort stratified by age are shown in Fig. 1A (OS) and 1B (DSS). As expected, OS was better at 5 and 10 years for men under the age of 55. DSS for the entire group was not significantly different for men younger or older than 55. To explore this further, we analyzed univariate 10 year OS and DSS Kaplan-Meier curves for patients with Gleason 5-7 tumors as stratified by age at diagnosis, and these are shown in Fig. 2A and 2B. Among all men with Gleason 5-7 tumors, the 10-year OS and DSS rates were 69% and 92%, respectively. As expected, when stratified by age, advancing age was associated with decreased OS compared to the youngest men, although DSS was similar between age groups. Figures 3A and 3B present OS and DSS for Gleason 8-10 tumors stratified by age at diagnosis. Among all men with Gleason 8-10 tumors, the 10-year OS and DSS rates were 48% and 70%, respectively. However, in contrast to intermediate grade tumors, young men with high grade tumors had significantly decreased OS and DSS when compared to older men.
Figure 1.
Survival Outcomes in men with prostate cancer by age at diagnosis (A) Overall survival after diagnosis. (B) Prostate cancer specific survival after diagnosis.
Figure 2.
Survival Outcomes in men with Gleason 5-7 prostate cancer by age at diagnosis (A) Overall survival after diagnosis. (B) Prostate cancer specific survival after diagnosis.
Figure 3.
Survival Outcomes in men with Gleason 8-10 prostate cancer by age at diagnosis (A) Overall survival after diagnosis. (B) Prostate cancer specific survival after diagnosis.
Table 2 gives the results of the multivariate analysis stratified by age, grade and stage while adjusting for race, year of diagnosis, and initial treatment type. When men with Gleason 5-7 tumors were stratified by grade and stage, the risk of all cause death predictably increased with each advancing age group in men with organ-confined disease, with the oldest men having the highest risk of death (HR 18.25, 95%CI 4.56-73.02), although older men had improved DSS in stage IV disease. However, among men with high grade and stage III and IV disease, the youngest men were approximately three times more likely to die of prostate cancer than all other age groups with locally advanced disease.
TABLE 2. Overall and prostate cancer-specific survival of patients with prostate cancer stratified by age, grade, and stage.
| Overall survival HR (95% CI) |
Prostate cancer-specific survival HR (95% CI) |
|||
|---|---|---|---|---|
| Gleason 5-7 | Gleason 8-10 | Gleason 5-7 | Gleason 8-10 | |
| All stages** | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 1.45 (0.95, 2.22) | 0.66 (0.50,0.86)† | 0.80 (0.50,1.27) | 0.64 (0.48,0.86)† |
| 55-64 | 2.10 (1.38, 3.19) † | 0.71 (0.54,0.92)† | 0.79 (0.50,1.24) | 0.58 (0.44,0.77)† |
| 65-74 | 3.53 (2.32, 5.37) † | 0.89 (0.69,1.16) | 0.92 (0.59, 1.45) | 0.60 (0.46,0.80)† |
| Stages I and II* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 6.28 (1.56,25.22) † | 1.03 (0.38,2.81) | 2.07 (0.29,14.95) | 1.36 (0.33,5.60) |
| 55-64 | 9.96 (2.49,39.84) † | 1.21 (0.45,3.25) | 2.29 (0.32,16.34) | 0.98 (0.24,3.95) |
| 65-74 | 18.25 (4.56,73.02) † | 1.70 (0.64,4.55) | 3.27 (0.46,23.29) | 1.06 (0.26, 4.25) |
| Stage III* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | †† | 0.38 (0.18, 0.78)† | †† | 0.30 (0.14,0.65)† |
| 55-64 | †† | 0.49 (0.24, 0.99)† | †† | 0.30 (0.14,0.63)† |
| 65-74 | †† | 0.73 (0.37, 1.47) | †† | 0.33 (0.16,0.70)† |
| Stages IV* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 0.75 (0.47, 1.18) | 0.71 (0.52, 0.97)† | 0.65 (0.40,1.06)† | 0.67 (0.49,0.93)† |
| 55-64 | 0.79 (0.50, 1.23) | 0.73 (0.54, 0.98)† | 0.60 (0.37,0.96)† | 0.61 (0.45,0.84)† |
| 65-74 | 0.96 (0.63, 1.52) | 0.83 (0.61,1.10) | 0.60 (0.38,0.96)† | 0.62 (0.46,0.85)† |
Cox PH regression; adjusted for race, year of diagnosis, and initial treatment type
Cox PH regression, adjusted for stage, race, year of diagnosis, and initial treatment type
HR incalculable due to no events in one or more age strata
Indicates p<0.05
In an attempt to minimize stage and grade bias, we performed a second multivariate analysis on the subgroup of men ages 35-74 who had undergone radical prostatectomy, as these men have tumor characteristics (pathologic stage and grade) determined from the radical prostatectomy specimen (Table 3). Trends similar but more pronounced than in the main analysis were observed in this subanalysis. Specifically, the youngest men are at least 5 times more likely to die of prostate cancer than any of their older counterparts with high grade, stage III disease.
Table 3. Overall and prostate cancer specific survival of patients undergoing radical prostatectomy stratified by age.
| Overall survival (HR) | Prostate cancer specific survival (HR) | |||
|---|---|---|---|---|
| Gleason 5-7 | Gleason 8-10 | Gleason 5-7 | Gleason 8-10 | |
| All stages** | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 6.41 (1.59, 25.8)† | 0.47 (0.27, 0.84)† | 3.15 (0.44,23.7) | 0.35 (0.19,0.65)† |
| 55-64 | 10.1 (2.53, 40.5)† | 0.55 (0.32, 0.96)† | 2.79 (0.39,19.9) | 0.29 (0.16,0.52)† |
| 65-74 | 20.5 (5.12, 81.9)† | 0.86 (0.49, 1.50) | 4.19 (0.56,29.9) | 0.35 (0.19,0.63)† |
| Stages I and II* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 5.95 (0.83,42.6) | 0.45 (0.14,1.47) | †† | 0.31 (0.07,1.33) |
| 55-64 | 9.07 (1.28,64.5)† | 0.41 (0.13,1.30) | †† | 0.16 (0.04,0.68)† |
| 65-74 | 20.0 (2.81,142.1)† | 0.72 (0.23,2.27) | †† | 0.18 (0.04,0.76)† |
| Stage T3* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | †† | 0.28 (0.12,0.64)† | †† | 0.17 (0.07,0.39)† |
| 55-64 | †† | 0.36 (0.16,0.82)† | †† | 0.16 (0.07,0.36)† |
| 65-74 | †† | 0.58 (0.26,1.30) | †† | 0.20 (0.09,0.44)† |
| Stages T4+* | ||||
| Age groups | ||||
| 35-44 | Referent | Referent | Referent | Referent |
| 45-54 | 1.25 (0.17,9.21) | 0.86 (0.30,2.36) | 0.57 (0.07,4.31) | 0.69 (0.24,1.98) |
| 55-64 | 1.79 (0.25,12.8) | 0.98 (0.36, 2.67) | 0.55 (0.07,4.06) | 0.58 (0.21,1.60) |
| 65-74 | 2.87 (0.40,20.6) | 1.36 (0.50,3.68) | 0.63 (0.09,4.57) | 0.68 (0.25,1.87) |
Cox PH regression; adjusted for race, year of diagnosis, and initial treatment type
Cox PH regression, adjusted for stage, race, year of diagnosis, and initial treatment type
HR incalculable due to no events in one or more age strata
Indicates p<0.05
Discussion
This population-based analysis demonstrates first that the diagnosis of prostate cancer in young men constitutes an increasing proportion of the number of men with newly diagnosed prostate cancer and that the primary treatment choice among young men and their physicians is radical prostatectomy over other modalities. Second, our analysis provides critical OS and DSS data beyond the prior institutional reports that assessed biochemical progression alone. The data support the intuitive concept that young men, with fewer comorbidities will have better OS as a group. This data shows that DSS at 10 years for the group as a whole is not significantly better for younger men. Other institutional series of outcomes in very young men suggest that biochemical relapse rates are superior for the youngest men compared to older men7. The current study focuses on survival outcomes rather than biochemical recurrence and surveys a substantially larger and more diverse range of patients, making direct comparison difficult. The advantage of this analysis is that is reflects practice patterns and outcomes from a broad range of providers and include a significantly larger cohort of men younger than 50 than any other reported series. This analysis also unexpectedly revealed that young men with high grade tumors have significantly worse DSS outcomes. Specifically, these young men with high-grade tumors had worse OS when diagnosed with stage III and IV disease and worse DSS in all stages, depending on primary treatment. Conversely, older men with Gleason 5-7 disease in general have worse outcomes, presumably related to other comorbidities. Specifically, these older men with low/intermediate-grade tumors have decreased OS in stage I/II disease and decreased DSS in stage IV disease.
Younger age at diagnosis has a positive impact on prognosis for most adult men and women diagnosed with malignancy. For all patients diagnosed with cancer, a subset of histologies are associated with worse outcome in young adult patients, including breast cancer, sarcoma, colon cancer, lymphoma and leukemia 1. In breast cancer, another hormone-dependent malignancy, young age is a risk factor independent of stage, grade, hormone receptor expression and mode of primary therapy 9, 10. Better tolerance for aggressive therapy, earlier recognition of the disease, and attendant lower grade and stage of disease have all been invoked as reasons why young men with prostate cancer have better relapse-free survival after primary therapy in other smaller institutional series 11.
There are several potential explanations for our present findings with regards to the more significant endpoints of OS and DSS. In our study, younger men were more likely to have extraprostatic disease, possibly reflecting the greater use of prostatectomy, and subsequent pathologic upstaging in these men. Despite the trend toward better outcomes with younger age for the group as a whole, the youngest men diagnosed with high grade disease paradoxically are at much higher risk for death from prostate cancer regardless of the form of therapy. Why men aged 35-44 with high-grade disease have dramatically higher cancer-specific mortality when compared to older men is unclear, although the relative lack of competing comorbidities in younger men likely plays a role in this finding. In support of our findings, an analysis of the CAPSURE dataset suggested that younger men presenting with metastatic prostate cancer were at higher risk of early death, with a hazard ratio of 0.47 (95%CI 0.28-0.78) for men over the age of 65 12. Family history of prostate cancer may also play a role in screening men earlier in life and could potentially enrich the population of young patients for high risk disease. However, in the PSA era, a family history of prostate cancer has been reported to impart no significant impact on biochemical relapse rates, suggesting that this factor is unlikely to play a significant role in our analysis.13, 14. Genetic factors, such as BRCA2 mutations, are associated with a higher risk of locally advanced disease and death from prostate cancer, although only small minority of patients carry the mutation at diagnosis 15, 16. Additionally, the youngest men may be diagnosed as a result of symptomatic disease, as opposed to a screening diagnosis, and thus harbor more aggressive and higher volume disease. However, the proportion of men under 45 did not change significantly over the period of analysis, despite a substantial increase in PSA screening, making it unlikely that symptomatic disease is more common in this group of patients. Lastly, young men may simply have biologically more aggressive disease, as these men are more likely to be African American and to present with metastatic disease, suggesting a shorter latency and a different biology. Despite the small number of very young men diagnosed with high grade disease, more completely defining a significant clinical phenotype in this set of very young men and its associated molecular signature may provide additional insights into the biology of aggressive prostate cancer 17.
Our study has potential limitations that merit review. First, there is the potential for misclassification of grade and stage, given that only a subset of men in our study underwent radical prostatectomy, the gold standard for obtaining accurate stage and grade. As shown in Table 3, we attempted to control for this potential shortcoming by analyzing only patients with post-prostatectomy pathologic data, and we found that the results were very similar to the analysis that included all patients, suggesting that misclassification bias minimally affected the results. Second, the SEER database does not report comorbidity measures, and the effects of comorbidity on OS were unmeasured. However the most significant results of this analysis are the opposite of what might be expected with increasing comorbidity associated with age, as younger men had equivalent or worse outcomes compared to older men. If we had been able to adjust for co-morbidity, it would likely have increased the magnitude of the hazard ratios associated with the youngest age group. Second, the group of patients with T4 and higher disease is heterogeneous, and that heterogeneity might lead to imbalances between the different age groups. In a secondary analysis, the dataset demonstrates that the number of men with node positive disease by age group was: 8.1% for ages 35-44, 6.1% for ages 45-54, 6.9% for ages 55-64, and 8.4% for ages 65-74. There does not appear to be a bias for a greater proportion of node-positive patients in the youngest men. The grouping of T4 patients with those with node-positive disease increases the heterogeneity of the group, but given that the large majority of men with T4 disease are likely to harbor nodal metastases, this appeared to be a logical grouping. Another consideration is that the SEER database lacks stage and grade information for approximately 40% of patients. We elected to analyze only those patients for whom complete data was available as it appears unlikely that age of the patient would change the availability of clinical staging information. The amount of missing data was 43%, 40%, 38%, 38% for the men aged 35-44, 45-54, 55-64 and 65-74, suggesting no clear bias for selective lack of information based on age. As noted above, the analysis of the group as a whole and patients treated with prostatectomy demonstrate very similar results, suggesting that the missing data compromises the conclusions. Lastly, the majority of young men diagnosed with prostate cancer are at low risk for cancer-specific mortality within 10 years of diagnosis, and the majority of men diagnosed with prostate cancer are 60 years of age or older; thus, the clinical applicability of these findings are limited to a defined, small, yet clinically relevant population of men.
The proportion of young men with prostate cancer will continue to rise with the current and evolving practices of screening and treatment. 18. The number of men with high grade cancer may also continue to increase, and finding ways to prevent cancer-related morbidity and mortality in young men, who will remain at risk for the greatest period of time, is critical. In this SEER based analysis, which reflects “real-world” practice, young men do not have a uniformly better cancer specific survival than older men, counter to most institutional series. Our finding that high grade cancer is associated with > 25% cancer related mortality at 10 years in men younger than 55 emphasizes the significant incidence of disease-related morbidity in this group of men. This data provides a strong argument for the need to consider multimodality therapy for young men with high risk disease and to support the ongoing neoadjuvant and adjuvant studies of systemic therapies which could improve the efficacy of local therapy and improve cure for these men. The paradoxical effect of very young age and high grade disease suggests the biology of prostate cancer in young men may be inherently different and may provide new insights into the development and behavior of the disease. More detailed studies of the clinical phenotype and molecular changes in primary tumors that develop in very young men might provide additional tools to improve screening in young men and answer the question of whether there are important biologic differences which could lead to different systemic and local treatment strategies in these men.
Acknowledgments
Support: VA Cooperative studies program (CSP 553) and Northwest Specialized Program of Research Excellence (P50 CA097186-06)
Footnotes
Financial disclosures: None
References
- 1.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57(4):242–55. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 2.Kotsis SV, Spencer SL, Peyser PA, Montie JE, Cooney KA. Early onset prostate cancer: predictors of clinical grade. J Urol. 2002;167(4):1659–63. [PubMed] [Google Scholar]
- 3.Khan MA, Han M, Partin AW, Epstein JI, Walsh PC. Long-term cancer control of radical prostatectomy in men younger than 50 years of age: update 2003. Urology. 2003;62(1):86–91. doi: 10.1016/s0090-4295(03)00404-7. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 4.Rosser CJ, Chichakli R, Levy LB, Kuban DA, Smith LG, Pisters LL. Biochemical disease-free survival in men younger than 60 years with prostate cancer treated with external beam radiation. J Urol. 2002;168(2):536–41. [PubMed] [Google Scholar]
- 5.Smith CV, Bauer JJ, Connelly RR, Seay T, Kane C, Foley J, et al. Prostate cancer in men age 50 years or younger: a review of the Department of Defense Center for Prostate Disease Research multicenter prostate cancer database. J Urol. 2000;164(6):1964–7. doi: 10.1016/s0022-5347(05)66929-7. [DOI] [PubMed] [Google Scholar]
- 6.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr, Amling CL, Elashoff D, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22(3):446–53. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 7.Loeb S, Hernandez DJ, Mangold LA, Humphreys EB, Agro M, Walsh PC, et al. Progression after radical prostatectomy for men in their thirties compared to older men. BJU Int. 2008;101(12):1503–6. doi: 10.1111/j.1464-410X.2008.07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39. [PubMed] [Google Scholar]
- 9.de la Rochefordiere A, Asselain B, Campana F, Scholl SM, Fenton J, Vilcoq JR, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341(8852):1039–43. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 10.Aebi S, Gelber S, Castiglione-Gertsch M, Gelber RD, Collins J, Thurlimann B, et al. Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet. 2000;355(9218):1869–74. doi: 10.1016/s0140-6736(00)02292-3. [DOI] [PubMed] [Google Scholar]
- 11.Freedland SJ, Presti JC, Jr, Kane CJ, Aronson WJ, Terris MK, Dorey F, et al. Do younger men have better biochemical outcomes after radical prostatectomy? Urology. 2004;63(3):518–22. doi: 10.1016/j.urology.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 12.Ryan CJ, Elkin EP, Cowan J, Carroll PR. Initial treatment patterns and outcome of contemporary prostate cancer patients with bone metastases at initial presentation: data from CaPSURE. Cancer. 2007;110(1):81–6. doi: 10.1002/cncr.22736. [DOI] [PubMed] [Google Scholar]
- 13.Kupelian PA, Reddy CA, Reuther AM, Mahadevan A, Ciezki JP, Klein EA. Aggressiveness of familial prostate cancer. J Clin Oncol. 2006;24(21):3445–50. doi: 10.1200/JCO.2006.05.7661. [DOI] [PubMed] [Google Scholar]
- 14.Roupret M, Fromont G, Bitker MO, Gattegno B, Vallancien G, Cussenot O. Outcome after radical prostatectomy in young men with or without a family history of prostate cancer. Urology. 2006;67(5):1028–32. doi: 10.1016/j.urology.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Tryggvadottir L, Vidarsdottir L, Thorgeirsson T, Jonasson JG, Olafsdottir EJ, Olafsdottir GH, et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J Natl Cancer Inst. 2007;99(12):929–35. doi: 10.1093/jnci/djm005. [DOI] [PubMed] [Google Scholar]
- 16.Edwards SM, Kote-Jarai Z, Meitz J, Hamoudi R, Hope Q, Osin P, et al. Two percent of men with early-onset prostate cancer harbor germline mutations in the BRCA2 gene. Am J Hum Genet. 2003;72(1):1–12. doi: 10.1086/345310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Gracia JL, Gloria Ruiz-Ilundain M, Garcia-Ribas I, Maria Carrasco E. The role of extreme phenotype selection studies in the identification of clinically relevant genotypes in cancer research. Cancer. 2002;95(7):1605–10. doi: 10.1002/cncr.10877. [DOI] [PubMed] [Google Scholar]
- 18.Kawachi MH, Bahnson RR, Barry M, Carroll PR, Carter HB, Catalona WJ, et al. Prostate cancer early detection. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2007;5(7):714–36. [PubMed] [Google Scholar]