Abstract
Histone deacetylases (HDACs) have been under intense scientific investigation for a number of years. However, only recently the unique class III HDACs, sirtuins, have gained increasing investigational momentum. Originally linked to longevity in yeast, sirtuins and more specifically, SIRT1 have been implicated in numerous biological processes having both protective and/or detrimental effects. SIRT1 appears to play a critical role in the process of carcinogenesis, especially in age-related neoplasms. Similarly, alterations in circadian rhythms as well as production of the pineal hormone melatonin have been linked to aging and cancer risk. Melatonin has been found act as a differentiating agent in some cancer cells and to lower their invasive and metastatic status. In addition, melatonin synthesis and release occurs in a circadian rhythm fashion and it has been linked to the core circadian machinery genes (Clock, Bmal1, Periods, and Cryptochromes). Melatonin has also been associated with chronotherapy, the timely administration of chemotherapy agents to optimize trends in biological cycles. Interestingly, a recent set of studies have linked SIRT1 to the circadian rhythm machinery through direct deacetylation activity as well as through the NAD+ salvage pathway. In this review, we provide evidence for a possible connection between sirtuins, melatonin, and the circadian rhythm circuitry and their implications in aging, chronomodulation and cancer.
Keywords: Sirtuins, circadian rhythm, melatonin, cancer, aging, chronotherapy
Introduction
Studies in recent years have begun to unravel the link between various biological processes and sirtuins such as SIRT1. In 1979, Klar and colleagues [1] discovered the first sirtuin gene (currently known as SIR2) in Saccharomyces cerevisiae and named it MAR1 (mating-type regulator 1). They reported that a spontaneous mutation in MAR1 caused sterility as it relieved silencing at the mating-type loci HMR and HML [1]. Later, a variety of additional mutations with sterile phenotypes were discovered by Rine and colleagues [2,3] and the nomenclature of silent information regulator (SIR) 1–4 was given to the set of the four genes responsible. SIR2 was found to suppress recombination between the 100–200 copies of the ribosomal RNA genes repeated in tandem on chromosome XII as well as play a role in silencing genes near telomeres [4,5]. Later studies found that SIR2 inhibits histone deacetylation [6]. Soon after these novel discoveries, SIR2 homologues were reported to be present in many organisms from yeast to humans [7]. At present, sirtuins are being investigated for their possible roles in a number of physiological processes. Recent studies have focused on deciphering the role of SIRT1, the most well-known sirtuin family member, in the process of carcinogenesis. Several studies have demonstrated a critical role of SIRT1 in the regulation of cell fate and the stress response in mammalian cells as it promotes cell survival by inhibiting apoptosis or cellular senescence induced by stresses including DNA damage or oxidative stress [2,8,9]. Only recently, ground-breaking research from several groups have suggested an important role for SIRT1 in circadian rhythms of biological systems as it was found to connect cellular metabolism to the circadian core clockwork machinery [10–13].
Several aspects of living systems (sleep-wake cycle, body temperature, metabolism, hormone synthesis, etc.) are regulated by the circadian clock machinery which depend on a network of genes and their periodic and rhythmic oscillations driven by the circadian timing system located in the suprachiasmatic nucleus (SCN) of the hypothalamus as well as peripheral oscillators located in most cells [14]. To regulate these rhythmic patterns, approximately 15 mammalian genes are synchronized to control 8–10% of the genes expressed in each tissue in a distinct fashion [14]. Briefly, the circadian clock machinery is composed of a central pacemaker which acts to synchronize the clock component genes including Clock, Bmal1, Periods (Pers), and Cryptochromes (Crys) [15]. A number of these genes have also been found to be altered in several cancers [16–18]. Further, it has been demonstrated that the transcriptional activator, CLOCK, contains histone acetyltransferase (HAT) activity as it was found to acetylate histone H3 and its dimerization partner BMAL1 at lysine 537 [19]. This suggested that CLOCK may regulate circadian rhythm genes through chromatin remodeling. In addition, recent studies have shown that SIRT1 regulates the CLOCK:BMAL1 complex [10–13].
Interestingly, melatonin, a pineal hormone in mammals, has a well-defined circadian rhythm of production with an approximate 24 hour oscillatory pattern. Serum melatonin levels have been found to be high at night (80–120pg/ml) and low during the day (2–20pg/ml) [20]. Studies suggest that reduced melatonin levels due to the exposure to light-at-night (LAN) is linked to an increased risk for several cancers including breast, endometrium, colon, prostate, non-Hodgkin lymphoma, etc. [21–25]. Melatonin, alone or in combination with other agents, has been shown to have chemopreventive, oncostatic and tumor inhibitory effects in a variety of in vitro and in vivo experimental models of neoplasia [26–32]
Recently, chronodisruption, “the maladjustment of time,” has become a topic of great interest. Chronodisruption, alterations in the temporal organization of order of biological rhythmicity over days and seasons [33], may predispose a person's risk for cancer [30,34,35]. In addition, melatonin functions as an endogenous messenger of biological time and may be critical in regulating the biological clock [35,36]. Therefore, chronotherapy, a novel and logical means to administer drugs which optimizes the timing of drug availability according to the circadian rhythm of its anti-cancer efficacy while limiting the effects on normal cells, has gained a great deal of attention. A number of rodent and clinical trials have found that precise timing of a number of chemotherapy drugs increases the anti-tumor activity as well as reducing the unwanted associated side-effects [34].
Thus, the connection between aging, sirtuins, clock genes and melatonin is beginning to be unraveled. Based on available scientific evidence and rational thinking, we have recently proposed that SIRT1 could be a critical player in age-related neoplasms and its modulation may resynchronize the deregulated core clock circuitry at the cellular level which may have implications in cancer management [37–39].
Aging Connection of Cancer
Within the last century the mean life expectancy of humans in the industrialized world has increased dramatically and it is predicted that by 2050 about 5% of the population in developed countries will be older than 85 years of age [40]. Unfortunately, aging is also linked to biological wear and tear leading to a variety of conditions including certain types of neoplasms. Thus, the risk for developing several types of cancers increases with increasing age. An explanation for this elevated cancer risk in older individuals has been attributed to the fact that progenitor cells from mature organisms accumulate molecular lesions which eventually evade the homeostatic control culminating into neoplastic situations [41]. A number of abnormal epigenetic signals also contribute to tumorigenesis such as DNA methylation and histone modifications [42]. Other biological changes of aging that may support cancer development include proliferative senescence which may result in the loss of apoptosis and the production of tumor growth factors and proteolytic enzymes that promote the growth and the spread of cancer [43].
Another contributing factor is believed to be the functionality of the immune system as it influences not only the tumor's microenvironment, but the tumor itself [44]. Immunosenescence, or the decline in immune function with age, appears to co-exist with tumor formation, but its relevance has been under debate. On one hand, immunosenescence has been associated with increased levels of interleukin-6 which is linked to tumors that elicit an immune response such as lymphomas and multiple myelomas. On the other hand, immunosenescence has also been suggested to create an environment that favors a less aggressive tumor which may be why the incidence of cancer levels off after a certain age [44]. Additionally, one study found fewer tumor-infiltrating lymphocytes in tumor samples from older women compared to younger subjects, indicative of a reduced immune response; on the other hand a more robust immune response might drive tumor progression due to growth or angiogenic factors secreted from immune cells [45,46] Further, chronic inflammation has been reported to precede or accompany a number of cancers, adding further controversy [47]. Although a definitive conclusion has yet to be drawn, strong evidence exists to suggest the activity level of the immune system plays a critical role in carcinogenesis.
The median age for cancer diagnosis in industrialized countries is approaching 70 years and it is expected to increase. The absolute number of cancer patients younger than 50 years of age is not expected to rise significantly over the next 50 years, but is expected to double in those over the age of 65 [48]. Because of these striking statistics, it is believe that a direct correlation between cancer risk and aging exists. An excellent example of an age-related neoplasm is prostate cancer (PCa). According to the Center for Disease Control and Prevention (CDC), PCa is one of the most common forms of cancer and is a leading cause of cancer deaths among men in the United States. In 2009, 192,280 new cases of PCa are expected to be diagnosed and 27,360 PCa related deaths will occur [49]. Further, according to recent estimates from the CDC, approximately 62% of all PCa cases are diagnosed in males 65 and older. Thus, it is now generally well accepted that aging is a major risk factor for PCa in addition to race and family history. Further, a number of cancer types such as acute myeloid leukemia, breast, non-small cell lung cancer, and ovarian cancers tend to become resistant to chemotherapy and/or more indolent with increasing age [48]. Other cancers with dramatically increased risk with increasing age include non-melanomatous skin cancer, Non-Hodgkin's lymphoma, and malignant brain tumors [44]. Because of these striking statistics, in the recent past, researchers have expended enormous effort to understand the mechanisms that connect aging with cancer. Such information could be immensely useful in development of novel strategies to manage age-related neoplasms.
Sirtuin Connection to Aging
As mentioned earlier, sirtuins, class III histone deacetylases (HDAC) have been shown to have a link with longevity [2,8,50,51]. Sirtuins are phylogenetically conserved from archeobacteria to humans and contain a catalytic domain of about 275 amino acids accompanied by unique additional N-terminal and/or C-terminal sequences of variable length [2]. Sirtuins utilize nicotinamide adenine dinucleotide (NAD+) to remove acetyl groups from various targets and transfer them to the 2'-OH of nicotinamide ribose, yielding 2”-O-acetyl-ADP ribose. The nicotinamide ribosyl bond is then cleaved to add a molecule of water to nicotinamide ribose [51]. Of the sirtuin family members, SIRT1 is the most well studied and has been shown to participate in a number of processes including apoptosis, senescence, lipid and glucose homeostasis, insulin secretion and axonal degradation ([2]; Table 1). Consistent with its diverse biological functions, SIRT1 has plentiful downstream targets including various FOXO factors, PGC-1α, androgen receptor (AR), p53, Ku70, NFκB and HES1 [52–61].
Table 1.
Localization and functions of sirtuins*
| Member | Activity | Localization | Size (kDa) | Function |
|---|---|---|---|---|
| Sirt1 | DAC | Nucleus | 62 | Chromatin modulation, gene expression, senescence, apoptosis, insulin secretion, neuronal differentiation, adipogenesis, development, glucose metabolism |
| Sirt2 | DAC and ART | Cytoplasmic | 41.5 | Cell cycle progression, adipocyte differentiation |
| Sirt3 | DAC and ART | Mitochondria | 43.6 | Cellular metabolism, apoptosis |
| Sirt4 | ART | Mitochondria | 35.2 | Regulation of insulin secretion, mitochondrial NAD(+) salvage |
| Sirt5 | DAC | Mitochondria | 33.9 | Regulation of urea cycle |
| Sirt6 | ART | Nucleus | 39.1 | Telomere maintenance, DNA repair |
| Sirt7 | None | Nucleolus | 44.8 | rDNA transcription |
There are seven reported sirtuin enzymes in mammals that act as either a mono-ADP-ribosyl transferase (ART), a NAD+-dependent deacetylase (DAC), or both. Although each sirtuin enzyme has an NAD+-dependent catalytic core domain, the N-and/or C-terminal sequences are of variable lengths giving rise to specific sizes. Each sirtuin has a specific localization and function.
The other members of the sirtuin family, SIRT 2 – 7, also play important roles in cell cycle control, metabolism, DNA repair, and rDNA transcription (Table 1) [2,51]. SIRT2 is predominantly cytoplasmic, SIRT3, 4, and 5 are mainly mitochondrial proteins and SIRT6 and 7 are present in the nucleus and nucleolus, respectively [2,51]. The sirtuin family of enzymes is unique and displays a broad range of biological functionality and distribution. To study the function of sirtuins in vivo, mice have been genetically modified. SIRT1 knockout mice are reported to be smaller in size, sterile, and have developmental defects of the retina and heart. In addition they display hyperacetylation of p53, defects in telomere length, and frequent prenatal and early postnatal death [62,63]. Interestingly, a recent study reported the number of intestinal polyps induced in mice carrying the Apcmin mutation were unaffected in SIRT1 knockout mice. Further, the same study found that in a classical two-stage carcinogensis protocol, the presence or absence of SIRT1 had no effect on the incidence or tumor load of skin papillomas [64]. The phenotype of genetically modified SIRT2 mice has not been reported. SIRT3 knockout mice are viable and fertile with no gross phenotypic abnormalities, but display mitochondrial hyperacetylation [65]. Mice lacking SIRT4 and SIRT5 have been shown to be viable and fertile. SIRT4 knockout mice show elevated mitochondrial GDH activity whereas SIRT5 knockout mice exhibit defects in the urea cycle [66]. Both of them fail to display mitochondrial hyperacetylation, but possess increased pancreatic glutamate dehydrogenase activity [65]. SIRT6 knockout mice are smaller in size with severe metabolic defects, and often succumbed prematurely to profound hypoglycaemia [66,67]. The phenotypes associated with SIRT7 knockout mice include decreased mean and medial lifespan, heart hypertrophy and inflammatory cardiomyopathy [68]. The associated phenotype of each specific sirtuin knockout mouse model aids in the discovery of their probable regulatory function(s).
SIRT1 Connection of Cancer
Originally discovered as a longevity factor (SIR2) in yeast, SIRT1 is now believed to play important role in development of certain cancers. We recently demonstrated that SIRT1 was over-expressed in human PCa cells and PCa tissue from patients, compared to normal prostate epithelial cells (PrEC) and adjacent normal prostate tissues, respectively [38]. Furthermore, chemical inhibition (by nicotinamide or sirtinol) as well as genetic knockdown (via short-hairpin shRNA mediated RNA interference) of SIRT1 was found to result in an inhibition of cell growth and viability in human PCa cells, but not in normal PrEC. Additionally we observed that the inhibition of SIRT1 resulted in an increase in acetylation and transcriptional activation of FOXO1 [38]. In a follow-up study, we also found that SIRT1 inhibition may have different downstream targets in cells with active p53 were it induces senescence, versus cells where p53 is inactive and in which it induces apoptosis [39]. Studies from other groups have reported similar findings in other cancer types [54,63,69–76]. A recent study conducted by Lara et al. [74] found that salermide, a reverse amide and a strong inhibitor of SIRT1 and SIRT2, induced apoptosis in a variety of human cancer cell lines, while having no effect in normal cells. This study also demonstrated that salermide's pro-apoptotic effects were due to the reactivation of pro-apoptotic genes which were epigenetically repressed by SIRT1 [74]. In another study, it was reported that SIRT1-deficient cells exhibited p53 hyperacetylation after DNA damage and increased ionizing radiation-induced thymocyte apoptosis in vivo [63]. Kojima and colleagues [69] found that treatment of various PCa cell lines with sirtinol (a chemical SIRT1 inhibitor) or shRNA directed toward SIRT1, resulted in a reduction in cell growth and an increased sensitivity to camptothecin and cisplatin. Sun et al. [75] reported that downregulation of SIRT1 using antisense oligonucleotides reduced the survival and increased radiation-induced anti-proliferation in human lung cancer cells.
In addition to the studies which examined events downstream of SIRT1, a few have looked at the upstream regulation of SIRT1 in various cancer model systems. Zhao and colleagues [73] found that DBC1 (deleted in breast cancer-1) promotes p53-mediated apoptosis through specific inhibition of SIRT1. Another study by Chen and colleagues [76] demonstrated that the tumor suppressor hypermethylated in cancer-1 (HIC-1) forms a transcriptional repression complex with SIRT1, which inhibits SIRT1 transcription. HIC-1 becomes epigenetically inactive in many cancers resulting in the upregulation of SIRT1 followed by the deacetylation and inactivation of p53, allowing for the bypass of apoptosis [76]. Interestingly, a number of studies have suggested that SIRT1 functions as a tumor suppressor [77–81]. Yuan and colleagues [77] have documented that c-MYC binds to the SIRT1 promoter to induce SIRT1 expression, but SIRT1 in turn then deacetylates c-MYC, resulting in a decrease in c-MYC stability. Further, they found that the reduction of c-MYC stability resulted in c-MYC's transformational capability being compromised in the presence of SIRT1. In another study, Firestein and colleagues [78] have found that ectopic induction of SIRT1 in a β-catenin-driven mouse model of colon cancer significantly reduced tumor formation, proliferation, and animal morbidity. These authors also found an inverse correlation between nuclear SIRT1 and the oncogenic form of β-catenin in human colon tumor specimens [78]. Moreover, Wang et al. [79] demonstrated that breast cancer-1 (BRCA1) binds to the SIRT1 promoter causing an increase in SIRT1 expression, followed by an inhibition of Survivin, which ultimately resulted in a greater inhibitory effect on BRCA1 mutant cancer cells than on BRCA1-wild-type cancer cells both in vitro and in vivo.
Collectively, the results of the studies summarized have shown both pro-proliferative as well as anti-proliferative function of SIRT1. The reason for the observed distinct and sometimes opposite functions of SIRT1 is not well understood. This may, however, be attributed to the complexity of the interplay that SIRT1 has on a number of processes or to the genetic differences among the model systems studied.
Circadian Rhythm: Its Regulation and Cancer Connection
Circadian rhythms, in the biochemical, physiological or behavioral processes of organisms, are rhythmic time periods that are repeated at approximately 24 hour intervals. Endogenously generated, circadian rhythms can also be modulated by external factors, the most important being daylight. On an organismal level, circadian rhythms allow organisms to anticipate and respond to precise and regular environmental changes. The basic regulatory system of circadian rhythms are evolutionally conserved from cyanobacteria to mammals and in most species these functions are observed in many critical physiological functions, including metabolism, cell growth, and immune responses [82].
Mammalian circadian rhythms are controlled by a number of metabolic and physiological components as well as certain genes, often collectively termed `clock genes', which appear to play a critical role in controlling the central circadian rhythm apparatus. This apparatus is composed of a pacemaker in the SCN of the brain which acts to synchronize clock component genes, including Clock, Bmal1, Period (Per), and Cryptochrome (Cry). Many peripheral cells also contain the circadian clock circuitry which can function on their own or in concert with the central SCN circuitry. Clock and Bmal1 genes encode PAS helix-loop-helix transcription factors which complex and bind E-box elements at the promoter regions of Per1–3 and Cry1–2 to induce their transcription as well as a number of other clock-controlled genes (CCGs). The expression of CCGs can be accomplished directly or indirectly through the activity of other non-circadian transcription factors containing E-boxes [83]. Interestingly, the transcription of Per1–3 and Cry1–2 act in a negative feedback loop to inhibit the transcriptional activity of the CLOCK:BMAL1 complex [15]. This is a securely regulated feedback loop that controls multiple aspects of life.
The same mechanisms that regulate the core clock components in the SCN are operative in peripheral tissues. It has been suggested that these two regulatory units are similar as they both are intracellular and rely on cycles of transcription, translation, protein modification and degradation [84]. Increasing evidence has suggested that altered circadian rhythm regulation plays a critical role in carcinogenesis. For example, mice with a deletion mutation in Per2 have a shorter circadian period [18] and an enhanced susceptibility to radiation-induced malignant lymphoma [16]. Another study has shown that the gene expression of Per1 and Per2 failed to maintain circadian rhythms in tumors in C3HFeJ/HeB mice with transplanted syngeneic mammary tumor [17]. Furthermore, studies have found that overexpression of mPER2 induced apoptosis in the mouse Lewis lung carcinoma cell line (LLC) and the mammary carcinoma cell line (EMT6), but not normal (NIH 3T3) cells [85]. In addition, overexpression of mPER2 in human pancreatic carcinoma cells has been shown to posses, i), growth inhibitory and pro-apoptotic effects in human pancreatic cancer cells, and, ii), synergistic growth inhibitory response when used in combination with cisplatin [86]. Chen and colleagues [87] observed that 95% of human breast tumors display no or dysregulated levels of PER1 and PER2 compared with adjacent normal cells. Other reports documented a link between circadian rhythm genes and sex steroids. Chu et al. [88] reported an association between Per3 variants and elevated serum levels of IGF-1 and variants of NPAS2, Per1, and CSNK1E were associated with increased levels of serum androgens. Another study from the same group demonstrated a significant 1.7-fold increase in PCa risk with a variant in Cry2 and this risk was increased to 4.1-fold in those men who also had greater insulin resistance [89]. In addition, a recent study by Hoffman and colleagues has suggested, based on both genetic association and functional analyses, that the circadian gene CRY2 may play an important role in non-Hodgkin lymphoma development [90]. Overall, numerous scientific studies have provided abundant evidence linking the circadian rhythm machinery to cancer development and progression, but this area of research still needs further intensive investigation.
SIRT1 Connection to Circadian Rhythms
Based on the results of a number studies, in a recent hypothesis paper, we theorized that that SIRT1 inhibition will impart an antiproliferative response in age-related cancers via resynchronization of deregulated core clock circuitry at the cellular level [38]. This hypothesis is based on and supported by several recent observations. In a set of recently published studies, SIRT1 was shown to function as a HDAC which counteracts the activity of the clock machinery. Asher et al. [10] reported that SIRT1 is required for the circadian transcription of Bmal1, Rorγ, Per2 and Cry1. In addition they found that SIRT1 bound to the CLOCK:BMAL1 complex in a circadian fashion and promoted the deacetylation and subsequent degradation of PER2. Nakahta et al. [11] observed that SIRT1 bound to the CLOCK:BMAL1 complex at circadian promoters and deacetylated BMAL1 at Lys537. Further, they claimed that inhibition of SIRT1 activity led to significant disturbances in the circadian cycle and acetylation of histone 3 (H3) and BMAL1 [11]. Finally, they reported that the HDAC activity of SIRT1 is regulated in a circadian manner both in vitro and in vivo [11]. Together, these observations suggest that the physical interaction between CLOCK/BMAL1/SIRT1 controls physiological functions at the circadian rhythm level.
Due to the dependency of SIRT1 on NAD+ which acts as a cofactor for its enzymatic activity, SIRT1 was hypothesized to be a link between metabolic activity and genome stability [91]. Therefore, a second set of studies recently investigated the circadian control of the NAD+ salvage pathway by the CLOCK:SIRT1 complex. Nakahata et al. [12] reported that intracellular NAD+ levels cycle with a 24 hour rhythm and that the CLOCK:BMAL1 complex regulates the circadian expression of NAMPT (nicotinamide phophoribosyltransferase), the rate-limiting step enzyme in the NAD+ salvage pathway [12]. Moreover, Ramsey and colleagues [13] reported similar findings and also demonstrated that inhibition of NAMPT promoted the oscillation of the clock gene Per2 by releasing CLOCK:BMAL1 from SIRT1 suppression. Collectively, the findings reported suggest that the oscillating biosynthesis of NAD+ constitutes a feedback loop that integrates the circadian clock with metabolism [12,13,92].
Melatonin Connection of Circadian Rhythm
Melatonin (N-acetyl-5-methoxytryptamine) is a small lipophilic molecule synthesized in humans primarily in the pineal gland, but also is produced in the retina, extraorbital lacrimal gland, Harderian gland, gastrointestinal tract, blood platelets, and bone marrow cells [22,93,94]. Melatonin signaling presumably influences all cells in the organism with one purpose being to convey circadian and seasonal information on the timing and duration of environmental photoperiods [95]. The multiple mechanism of actions of melatonin include, (i), signaling through G-protein coupled receptors (MT1 and MT2) to decrease the linoelic acid (LA) uptake, (ii), inducing QR2 (a detoxifying enzyme), (iii) functioning as a scavenger of reactive oxygen and reactive nitrogen species, (iv), increasing calmodulin degradation, (v), binding to nuclear receptors (RZR/RORα and RZRβ) to alter transcription of target genes, and, (vi), as a modulator of hemopoiesis and immune cell production and function [29,96,97]. Melatonin is principally secreted at night and is deemed the “chemical expression of darkness” because of specialized photoreceptive cells in the retina detect light and suppress its production [98–100]. More importantly its synthesis displays a circadian pattern that is generated by a primary circadian clock located in the SCN of the hypothalamus region of the brain [29,101]. Specifically, melatonin is synthesized from tryptophan under the control of various enzymes that are inhibited by light and stimulated by dark [29,93,99].
Since melatonin receptors are present in the SCN and because melatonin production in mammals exhibits a circadian rhythm, very early it was speculated that the day:night rhythm of melatonin production may be associated with the circadian rhythm machinery. Nevertheless, rather few studies have investigated the association between melatonin and the circadian rhythm clock components. Torres-Farfan et al. [102] assessed the patterns of clock gene proteins in melatonin-proficient mice (C3H) versus melatonin-deficient mice (C57BL). This study found PER1-, CRY2- and BMAL1-protein levels to be consistently lower in the adrenal cortex of C57BL mice compared to the adrenal cortex of C3H mice. In addition, a study of primary neuronal cultures prepared from striatum found that melatonin reduced the expression of PER1 and CLOCK, but had no effect on BMAL1 and these effects were reversed by a mutation in the MT1 receptor [103]. Another study evaluated the response of melatonin, among other things, to light pulses in mice with a ClockΔ19 mutation. In ClockΔ19 mice, an A to T transversion causes the elimination of 51 amino acids in the COOH-terminal glutamine-rich region of the CLOCK protein, rendering the protein inactive [104]. These mice were then bred with CBA mice to produce melatonin synthesizing CLOCK null mice. The authors found that these animals have endogenous rhythmic melatonin patterns that can be altered by light despite producing a protein that fails to initiate the transcription of Clock and other clock-controlled genes [104]. More recently, Zeman et al. [105] investigated the effects of rhythmic melatonin administration on clock gene expression in hypertensive TGR(mRen2)27 rats. They found melatonin to have a phase-dependent effect on clock gene expression in the heart as it, (i), reduced the expression of Per2 during the dark phase and increased the expression of Per2 during the light phase and, (ii), decreased Bmal1 expression during the light phase while increasing Bmal1 expression during the dark phase. They suggested that exogenous melatonin, applied during the dark period, results in strong synchronization of Per2 and Bmal1 expression in the heart [105]. The data from these investigations suggest that melatonin may be involved in the control of certain clock genes, but the exact mechanisms have yet to be elucidated.
To date, a limited number of studies have investigated the relationship between SIRT1 and melatonin. Gutierrez-Cuesta et al. [106] examined the effects of melatonin on pro-survival processes in senescence-accelerated mice (SAMP8) and senescence-accelerated resistant mice (SAMR1). The mice were give melatonin (10mg/kg) supplementation in the drinking water from the end of the first month of age until the end of the ninth month. These workers found that melatonin increased the protein levels of SIRT1 and subsequently decreased the levels of acetylated p53 and acetylated NFκB in SAMP8 mice, but the effects of melatonin on SAMR1 mice were not reported. The authors suggested that melatonin suppressed oxidative stress in SAMP8 mice allowing SIRT1 levels to increase to the levels observed in SAMR1 mice [106]. Based on this study, the authors concluded that melatonin improves pro-survival signals and reduces pro-apoptotic processes via alterations in α-secretase, amyloid-β aggregates, Bid, and Bcl-2XL. Cleary, little information is available regarding the effects of melatonin on SIRT1 and systematic studies are needed in this area.
Melatonin's Connection with Aging and Cancer
Like SIRT1, melatonin production has an association with aging. As animals including humans age, their ability to retain the night-day regulation of melatonin synthesis begins to deteriorate such that in old animals and elderly humans the day:night differences in pineal melatonin production and circulating melatonin levels are severely dampened due to the compromised ability of the pineal to produce melatonin nightly. This is believed to be due to reduced adrenergic innervation as well as reductions in the β-adrenergic receptor number and density on the surface of the pinealocytes [95]. The weakening of many other circadian rhythms (sleep/wake cycle, core body temperature, performance, etc.) in the elderly, not only melatonin synthesis [20,107,108], are likely related to changes in neurons of the SCN and appear to be associated with increased cancer susceptibility [22].
As previously stated, aging is a risk factor for cancer. Interestingly it has been reported that reduced melatonin levels may also be a risk factor for some cancer types. In 1978, Cohen and associates [109] proposed that a reduction in pineal function (i.e., reduction in melatonin secretion), may induce a state of relative hyperestrogenism, with the early and prolonged exposure of the breast tissue to the estrogens being involved in the initiation of the breast carcinogenesis. This hypothesis was later supported by the outcomes of a number of epidemiological studies which suggested that a reduction in melatonin levels due to occupational conditions such as working the night shift resulted in an increase in the risk for breast, endometrial, colorectal, non-Hodgkin lymphoma and prostate cancer [22,24,25,35,110,111]. Bartsch and colleagues [112] analyzed the circadian rhythms of melatonin and 6-sulfatoxymelatonin (aMT6s) in serum and urine of young men, elderly patients with benign prostatic hyperplasia (BPH) and of patients of similar age with primary PCa. This study found that levels of melatonin were significantly depressed in PCa (40–60%) as compared to BPH or normal individuals, indicating that the reduction in serum melatonin in PCa is due to a reduced pineal activity.
Physiological and pharmacological concentrations of melatonin have been reported to have chemopreventive, oncostatic and tumor inhibitory effects in a variety of in vitro and in vivo experimental models of neoplasia [27–29,31,113–116]. For example, Petranka et al. [117] demonstrated an oncostatic role of melatonin and found that melatonin caused a 20–25% reduction in ovarian cancer cell number. Another study demonstrated that melatonin significantly inhibited estrogen receptor-positive endometrial cancer cell growth, but had no effect on those cells which were estrogen receptor negative [118]. Moreover, melatonin causes a significant growth inhibition of breast cancer (MCF-7) cells, which seems to be transforming growth factor beta 1 (TGFβ1)-dependent [115]. It also was reported that HepG2 human hepatocarcinoma cells are sensitive to the apoptotic effects of melatonin and the pro-apoptotic effects of melatonin were related to cytosolic cytochrome c release, upregulation of Bax, and induction of caspase-9 activity [32]. Anisimov et al. [113] used melatonin in an in vivo rat model to test whether it would inhibit intestinal carcinogenesis induced by 1,2-dimethylhydrazine (DMH). They found that melatonin reduced the number of tumors per rat in the ascending and descending colon when induced by DMH. Oral administration of melatonin via the drinking water was also reported to be effective in suppressing 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary tumor growth [119]. Finally, melatonin treatment induced apoptotic death in LNCaP cells via modulation of the p38 and JNK pathways [28]. A number of clinical studies have also investigated the effects of melatonin on certain cancer types (breast, non-small lung cancer, skin, kidney and solid tumors with brain metastases). These studies have suggested the usefulness of melatonin as a single agent as well as in an adjuvant setting to increase the efficacy and decrease the side effects of chemotherapeutic drugs [29]. There is, in fact, a vast literature documenting the ability of melatonin to reduce the toxicity of commonly-used cancer chemotherapeutic agents while at the same time possibly increasing their efficacy [120].
Melatonin, Circadian Rhythm Genes, and Cancer
Limited evidence has suggested that melatonin-mediated modulation of circadian rhythms may be associated with its anti-cancer properties. Recently, Otalora and colleagues [121] assessed whether body temperature, a circadian rhythm marker, is impaired by melanoma progression, and whether exogenous melatonin could restrict the tumor growth and restore circadian rhythmicity. In this study, C57 mice were subcutaneously inoculated with murine B16 melanoma cells and implanted with a temperature data logger. The animals were subjected to a 12:12 light:dark cycle or to continuous light, with or without melatonin supplementation in the drinking water (2mg/kg BW/day). The authors found that nocturnal melatonin administration increased the body temperature rhythm amplitude and improved phase stability, reduced melanoma tumor weight and prevented the tumor from disseminating. In addition, exposure to continuous light, which reduces the endogenous melatonin synthesis and alters circadian rhythmicity, caused a significantly increased tumor malignancy, reduced survival and free-running rhythms. Based on the outcome of this study, the authors suggested that melatonin due to it rhythm stabilizing actions and its anti-cancer effects, may be a useful therapy for melanoma [121]. Additional studies are required to further clarify the ability of melatonin to modulate the circadian rhythm system in various cancer models. The preliminary findings are consistent with the ability of melatonin to induce circadian synchronization which, in turn, may improve its (or other chemotherapeutic agents) ability to inhibit tumor progression.
Chronotherapy
A plethora of information has suggested that chronodisruption, a disturbance of the temporal organization or order of physiology, endocrinology, metabolism and behavior, contributes to development and/or progression of several cancer types including colorectal, breast, prostate and endometrial neoplasms [22,24,111]. Since disturbances of the circadian system have been shown to contribute to cancer risk, it seems likely that the proper timing of administering anti-cancer drugs and procedures should be more frequently considered when treating cancer patients. The goal of chronotherapy is to synchronize the time of drug administration with the interval of greatest cancer cell susceptibility and least toxicity to the chemotherapy [122]. The precise timing of drug administration takes advantage of the asynchronies in cell proliferation and drug metabolic rhythms between normal and malignant tissues, and in turn, minimizes the damage to host tissues and maximizes drug toxicity to tumors [123].
A number of situations can be exploited to increase the efficacy of chemotherapy. A few examples include the rhythm of tumor blood flow, the rhythmic release of cytokines and hematologic growth factors from tumors, as well as the periodicity of the cell cycle, all of which can be distinct from normal tissues or cells [34,123]. Chemotherapeutic drugs such as 5-fluorouracil (5-FU), floxuridine, doxorubicin, melphalan and cisplatin are best tolerated and achieve the greatest antitumor response when administered in a circadian fashion, at certain time of the day [34,124]. A number of clinical trials have tested the effectiveness of chronotherapy [124–128]. In one study of more than 2,000 patients with metastatic colorectal cancer, chronotherapy was shown to reduce drug toxicity, improve performance, and was two to eight-fold more effective than conventional therapy [125]. Rivard and colleagues [126] have found that chronotherapy increased the survival time of children with acute lymphoblastic leukemia. Moreover, in patients with advanced ovarian cancer, it was found that administration of doxorubicin or theprubicin at 06:00 hours followed by cisplatin between 16:00 and 20:00 hours resulted in significantly less severe hematological suppression and renal toxicity when compared to treatment given 12 hours apart [127,128]. The general goal of chronotherapy is to determine the most opportunistic time of administration of a drug, based on the stage of the circadian timing system, and treat the tumor more aggressively with larger and more frequent chemotherapy doses. In addition, the chromodulation method has been applied to radiotherapy and may also be used with immunotherapy [34,129].
In slow growing or well-differentiated tumors, circadian periodicity in cellular proliferation and metabolic activity is usually retained, but with reduced amplitude and occasional shift in phase. On the contrary, rapidly growing or advanced stage tumors have been found to lose circadian organization [34]. Thus, in addition to the anti-proliferative activities, melatonin may also serve as a marker of chronodisruption and aid in determining the proper chronotherapy regime. Further, it may be worthwhile to investigate the usefulness of chronomodulation and chronotherapy in cancer management in older patients who may have more disrupted circadian rhythma (versus relatively younger patients).
Concluding Remarks
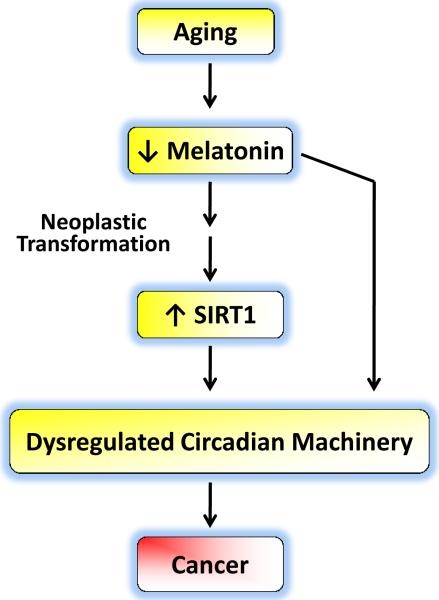
Unfortunately, one of the consequences of aging is an increased risk for cancer, but scientists are making great strides in not only uncovering the cause of this association, but also in exploiting that relationship for therapeutic benefits. One such link appears to be the sirtuin family of HDACs, which was first found to be associated with aging. SIRT1, the most studied of the group of sirtuins, has now been implicated in a number of pathways as well as in a number of biological processes including carcinogenesis. SIRT1 has also been linked to the regulation of a core set of circadian rhythm genes. Interestingly, reduced production of melatonin in mammals causes deregulation of the circadian rhythm machinery as well as an increasing risk for cancer. The implications of connecting sirtuins, aging, core clock components and melatonin are becoming evident (Fig. 1). Thus far, the literature has provided an excellent base on which to plan subsequent experiments. Investigators must continue their concerted efforts to unravel the molecular mechanisms connecting aging and cancer with a specific focus on SIRT1, melatonin and circadian rhythms. We believe that the payoffs from these efforts will be enormous for the management of age-related diseases including cancers.
Fig. 1.
Aging is associated with reduced levels of melatonin and various age-related neoplasms have elevated levels of SIRT1. The enhanced SIRT1 levels in several cancers occur at a time when melatonin levels are reduced. Reduced melatonin levels as well as increased SIRT1 activity in cancer cells may well lead to alterations in the biological clock causing a dysregulation of circadian rhythms thereby leading to uncontrolled cell cycle progression, finally culminating in cancer development. Thus, exogenous melatonin administration may lead to SIRT1 inhibition that could be responsible for melatonin's anti-cancer effects.
Acknowledgements
This work was supported in part by National Institutes of Health grants (T32ES00715 and 1F31AT005393).
References
- 1.KLAR AJ, FOGEL S, MACLEOD K. MAR1-a Regulator of the HMa and HMalpha Loci in SACCHAROMYCES CEREVISIAE. Genetics. 1979;93:37–50. doi: 10.1093/genetics/93.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MICHAN S, SINCLAIR D. Sirtuins in mammals: insights into their biological function. Biochem J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.RINE J, HERSKOWITZ I. Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 1987;116:9–22. doi: 10.1093/genetics/116.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.GOTTLIEB S, ESPOSITO RE. A new role for a yeast transcriptional silencer gene, SIR2, in regulation of recombination in ribosomal DNA. Cell. 1989;56:771–776. doi: 10.1016/0092-8674(89)90681-8. [DOI] [PubMed] [Google Scholar]
- 5.APARICIO OM, BILLINGTON BL, GOTTSCHLING DE. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;20:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 6.BRAUNSTEIN M, ROSE AB, HOLMES SG, et al. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- 7.BRACHMANN CB, SHERMAN JM, DEVINE SE, et al. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 8.LONGO VD, KENNEDY BK. Sirtuins in aging and age-related disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 9.YING W. NAD+ and NADH in cellular functions and cell death. Front Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 10.ASHER G, GATFIELD D, STRATMANN M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 11.NAKAHATA Y, KALUZOVA M, GRIMALDI B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NAKAHATA Y, SAHAR S, ASTARITA G, et al. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.RAMSEY KM, YOSHINO J, BRACE CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.ALBRECHT U. The mammalian circadian clock: a network of gene expression. Front Biosci. 2004;9:48–55. doi: 10.2741/1196. [DOI] [PubMed] [Google Scholar]
- 15.LEE C, ETCHEGARAY JP, CAGAMPANG FR, et al. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 16.FU L, PELICANO H, LIU J, et al. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 17.YOU S, WOOD PA, XIONG Y, et al. Daily coordination of cancer growth and circadian clock gene expression. Breast Cancer Res Treat. 2005;91:47–60. doi: 10.1007/s10549-004-6603-z. [DOI] [PubMed] [Google Scholar]
- 18.ZHENG B, LARKIN DW, ALBRECHT U, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400:169–173. doi: 10.1038/22118. [DOI] [PubMed] [Google Scholar]
- 19.DOI M, HIRAYAMA J, SASSONE-CORSI P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125:497–508. doi: 10.1016/j.cell.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 20.REITER RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 21.BLASK DE, DAUCHY RT, SAUER LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27:179–188. doi: 10.1385/ENDO:27:2:179. [DOI] [PubMed] [Google Scholar]
- 22.ERREN TC, REITER RJ. A generalized theory of carcinogenesis due to chronodisruption. Neuro Endocrinol Lett. 2008;29:815–821. [PubMed] [Google Scholar]
- 23.LAHTI TA, PARTONEN T, KYYRONEN P, et al. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123:2148–2151. doi: 10.1002/ijc.23566. [DOI] [PubMed] [Google Scholar]
- 24.REITER RJ, TAN DX, KORKMAZ A, et al. Light at night, chronodisruption, melatonin suppression, and cancer risk: a review. Crit Rev Oncog. 2007;13:303–328. doi: 10.1615/critrevoncog.v13.i4.30. [DOI] [PubMed] [Google Scholar]
- 25.VISWANATHAN AN, SCHERNHAMMER ES. Circulating melatonin and the risk of breast and endometrial cancer in women. Cancer Lett. 2009;281:1–7. doi: 10.1016/j.canlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BLASK DE, SAUER LA, DAUCHY RT. Melatonin as a chronobiotic/anticancer agent: cellular, biochemical, and molecular mechanisms of action and their implications for circadian-based cancer therapy. Curr Top Med Chem. 2002;2:113–132. doi: 10.2174/1568026023394407. [DOI] [PubMed] [Google Scholar]
- 27.GIRGERT R, HANF V, EMONS G, et al. Membrane-bound melatonin receptor MT1 down-regulates estrogen responsive genes in breast cancer cells. J Pineal Res. 2009;47:23–31. doi: 10.1111/j.1600-079X.2009.00684.x. [DOI] [PubMed] [Google Scholar]
- 28.JOO SS, YOO YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 29.JUNG B, AHMAD N. Melatonin in cancer management: progress and promise. Cancer Res. 2006;66:9789–9793. doi: 10.1158/0008-5472.CAN-06-1776. [DOI] [PubMed] [Google Scholar]
- 30.KUBO T, OZASA K, MIKAMI K, et al. Prospective cohort study of the risk of prostate cancer among rotating-shift workers: findings from the Japan collaborative cohort study. Am J Epidemiol. 2006;164:549–555. doi: 10.1093/aje/kwj232. [DOI] [PubMed] [Google Scholar]
- 31.LEON-BLANCO MM, GUERRERO JM, REITER RJ, et al. Melatonin inhibits telomerase activity in the MCF-7 tumor cell line both in vivo and in vitro. J Pineal Res. 2003;35:204–211. doi: 10.1034/j.1600-079x.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 32.MARTIN-RENEDO J, MAURIZ JL, JORQUERA F, et al. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 33.ERREN TC, REITER RJ. Defining chronodisruption. J Pineal Res. 2009;46:245–247. doi: 10.1111/j.1600-079X.2009.00665.x. [DOI] [PubMed] [Google Scholar]
- 34.MORMONT MC, LEVI F. Cancer chronotherapy: principles, applications, and perspectives. Cancer. 2003;97:155–169. doi: 10.1002/cncr.11040. [DOI] [PubMed] [Google Scholar]
- 35.STEVENS RG. Electric light causes cancer? Surely you're joking, Mr. Stevens. Mutat Res. 2009;682:1–6. doi: 10.1016/j.mrrev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 36.ERREN TC, REITER RJ, PIEKARSKI C. Light, timing of biological rhythms, and chronodisruption in man. Naturwissenschaften. 2003;90:485–494. doi: 10.1007/s00114-003-0468-6. [DOI] [PubMed] [Google Scholar]
- 37.JUNG-HYNES B, NIHAL M, ZHONG W, et al. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284:3823–3832. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.JUNG-HYNES B, AHMAD N. SIRT1 controls circadian clock circuitry and promotes cell survival: a connection with age-related neoplasms. FASEB J. 2009;23:2803–2809. doi: 10.1096/fj.09-129148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.JUNG-HYNES B, AHMAD N. Role of p53 in the anti-proliferative effects of Sirt1 inhibition in prostate cancer cells. Cell Cycle. 2009;8:1478–1483. doi: 10.4161/cc.8.10.8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.TULJAPURKAR S, LI N, BOE C. A universal pattern of mortality decline in the G7 countries. Nature. 2000;405:789–792. doi: 10.1038/35015561. [DOI] [PubMed] [Google Scholar]
- 41.FRAGA MF, AGRELO R, ESTELLER M. Cross-talk between aging and cancer: the epigenetic language. Ann N Y Acad Sci. 2007;1100:60–74. doi: 10.1196/annals.1395.005. [DOI] [PubMed] [Google Scholar]
- 42.KORKMAZ A, SANCHEZ-BARCELO EJ, TAN DX, et al. Role of melatonin in the epigenetic regulation of breast cancer. Breast Cancer Res Treat. 2009;115:13–27. doi: 10.1007/s10549-008-0103-5. [DOI] [PubMed] [Google Scholar]
- 43.HORNSBY PJ. Replicative senescence and cancer. Cancer Treat Res. 2005;124:53–73. doi: 10.1007/0-387-23962-6_3. [DOI] [PubMed] [Google Scholar]
- 44.ERSHLER WB. Cancer: a disease of the elderly. J Support Oncol. 2003;1:5–10. [PubMed] [Google Scholar]
- 45.KURTZ JM. How to predict the risk of local relapse in the preserved breast. Recent Results Cancer Res. 1996;140:263–72. doi: 10.1007/978-3-642-79278-6_29. 263–272. [DOI] [PubMed] [Google Scholar]
- 46.HADAR EJ, ERSHLER WB, KREISLE RA, et al. Lymphocyte-induced angiogenesis factor is produced by L3T4+ murine T lymphocytes, and its production declines with age. Cancer Immunol Immunother. 1988;26:31–34. doi: 10.1007/BF00199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.GONDA TA, TU S, WANG TC. Chronic inflammation, the tumor microenvironment and carcinogenesis. Cell Cycle. 2009;8:2005–2013. doi: 10.4161/cc.8.13.8985. [DOI] [PubMed] [Google Scholar]
- 48.BALDUCCI L, ERSHLER WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- 49.JEMAL A, SIEGEL R, WARD E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 50.MARMORSTEIN R. Structure and chemistry of the Sir2 family of NAD+-dependent histone/protein deactylases. Biochem Soc Trans. 2004;32:904–909. doi: 10.1042/BST0320904. [DOI] [PubMed] [Google Scholar]
- 51.SAUVE AA, WOLBERGER C, SCHRAMM VL, et al. The biochemistry of sirtuins. Annu Rev Biochem. 2006;75:435–465. doi: 10.1146/annurev.biochem.74.082803.133500. [DOI] [PubMed] [Google Scholar]
- 52.FRESCAS D, VALENTI L, ACCILI D. Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes. J Biol Chem. 2005;280:20589–20595. doi: 10.1074/jbc.M412357200. [DOI] [PubMed] [Google Scholar]
- 53.JEONG J, JUHN K, LEE H, et al. SIRT1 promotes DNA repair activity and deacetylation of Ku70. Exp Mol Med. 2007;39:8–13. doi: 10.1038/emm.2007.2. [DOI] [PubMed] [Google Scholar]
- 54.YANG Y, HOU H, HALLER EM, et al. Suppression of FOXO1 activity by FHL2 through SIRT1-mediated deacetylation. EMBO J. 2005;24:1021–1032. doi: 10.1038/sj.emboj.7600570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.BERESHCHENKO OR, GU W, DALLA-FAVERA R. Acetylation inactivates the transcriptional repressor BCL6. Nat Genet. 2002;32:606–613. doi: 10.1038/ng1018. [DOI] [PubMed] [Google Scholar]
- 56.BOURAS T, FU M, SAUVE AA, et al. SIRT1 deacetylation and repression of p300 involves lysine residues 1020/1024 within the cell cycle regulatory domain 1. J Biol Chem. 2005;280:10264–10276. doi: 10.1074/jbc.M408748200. [DOI] [PubMed] [Google Scholar]
- 57.BRUNET A, SWEENEY LB, STURGILL JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 58.KOBAYASHI Y, FURUKAWA-HIBI Y, CHEN C, et al. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 2005;16:237–243. [PubMed] [Google Scholar]
- 59.LANGLEY E, PEARSON M, FARETTA M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J. 2002;21:2383–2396. doi: 10.1093/emboj/21.10.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.LUO J, NIKOLAEV AY, IMAI S, et al. Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell. 2001;107:137–148. doi: 10.1016/s0092-8674(01)00524-4. %19. [DOI] [PubMed] [Google Scholar]
- 61.VAZIRI H, DESSAIN SK, NG EE, et al. hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–159. doi: 10.1016/s0092-8674(01)00527-x. %19. [DOI] [PubMed] [Google Scholar]
- 62.MCBURNEY MW, YANG X, JARDINE K, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23:38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.CHENG HL, MOSTOSLAVSKY R, SAITO S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.BOILY G, HE XH, PEARCE B, et al. SirT1-null mice develop tumors at normal rates but are poorly protected by resveratrol. Oncogene. 2009 doi: 10.1038/onc.2009.147. [DOI] [PubMed] [Google Scholar]
- 65.LOMBARD DB, ALT FW, CHENG HL, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.FINKEL T, DENG CX, MOSTOSLAVSKY R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.MOSTOSLAVSKY R, CHUA KF, LOMBARD DB, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 68.VAKHRUSHEVA O, SMOLKA C, GAJAWADA P, et al. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 69.KOJIMA K, OHHASHI R, FUJITA Y, et al. A role for SIRT1 in cell growth and chemoresistance in prostate cancer PC3 and DU145 cells. Biochem Biophys Res Commun. 2008;373:423–428. doi: 10.1016/j.bbrc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 70.OTA H, TOKUNAGA E, CHANG K, et al. Sirt1 inhibitor, Sirtinol, induces senescence-like growth arrest with attenuated Ras-MAPK signaling in human cancer cells. Oncogene. 2006;25:176–185. doi: 10.1038/sj.onc.1209049. [DOI] [PubMed] [Google Scholar]
- 71.WANG C, WANG MW, TASHIRO S, et al. Roles of SIRT1 and phosphoinositide 3-OH kinase/protein kinase C pathways in evodiamine-induced human melanoma A375-S2 cell death. J Pharmacol Sci. 2005;97:494–500. doi: 10.1254/jphs.fpj04055x. [DOI] [PubMed] [Google Scholar]
- 72.WANG C, CHEN L, HOU X, et al. Interactions between E2F1 and SirT1 regulate apoptotic response to DNA damage. Nat Cell Biol. 2006;8:1025–1031. doi: 10.1038/ncb1468. [DOI] [PubMed] [Google Scholar]
- 73.ZHAO W, KRUSE JP, TANG Y, et al. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.LARA E, MAI A, CALVANESE V, et al. Salermide, a Sirtuin inhibitor with a strong cancer-specific proapoptotic effect. Oncogene. 2009;28:781–791. doi: 10.1038/onc.2008.436. [DOI] [PubMed] [Google Scholar]
- 75.SUN Y, SUN D, LI F, et al. Downregulation of Sirt1 by antisense oligonucleotides induces apoptosis and enhances radiation sensitization in A549 lung cancer cells. Lung Cancer. 2007;58:21–29. doi: 10.1016/j.lungcan.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 76.CHEN WY, WANG DH, YEN RC, et al. Tumor suppressor HIC1 directly regulates SIRT1 to modulate p53-dependent DNA-damage responses. Cell. 2005;123:437–448. doi: 10.1016/j.cell.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 77.YUAN J, MINTER-DYKHOUSE K, LOU Z. A c-Myc-SIRT1 feedback loop regulates cell growth and transformation. J Cell Biol. 2009;20:203–211. doi: 10.1083/jcb.200809167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.FIRESTEIN R, BLANDER G, MICHAN S, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS ONE. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.WANG RH, ZHENG Y, KIM HS, et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol Cell. 2008;32:11–20. doi: 10.1016/j.molcel.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DAI Y, NGO D, FORMAN LW, et al. Sirtuin 1 is required for antagonist-induced transcriptional repression of androgen-responsive genes by the androgen receptor. Mol Endocrinol. 2007;21:1807–1821. doi: 10.1210/me.2006-0467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.FU M, LIU M, SAUVE AA, et al. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol. 2006;26:8122–8135. doi: 10.1128/MCB.00289-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.SHIMBA S, WATABE Y. Crosstalk between the AHR signaling pathway and circadian rhythm. Biochem Pharmacol. 2009;77:560–565. doi: 10.1016/j.bcp.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 83.GACHON F, OLELA FF, SCHAAD O, et al. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4:25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 84.GERY S, KOEFFLER HP. The role of circadian regulation in cancer. Cold Spring Harb Symp Quant Biol. 2007;72:459–464. doi: 10.1101/sqb.2007.72.004. [DOI] [PubMed] [Google Scholar]
- 85.HUA H, WANG Y, WAN C, et al. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97:589–596. doi: 10.1111/j.1349-7006.2006.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.ODA A, KATAYOSE Y, YABUUCHI S, et al. Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res. 2009;29:1201–1209. [PubMed] [Google Scholar]
- 87.CHEN ST, CHOO KB, HOU MF, et al. Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis. 2005;26:1241–1246. doi: 10.1093/carcin/bgi075. [DOI] [PubMed] [Google Scholar]
- 88.CHU F, CHOU PM, ZHENG X, et al. Control of multidrug resistance gene mdr1 and cancer resistance to chemotherapy by the longevity gene sirt1. Cancer Res. 2005;65:10183–10187. doi: 10.1158/0008-5472.CAN-05-2002. [DOI] [PubMed] [Google Scholar]
- 89.CHU LW, ZHU Y, YU K, et al. Variants in circadian genes and prostate cancer risk: a population-based study in China. Prostate Cancer Prostatic Dis. 2008;11:342–348. doi: 10.1038/sj.pcan.4501024. [DOI] [PubMed] [Google Scholar]
- 90.HOFFMAN AE, ZHENG T, STEVENS RG, et al. Clock-cancer connection in non-Hodgkin's lymphoma: a genetic association study and pathway analysis of the circadian gene cryptochrome 2. Cancer Res. 2009;69:3605–3613. doi: 10.1158/0008-5472.CAN-08-4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.GRIMALDI B, NAKAHATA Y, KALUZOVA M, et al. Chromatin remodeling, metabolism and circadian clocks: the interplay of CLOCK, and SIRT1. Int J Biochem Cell Biol. 2009;41:81–86. doi: 10.1016/j.biocel.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 92.WIJNEN H. Circadian rhythms. A circadian loop asSIRTs itself. Science. 2009;324:598–599. doi: 10.1126/science.1174132. [DOI] [PubMed] [Google Scholar]
- 93.HARDELAND R. Melatonin, hormone of darkness and more: occurrence, control mechanisms, actions and bioactive metabolites. Cell Mol Life Sci. 2008;65:2001–2018. doi: 10.1007/s00018-008-8001-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.REITER RJ, PAREDES SD, MANCHESTER LC, et al. Reducing oxidative/nitrosative stress: a newly-discovered genre for melatonin. Crit Rev Biochem Mol Biol. 2009;44:175–200. doi: 10.1080/10409230903044914. [DOI] [PubMed] [Google Scholar]
- 95.BARTSCH C, BARTSCH H, FLUCHTER SH, et al. Diminished pineal function coincides with disturbed circadian endocrine rhythmicity in untreated primary cancer patients. Consequence of premature aging or of tumor growth? Ann N Y Acad Sci. 1994;719:502–25. doi: 10.1111/j.1749-6632.1994.tb56855.x. 502–525. [DOI] [PubMed] [Google Scholar]
- 96.PEYROT F, DUCROCQ C. Potential role of tryptophan derivatives in stress responses characterized by the generation of reactive oxygen and nitrogen species. J Pineal Res. 2008;45:235–246. doi: 10.1111/j.1600-079X.2008.00580.x. [DOI] [PubMed] [Google Scholar]
- 97.TAN DX, MANCHESTER LC, TERRON MP, et al. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42:28–42. doi: 10.1111/j.1600-079X.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 98.JASSER SA, BLASK DE, BRAINARD GC. Light during darkness and cancer: relationships in circadian photoreception and tumor biology. Cancer Causes Control. 2006;17:515–523. doi: 10.1007/s10552-005-9013-6. [DOI] [PubMed] [Google Scholar]
- 99.REITER RJ. Melatonin: the chemical expression of darkness. Mol Cell Endocrinol. 1991;79:C153–C158. doi: 10.1016/0303-7207(91)90087-9. [DOI] [PubMed] [Google Scholar]
- 100.VAN GELDER RN. Non-visual photoreception: sensing light without sight. Curr Biol. 2008;18:R38–R39. doi: 10.1016/j.cub.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 101.PANDI-PERUMAL SR, SRINIVASAN V, MAESTRONI GJ, et al. Melatonin: Nature's most versatile biological signal? FEBS J. 2006;273:2813–2838. doi: 10.1111/j.1742-4658.2006.05322.x. [DOI] [PubMed] [Google Scholar]
- 102.TORRES-FARFAN C, SERON-FERRE M, DINET V, et al. Immunocytochemical demonstration of day/night changes of clock gene protein levels in the murine adrenal gland: differences between melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2006;40:64–70. doi: 10.1111/j.1600-079X.2005.00279.x. [DOI] [PubMed] [Google Scholar]
- 103.IMBESI M, ARSLAN AD, YILDIZ S, et al. The melatonin receptor MT1 is required for the differential regulatory actions of melatonin on neuronal `clock' gene expression in striatal neurons in vitro. J Pineal Res. 2008;46:87–94. doi: 10.1111/j.1600-079X.2008.00634.x. [DOI] [PubMed] [Google Scholar]
- 104.KENNAWAY DJ, VOULTSIOS A, VARCOE TJ, et al. Melatonin and activity rhythm responses to light pulses in mice with the Clock mutation. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1231–R1240. doi: 10.1152/ajpregu.00697.2002. [DOI] [PubMed] [Google Scholar]
- 105.ZEMAN M, SZANTOOVA K, STEBELOVA K, et al. Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive TGR(mRen2)27 rats. J Hypertens. 2009;27(Suppl 6):S21–6. doi: 10.1097/01.hjh.0000358833.41181.f6. S21–S26. [DOI] [PubMed] [Google Scholar]
- 106.GUTIERREZ-CUESTA J, TAJES M, JIMENEZ A, et al. Evaluation of potential pro-survival pathways regulated by melatonin in a murine senescence model. J Pineal Res. 2008;45:497–505. doi: 10.1111/j.1600-079X.2008.00626.x. [DOI] [PubMed] [Google Scholar]
- 107.REITER RJ, CRAFT CM, JOHNSON JE, JR., et al. Age-associated reduction in nocturnal pineal melatonin levels in female rats. Endocrinology. 1981;109:1295–1297. doi: 10.1210/endo-109-4-1295. [DOI] [PubMed] [Google Scholar]
- 108.SACK RL, LEWY AJ, ERB DL, et al. Human melatonin production decreases with age. J Pineal Res. 1986;3:379–388. doi: 10.1111/j.1600-079x.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 109.COHEN M, LIPPMAN M, CHABNER B. Role of pineal gland in aetiology and treatment of breast cancer. Lancet. 1978;2:814–816. doi: 10.1016/s0140-6736(78)92591-6. [DOI] [PubMed] [Google Scholar]
- 110.KOLSTAD HA. Nightshift work and risk of breast cancer and other cancers--a critical review of the epidemiologic evidence. Scand J Work Environ Health. 2008;34:5–22. doi: 10.5271/sjweh.1194. [DOI] [PubMed] [Google Scholar]
- 111.STEVENS RG. Light-at-night, circadian disruption and breast cancer: assessment of existing evidence. Int J Epidemiol. 2009;38:963–970. doi: 10.1093/ije/dyp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.BARTSCH C, BARTSCH H, FLUCHTER SH, et al. Evidence for modulation of melatonin secretion in men with benign and malignant tumors of the prostate: relationship with the pituitary hormones. J Pineal Res. 1985;2:121–132. doi: 10.1111/j.1600-079x.1985.tb00633.x. [DOI] [PubMed] [Google Scholar]
- 113.ANISIMOV VN, POPOVICH IG, ZABEZHINSKI MA. Melatonin and colon carcinogenesis: I. Inhibitory effect of melatonin on development of intestinal tumors induced by 1,2-dimethylhydrazine in rats. Carcinogenesis. 1997;18:1549–1553. doi: 10.1093/carcin/18.8.1549. [DOI] [PubMed] [Google Scholar]
- 114.BEJARANO I, REDONDO PC, ESPINO J, et al. Melatonin induces mitochondrial-mediated apoptosis in human myeloid HL-60 cells. J Pineal Res. 2009;46:392–400. doi: 10.1111/j.1600-079X.2009.00675.x. [DOI] [PubMed] [Google Scholar]
- 115.CUCINA A, PROIETTI S, D'ANSELMI F, et al. Evidence for a biphasic apoptotic pathway induced by melatonin in MCF-7 breast cancer cells. J Pineal Res. 2009;46:172–180. doi: 10.1111/j.1600-079X.2008.00645.x. [DOI] [PubMed] [Google Scholar]
- 116.DAUCHY RT, BLASK DE, DAUCHY EM, et al. Antineoplastic effects of melatonin on a rare malignancy of mesenchymal origin: melatonin receptor-mediated inhibition of signal transduction, linoleic acid metabolism and growth in tissue-isolated human leiomyosarcoma xenografts. J Pineal Res. 2009;47:32–42. doi: 10.1111/j.1600-079X.2009.00686.x. [DOI] [PubMed] [Google Scholar]
- 117.PETRANKA J, BALDWIN W, BIERMANN J, et al. The oncostatic action of melatonin in an ovarian carcinoma cell line. J Pineal Res. 1999;26:129–136. doi: 10.1111/j.1600-079x.1999.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 118.KANISHI Y, KOBAYASHI Y, NODA S, et al. Differential growth inhibitory effect of melatonin on two endometrial cancer cell lines. J Pineal Res. 2000;28:227–233. doi: 10.1034/j.1600-079x.2000.280405.x. [DOI] [PubMed] [Google Scholar]
- 119.KOTHARI LS. Influence of chronic melatonin on 9,10-dimethyl-1,2-benzanthracene-induced mammary tumors in female Holtzman rats exposed to continuous light. Oncology. 1987;44:64–66. doi: 10.1159/000226445. [DOI] [PubMed] [Google Scholar]
- 120.REITER RJ, TAN DX, SAINZ RM, et al. Melatonin: reducing the toxicity and increasing the efficacy of drugs. J Pharm Pharmacol. 2002;54:1299–1321. doi: 10.1211/002235702760345374. [DOI] [PubMed] [Google Scholar]
- 121.OTALORA BB, MADRID JA, ALVAREZ N, et al. Effects of exogenous melatonin and circadian synchronization on tumor progression in melanoma-bearing C57BL6 mice. J Pineal Res. 2008;44:307–315. doi: 10.1111/j.1600-079X.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 122.ERIGUCHI M, LEVI F, HISA T, et al. Chronotherapy for cancer. Biomed Pharmacother. 2003;57(Suppl 1):92s–95s. doi: 10.1016/j.biopha.2003.08.012. 92s–95s. [DOI] [PubMed] [Google Scholar]
- 123.FU L, LEE CC. The circadian clock: pacemaker and tumour suppressor. Nat Rev Cancer. 2003;3:350–361. doi: 10.1038/nrc1072. [DOI] [PubMed] [Google Scholar]
- 124.GRANDA TG, LEVI F. Tumor-based rhythms of anticancer efficacy in experimental models. Chronobiol Int. 2002;19:21–41. doi: 10.1081/cbi-120002589. [DOI] [PubMed] [Google Scholar]
- 125.WOOD PA, HRUSHESKY WJ. Circadian rhythms and cancer chemotherapy. Crit Rev Eukaryot Gene Expr. 1996;6:299–343. doi: 10.1615/critreveukargeneexpr.v6.i4.10. [DOI] [PubMed] [Google Scholar]
- 126.RIVARD GE, INFANTE-RIVARD C, DRESSE MF, et al. Circadian time-dependent response of childhood lymphoblastic leukemia to chemotherapy: a long-term follow-up study of survival. Chronobiol Int. 1993;10:201–204. doi: 10.3109/07420529309073888. [DOI] [PubMed] [Google Scholar]
- 127.HRUSHESKY WJ. Circadian timing of cancer chemotherapy. Science. 1985;228:73–75. doi: 10.1126/science.3883493. [DOI] [PubMed] [Google Scholar]
- 128.LEVI F, BENAVIDES M, CHEVELLE C, et al. Chemotherapy of advanced ovarian cancer with 4'-O-tetrahydropyranyl doxorubicin and cisplatin: a randomized phase II trial with an evaluation of circadian timing and dose-intensity. J Clin Oncol. 1990;8:705–714. doi: 10.1200/JCO.1990.8.4.705. [DOI] [PubMed] [Google Scholar]
- 129.HAUS E. Chronobiology of the mammalian response to ionizing radiation. Potential applications in oncology. Chronobiol Int. 2002;19:77–100. doi: 10.1081/cbi-120002592. [DOI] [PubMed] [Google Scholar]