Abstract
Background
Reports show higher prevalence of albuminuria among Hispanics compared to whites. Differences by country of origin or genetic background are unknown.
Methods and Results
In MESA, we studied the associations of both genetic ancestry and country of origin with albumin to creatinine ratio among 1,417 Hispanic vs. White participants using multivariable linear regression and back transforming beta-coefficients into relative difference (%RD, 95%CI). Percentage European, Native American and African ancestry components for Hispanics were estimated using genetic admixture analysis.
The proportions of European, Native American and African genetic ancestry differed significantly by country of origin (p-value<0.0001); Mexican/Central Americans had the highest Native American (41±13%), Puerto Ricans had the highest European (61±15 %), and Dominicans had the highest African (39±21%) ancestry. Hispanic ethnicity was associated with higher albumin/creatinine ratio compared to whites, but the association varied country of origin (adjusted p interaction=0.04). Mexican/Central Americans and Dominicans had higher albumin/creatinine ratio compared to whites after adjustment (RD 19%, 2-40% and (RD 27%, 1-61%), but not Puerto Ricans (RD 8%, −12-34%). Higher Native American ancestry was associated with higher albuminuria after age and sex adjustment among all Hispanics (RD 11%, 1-21%), but was attenuated after further adjustment. Higher European ancestry was independently associated with lower albumin/creatinine ratio among Puerto Ricans (−21%, −34 to −6), but not among Mexican/Central Americans and Dominicans.
Conclusions
Hispanics are a heterogeneous group with varying genetic ancestry. Risks of albuminuria differ across country of origin groups. These differences may be due, in part, to differences in genetic ancestral components.
Keywords: genetics, kidney, albuminuria, ancestry
Introduction
Hispanics have a higher incidence of end stage renal disease (ESRD) compared to whites in the United States,1 despite reports that Hispanics have lower prevalence of chronic kidney disease (CKD)2, 3. Although this discrepancy may be explained by Hispanics having faster rates of progression from CKD to ESRD,4 it may also be complicated by the use of different definitions to describe “Hispanics”. For example, CKD prevalence estimates have focused on Mexican-Americans3 while ESRD estimates have included a wider group of Hispanics.1 Since Hispanic subgroups in the United States differ culturally, socially and perhaps genetically,5, 6 categorization of Hispanics into one homogeneous group could lead to spurious inferences that may not generalize.
Differences between Hispanics and whites have also been reported for albuminuria, a marker of early kidney damage, and a known risk factor for CKD progression.7 Studies from the National Health and Nutrition Survey showed that albuminuria is more common among Hispanics of Mexican origin compared to whites.8, 9 This has also been documented in other studies with representation from several Hispanic subgroups.4, 10 None of these studies, however, has evaluated whether the risk of albuminuria is uniform across Hispanic subgroups of differing ancestral origin or whether differences impact comparisons with non-Hispanic whites.
In addition, it is unknown whether the reported differences in albuminuria between Hispanics and whites are associated, at least in part, with genetic predisposition. Hispanics are known to be genetically admixed with European, Native American and African ancestry, and the degree of admixture may vary by country of origin.5, 11-13 Determination of individual genetic ancestry in an admixed population such as Hispanics can be obtained using a series of markers informative for ancestry by genetic admixture analysis.14-20 This method quantifies the proportion of an individual’s genome that is of a given ancestral origin. Admixture analysis may offer insights into whether genetic ancestry varies significantly by country of origin among Hispanics, and whether genetic ancestry explains, at least in part, differences in kidney disease risk factors such as albuminuria. It may also allow the study of potential genetics vs. sociodemographic contributions to these associations. In addition, genetic admixture analysis may provide a method for future gene identification by genetic admixture mapping.11, 21, 22
We designed this study in the Multi-Ethnic Study of Atherosclerosis to determine: (1) whether genetically determined individual African, Native American or European ancestry differs by country of origin among Hispanics; (2) whether country of origin is associated with differences in cardiovascular risk factors and albuminuria within Hispanic subgroups and when compared to whites; and (3) whether genetically determined individual ancestry is independently associated with albuminuria among Hispanics.
Methods
Subjects
The Multi-Ethnic Study of Atherosclerosis (MESA) is a large NHLBI sponsored study designed to understand subclinical cardiovascular disease and its progression in a multi-ethnic cohort. Details on recruitment and design have been previously published.23 Briefly, MESA recruited 6,814 men and women who were between 45 and 84 years old, were free of cardiovascular disease and who self identified as white, African American, Hispanic or Chinese-American. Subjects were recruited from Baltimore City and Baltimore County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles County, California; Northern Manhattan and the Bronx, New York; and St. Paul, Minnesota between July 2000 and August 2002. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
A subcohort of 2880 MESA subjects were selected for genetic studies from subjects who gave informed consent for DNA extraction and genetic sub-study and had samples in the study DNA laboratory with sufficient DNA. Participants were balanced by ethnic group representation (approximately 720 per group) and equality by gender. For these analyses, we included self-identified Hispanics and non-Hispanic whites who were successfully genotyped and had a measure of albuminuria (705 Hispanics and 712 Whites) at baseline, for a total N=1,417.
Primary Predictors
Self reported race/ethnicity and self-reported country of origin were assessed at baseline by questionnaire.23 Individual genetic ancestry was determined by admixture analysis as described below.
Primary Outcome
The primary outcome of this study was albuminuria calculated as albumin to creatinine ratio in mg/g from a spot collection at baseline. Albumin to creatinine ratio was log transformed due to the skewed distribution and used as a continuous variable. Urine albumin and creatinine were measured by nephelometry and the rate Jaffe reaction, respectively. Microalbuminuria was defined as ≥30 mg/g and macroalbuminuria was defined as ≥300 mg/g.
DNA Extraction and Genotyping
DNA was extracted from peripheral leukocytes isolated from packed cells of anticoagulated blood by use of a commercially available DNA isolation kit (Puregene; Gentra Systems, Minneapolis, MN). The DNA was quantified by determination of absorbance at 260 nm followed by PicoGreen analysis (Molecular Probes, Inc., Eugene, OR). Two vials of DNA were stored per participant at −70 degrees centigrade. Genotyping was performed by Illumina Genotyping Services (Illumina Inc., San Diego, CA) using their proprietary GoldenGate assay. Details on quality control have been previously published.24
Selection of ancestry informative markers (AIMs)
Ancestry informative markers (AIMs) are single nucleotide polymorphisms (SNPs) that are known to have different allele frequencies between ancestral populations. AIMs in MESA were selected to maximize differential allele frequency between 2 or more of the 4 ethnic groups, and to distribute the SNPs as evenly across the genome as possible. AIMS in MESA were genotyped in two panels. In MESA Panel 1, AIMs were selected from an Illumina proprietary SNP database to maximize the difference in allele frequencies between any pair of ethnic groups: Caucasian- vs African-American; Caucasian- vs Chinese-American; African- vs Chinese-American. For MESA CG Panel 2, additional makers informative for Native American ancestry were selected from published lists.25, 26 A total of 199 SNPs were successfully genotyped.
Determination of Individual Ancestry
We estimated individual ancestry using 171 AIMS in MESA. We excluded markers that had no ancestral information in HapMap (N= 3) and markers in the X chromosome (N=25) in order to be able to look for gender interactions. We used individual level genotype data from three ancestral populations: 60 Yorubans, 60 Caucasians (CEPH) from the HapMap genome project (http://www.hapmap.org), and 320 Native American ancestors (ancestral data provided by Dr. Seldin and Dr. Choudhry). 25, 26 Determinations of deviations from Hardy-Weinberg Equilibrium (HWE) were evaluated separately for the ancestry informative markers (AIMs). Information on HWE and on informativeness of the markers have been previously reported.24
Individual admixture proportions were calculated using a Markov Chain-Monte Carlo method27, 28 with the program STRUCTURE 2.1 with a burn-in length of 50,000 and 50,000 iterations after burn-in, assuming independent allele frequencies. We used K=3 (three parental populations) based on prior studies that Hispanics are mainly admixed with Caucasian, Native American and African ancestries. 12, 25 This was confirmed with several iterations using K=2, 4, and 5, and a K=3 was confirmed as the best fit. We also looked at the summary statistics plots of various key summary statistics including Fst, alpha and likelihoods to make sure that they have come to equilibrium and the MCMC chain has converged. 24
Covariates
Information on age, self-reported race/ethnicity, level of education, annual household income, and smoking history was obtained using standardized questionnaires.23 Blood pressure measurements were obtained using the Dinamap® automated blood pressure device (Dinamap Monitor Pro 100®). Three sequential measures were obtained and the average of the second and third measurements was recorded. Hypertension was defined as systolic pressure ≥140 mm Hg, diastolic pressure ≥90 mm Hg, or current use of antihypertensive medication. Diabetes was defined as either self report of diabetes diagnosed by a physician, or fasting glucose ≥126 mg/dl or use of oral hypoglycemic medication or insulin. Cigarette smoking was defined as current or former, or never. Height and weight were measured with participants wearing light clothing and no shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Fasting blood was collected and stored at −70°F until needed for the appropriate assays, including total cholesterol, HDL cholesterol, triglycerides, and glucose. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald equation.29
Statistical Analysis
We first compared participant characteristics by self reported race/ethnicity (white vs. all Hispanics) using t-test or chi-squared where appropriate.
In a second step, we determined individual percent African, Native American and European ancestry for each participant. We have previously reported evidence of population substructure among Hispanics (with 61 AIMS deviating from HWE and 14 pairs of AIMS for Hispanics having pairwise linkage disequilibrium ≥ 0.20 (r2)).24 We investigated whether ancestral estimates varied significantly by country of origin using the Kruskall-Wallis test, and we present individual admixture estimates stratified by country of origin.
We then categorized Hispanics into three main subgroups by country of origin as follows: Mexicans and Central Americans, Puerto Ricans, and Dominicans. We grouped Mexicans with Central Americans because they were genetically similar and had similar risk factor profiles. For these subgroup analyses, we excluded those who reported being from Cuba, South America, or other due to the small numbers in each group. In addition, for South Americans, the numbers for each individual country were too small and South American countries differ socially and culturally and perhaps in the prevalence of kidney disease risk factors.30 We then compared participant characteristics by Hispanic subgroups using t-test or chi-square where appropriate.
In a third step, we tested the association of Hispanic ethnicity with log transformed ratio of albumin to creatinine as a continuous variable in a series of nested models using linear regression using baseline exam data. Beta coefficients were back-transformed into relative difference (RD), in percent. We first adjusted for age and sex only, then by income and education. Finally, we added adjustment for systolic blood pressure, body mass index, HDL and total cholesterol, triglycerides, hypertension, diabetes, smoking, and fasting glucose. We first conducted these analyses within Hispanics only to assess for differences by Hispanic subgroup, with Mexicans/Central Americans as the referent group. We then repeated the analyses including whites and tested the association of Hispanic ethnicity (all Hispanics) with albumin to creatinine ratio compared to whites as referent. To understand whether differences within Hispanic subgroups would also be apparent when assessing differences in albuminuria between Hispanics and whites, we tested for an interaction by country of origin, and stratified by Hispanic subgroup (Mexican and Central American, Puerto Rican and Dominican).
Finally, we studied the association of European and Native American ancestry and albuminuria within all Hispanics together and then within Hispanic subgroups. We tested for interactions by country of origin. Since only K-1 ancestral populations are needed to describe ancestral contributions, we used European and Native American ancestry for the main analyses. We used linear regression and studied the associations per one standard deviation increase
Results
Of the 1417 participants, mean age was 61 years, 40% had hypertension, 12% were diabetic, and 52% were smokers. Based on self reported race/ethnicity, compared to whites, Hispanics had a higher BMI, larger waist circumference, lower HDL cholesterol, higher triglycerides, and were more likely to be in the lower income and educational categories. (Table 1). Overall, 134 (9.5%) of participants had albumin to creatinine ratio >30 mg/g, and 31 (2.2%) had macroalbuminuria (albumin to creatinine ratio >300mg/g). Characteristics of Hispanic and White MESA participants in this study, who were randomly selected, did not significantly differ from those of the overall MESA cohort.
Table 1.
Baseline Characteristics of Self-Identified Whites and Hispanics in MESA
| Variables | White N=712 |
Hispanic N=705 |
P value |
|---|---|---|---|
| Mean (SD) or N (%) | |||
| Age | 62 (10) | 61 (10) | 0.55 |
| Male | 332 (47%) | 324 (46%) | 0.80 |
| Income ($) | <0.001 | ||
| <12,000 | 25 (4%) | 149 (21%) | |
| 12,000-25,000 | 85 (12%) | 212 (30%) | |
| 25,000-50,000 | 185 (26%) | 203 (29%) | |
| 50,000+ | 403 (57%) | 118 (17%) | |
| Education | <0.001 | ||
| <high school | 33 (5%) | 320 (45%) | |
| High school | 125 (18%) | 146 (21%) | |
| Some college | 201 (28%) | 173 (24%) | |
| College or more | 351 (49%) | 66 (9%) | |
| Body Mass Index (kg/m2) |
29 (5) | 30 (5) | <0.001 |
| Waist circumference (cm) |
98 (15) | 100 (13) | <0.001 |
| Waist-to-hip ratio | 0.92 (0.09) | 0.95 (0.07) | <0.001 |
| Low Density Lipoprotein (mg/dL) |
117 (31) | 120 (32) | 0.12 |
| High Density Lipoprotein (mg/dL) |
53 (16) | 48 (13) | <0.001 |
| Systolic Blood Pressure (mmHg) |
124 (21) | 126 (22) | 0.12 |
| Total cholesterol (mg/dL) |
196 (35) | 199 (38) | 0.11 |
| Triglycerides (mg/dL) |
133 (83) | 162 (118) | <0.001 |
| Serum Creatinine (mg/dL) |
0.95 (0.19) | 0.92 (0.44) | 0.12 |
| Serum Cystatin C (mg/L) |
0.90 (0.19) | 0.92 (0.36) | 0.35 |
| eGFR (by MDRD in ml/min/1.73m2) |
76 (22) | 82 (18) | <0.001 |
| eGFR (by Cystatin C in ml/min) |
91 (21) | 91 (21) | 0.83 |
| Diabetes | 46 (6%) | 121 (17%) | <0.001 |
| Fasting Glucose mg/dL |
90 (19) | 103 (40) | <0.001 |
| Smoking | 416 (58%) | 318 (45%) | <0.001 |
| Hypertension | 277 (39%) | 295 (42%) | 0.26 |
Genetic Ancestry and Country of Origin
Among all Hispanics, average Native American ancestry was 32% (±17) and mean African ancestry was 15% (±18). However, these proportions varied significantly by country of origin (Figure 1), p value <0.0001. Within the major Hispanic subgroups in MESA, Mexican and Central Americans had a very low African ancestral component (mean 7 ± 9 %), with the remainder of their genome mostly comprised of European (mean 52 ± 13 %) and Native American ancestry (41 ± 13%). Puerto Ricans had 23 ± 16% African, 61 ± 15 % European, and 16 ± 8 % Native American ancestry, whereas Dominicans had the highest proportion of African Ancestry (39 ± 21%), with 49 ± 19 % European, and only 11± 5% Native American ancestry. Among South Americans (N=46), Native American ancestry was 34 % ± 19 %, 56 ± 20 % was European and 10 ± 15 % ancestry was of African origin, and the South Americans reported being from several different countries.
Figure 1.
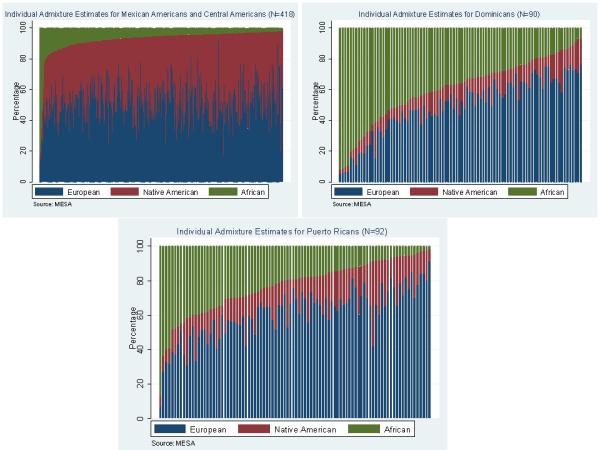
Individual Ancestry Estimates by Country of Origin For Mexican/Central Americans: Mexicans= 368, El Salvador= 19, Guatemala= 11, Nicaragua= 7, Panama= 6
Participant Characteristics by Country of Origin
We compared anthropomorphic characteristics and cardiovascular risk factors among Hispanics subgroups. (Table 2) Hispanics from Mexico and Central America had significantly larger waist to hip ratios and higher triglyceride levels compared to Puerto Ricans and Dominicans. Mexicans/Central Americans had higher levels of fasting glucose compared to Puerto Ricans. Interestingly, Mexican/Central Americans had much lower rates of smoking compared to Dominicans or Puerto Ricans.
Table 2.
Characteristics of Hispanics by Country of Origin in MESA
| Characteristics | Mexican & Central American N=418 |
Puerto Rican N=92 |
Dominican N=90 |
|---|---|---|---|
| Mean (SD) or % | |||
| Body mass index | 30 (5) |
30 (5) |
28* (4) |
|
Waist
Circumference (cm) |
102 (13) |
100 (12) |
96† (12) |
| Waist-to-hip ratio | 0.96 (0.07) | 0.94† (0.08) |
0.93* (0.08) |
| LDL cholesterol | 119 (33) | 119 (27) |
123 (37) |
| HDL cholesterol | 46 (12) | 49 (12) |
48 (11) |
| Systolic Blood Pressure |
126 (23) |
122 (19) |
127 (24) |
| Total Cholesterol |
200 (39) |
195 (29) |
195 (40) |
| Triglycerides | 176 (119) |
134† (62) |
123* (65) |
| eGFR (MDRD) | 84 (19) |
80 (17) |
80 (15) |
| eGFR (Cystatin C) | 91 (21) |
89 (18) |
95 (20) |
| Diagnosed Diabetes (%) |
20 | 15 | 16 |
| Fasting Glucose | 106 (42) |
107 (48) |
96 (27)† |
| Smoking (%) | 44 | 58† | 58† |
| Hypertension (%) | 40 | 43 | 39 |
p-value = 0.05-0.001 for comparison with Mexican/Central American
p-value = <0.001 for comparison with Mexican/Central American
Country of Origin and Albuminuria
Prevalence of albuminuria (>30 mg/g) varied by country of origin, with 14.1% among Mexican/Central Americans, 5.4% among Puerto Ricans, and 16.7% among Dominicans, p-value 0.05. Compared to Mexicans/Central Americans, Puerto Ricans had significantly lower levels of albuminuria, with a relative difference of −26% (p-value 0.03). This association persisted after adjustment for income and education (RD −23%, p-value 0.05). Although the association was attenuated after adjustment for comorbidities, it was still in the same direction of lower albuminuria for Puerto Ricans compared to Mexican/Central Americans (RD −18%, p-value 0.12). Dominicans also had lower levels of albuminuria compared to Mexican/Central Americans, but this difference was not statistically significant in unadjusted or adjusted models (RD −13%, p value 0.31 and RD −6%, p-value 0.64 respectively).
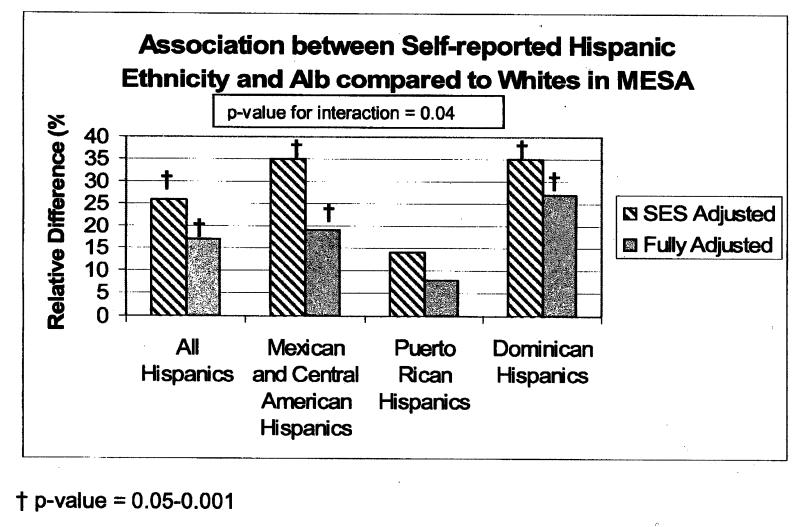
We then studied the association of Hispanic ethnicity and albumin to creatinine ratio compared to whites. Overall, Hispanics had higher albumin to creatinine ratios compared to whites, with 12.3% Hispanics having albumin/creatinine ratio >30 mg/g vs. 6.7% among whites (p-value <0.001). Even after adjustment for sociodemographic factors and comorbidities, Hispanic ethnicity was associated with higher albumin to creatinine ratio. However, these associations were not uniform across country of origin subgroups (adjusted p value for interaction 0.04). Mexicans/Central Americans and Dominicans, had higher albumin to creatinine ratio compared to whites after adjustment. However, Puerto Ricans did not have significant differences in albumin to creatinine ratio compared to whites. (Figure 2)
Figure 2.
The Association between Self-reported Hispanic Ethnicity (All Hispanics and by Country of Origin) and Albuminuria compared to Whites in MESA
Genetic Ancestry and Albuminuria
Increasing Native American ancestry was associated with higher levels of albuminuria in age and sex adjusted analyses among all Hispanics. (Table 3) This association was attenuated after adjustment for income and education, and was further attenuated after full adjustment, but the direction of the association remained the same. There was no statistically significant interaction by country of origin in the association of Native American ancestry with albumin to creatinine ratio (p-value=0.13). Among Mexicans/Central Americans and Puerto Ricans, Native American ancestry was directionally associated with increased albumin to creatinine ratio, though not statistically significant. In contrast, the estimates were in the opposite direction for Dominicans, though not statistically significant. (Table 3)
Table 3.
The Association of Native American and European Ancestry (per 10% increase) and Albuminuria Among Hispanics
| Native American Ancestry per 10% increase (95% CI) |
p-value | European Ancestry per 10% increase (95% CI) |
p-value | |
|---|---|---|---|---|
| All Hispanics (N= 705) | ||||
| Age and sex adjusted | 6% (1% to 11%) |
0.02 | −6% (−12% to 0%) |
0.03 |
| SES adjusted* | 5% (0% to 10%) |
0.07 | −5% (−11% to 1%) |
0.08 |
| Adjusted** | 3% (−2% to 8%) |
0.19 | −3% (−8% to 2%) |
0.26 |
| Mexican/Central American Hispanics (N= 418) | ||||
| Age and sex adjusted | 9% (−1% to 18%) |
0.09 | −4% (−14% to 6%) |
0.40 |
| SES adjusted* | 8% (−2% to 18%) |
0.13 | −3% (−13% to 7%) |
0.57 |
| Adjusted** | 5% (−4% to 14%) |
0.24 | −3% (−12% to 7%) |
0.59 |
| Puerto Rican Hispanics (N= 92) | ||||
| Age and sex adjusted | 6% (−17% to 29%) |
0.64 | −14% (−25% to −3%) |
0.011 |
| SES adjusted* | 4% (−19% to 26%) |
0.73 | −13% (−24% to −2%) |
0.02 |
| Adjusted** | 7% (−15% to 30%) |
0.53 | −14% (−24% to −3%) |
0.02 |
| Dominican Hispanics (N= 90) | ||||
| Age and sex adjusted | −16% (−57% to 26%) |
0.46 | 2% (−10% to 14%) |
0.75 |
| SES adjusted* | −14% (−57% to 28%) |
0.51 | 1% (−11% to 14%) |
0.82 |
| Adjusted** | −13% (−56% to 29%) |
0.54 | −2% (−15% to 10%) |
0.69 |
= adjusted for age, sex, income, and education
adjusted for age, sex, income, education, body mass index (BMI), high density lipids (HDL), total cholesterol, triglycerides, hypertension, diabetes, smoking
Higher European ancestry was associated with lower albumin to creatinine ratio for all Hispanics. Although the effect was attenuated by further adjustment, the direction of the estimates remained consistent. Although there was no statistically significant interaction by country of origin (p-value=0.89), higher European ancestry was most strikingly protective among Puerto Ricans, where higher European ancestry was associated with lower albumin to creatinine ratio, even after full adjustment. The association of European ancestry and albumin to creatinine ratio was not significant after adjustment among Mexican/Central Americans and Dominicans, but the direction of the association was consistent across groups. (Table 3)
As a confirmatory analysis, we repeated our analyses using African ancestry as our predictor. There was no significant association between African ancestry and albuminuria among all Hispanics in unadjusted or unadjusted analyses (adjusted RD −0.5 (95%CI −5% to 4%)). Interestingly, higher African ancestry was associated with higher albuminuria among Puerto Ricans, RD 11% (0.1% to 21%) after full adjustment. There were no associations between African ancestry and albuminuria among Dominicans (RD 3% (−8% to 14%)) or Mexican/Central Americans (RD −5% (−19% to 7%)).
There were no significant interactions by gender for the association of Native American or European ancestry and albuminuria (p-values 0.31 and 0.83 respectively).
Discussion
Using genetic admixture analysis, we found that Hispanics in MESA are admixed with European, African and Native American ancestries and that the proportion of each ancestral group varies strikingly by country of origin, with Mexicans/Central Americans having the largest Native American component, Dominicans having the largest African component, and Puerto Ricans having the highest European component. Moreover, we found that cardiovascular and renal risk factor profiles differ by country of origin among Hispanic subgroups, with Mexicans/Central Americans having more truncal obesity, worse lipid profile, and the highest levels of albuminuria compared to Dominicans or Puerto Ricans, but lower prevalence of smoking. Differences observed among Hispanics subgroups greatly impact the association of Hispanic ethnicity and urinary albumin to creatinine ratio compared to whites, where Hispanic ethnicity was associated with significantly higher levels of albumin to creatinine ratio among Mexican/Central Americans and Dominican Hispanics but not Puerto Ricans. Most interestingly, we found that higher European ancestry may be associated with lower albuminuria, particularly among Puerto Ricans, and that Native American ancestry may, at least in part, be associated with higher levels of albuminuria among Hispanics.
Differences in the genetic ancestral component by country of origin have been documented in prior studies of Mexican-Americans and Puerto Ricans.13, 25, 31, 32 Our study extends these findings to other Hispanic subgroups in a large, community-based cohort in the United States. We found that the differences by country of origin are also apparent in risk factor profiles in MESA. Whether the differences in genetic ancestral component among Hispanic subgroups account for these observed differences is still unclear.30, 33 It is also possible that social, cultural and environmental factors may account for the differences observed in risk factor profile. Our findings have important implications for future epidemiological and genetic studies among Hispanics. Future analyses should take into account country of origin when studying Hispanic populations.
Our observation that Hispanics have higher levels of albuminuria than whites is consistent with prior reports.8, 9, 34 However, most national studies have focused on Mexican Americans.8, 9 Our study is the first to show that levels of albuminuria among Hispanics may vary by country of origin, and that these observed differences significantly impact the association of Hispanic ethnicity and albuminuria when compared to whites. Since albuminuria is a known important risk factor for adverse cardiovascular events and kidney disease progression,7 our findings highlight the importance of recognizing the heterogeneity of Hispanic subgroups. That is, future studies should be aimed at understanding whether different Hispanic subgroups have different risk of kidney disease onset, progression, and whether the mediators may vary by country of origin. Accurately describing Hispanic subgroups may aid in the understanding of the conundrum of lower CKD prevalence but higher ESRD incidence among Hispanics in the United States.
Our study is the first to report the association of genetic ancestry and albuminuria among Hispanics. The observation that European ancestry may be protective for albuminuria, particularly among Puerto Ricans, may suggest that alleles more commonly found in Europeans reduce risk of albuminuria. One study of Hispanics patients with systemic lupus erythematosus has suggested that differences in the frequency of lupus nephritis correlated with the relative proportion of non-European admixture.35 However, these findings may also be due to unmeasured environmental factors. For example, the fact that European ancestry was significantly associated with lower albumin to creatinine ratio among Puerto Ricans, but not among other groups, could be explained by differences in social or environmental factors unique to Puerto Ricans with higher European ancestry (i.e. diet, socioeconomic status, neighborhood, acculturation). Our findings that Native American ancestry may, at least in part, be associated with higher levels of albuminuria is also noteworthy. Native Americans in the United States have been reported to have over 20% prevalence of albuminuria.36 If higher Native American ancestry is associated with higher albuminuria among Hispanics, it is possible that genetic factors due to a common Native American ancestry may play some role in explaining this observation. The attenuation by measures of socioeconomics and comorbidities could suggest mediation of the pathway by these factors, confounding, or an important gene-environment interaction that was not elucidated in this study. The heritability of albuminuria among Mexican Americans with high degree of Native American ancestry has been shown in prior studies37, and certain loci have been associated with albuminuria38 and other risk factors among Mexican Americans.39 These suggest that albuminuria may be a phenotype amenable to admixture mapping among Hispanics, a method that has proven fruitful among African Americans.21, 22, 40 However, these maps need to take into account the 3 ancestral populations among Hispanics.11, 41
The strengths of our study include a large, community-based, well characterized, multi-ethnic cohort. Participants were mostly healthy, had a wide age range, low rates of CKD, and had no known cardiovascular disease at study entry. Our study used 171 ancestry informative markers well selected for allele frequency differences between populations. Some error may still be present in the estimate, but prior studies have shown that accurate estimates may be obtained with 100 AIMS.42 However, our findings should be interpreted with caution given that we were likely limited by power in our subgroup analyses. In particular, Dominican and Puerto Rican subgroups were much smaller than Mexican/Central American, thus potentially biasing results toward the null. There may have also been some misclassification as we used self-reported country of origin for categorization into genetic groups. Although our study represents the major Hispanic subgroups in the United States (http://www.census.gov/population/www/socdemo/hispanic/reports.html), most Hispanics were recruited from only three sites. Most Dominicans and Puerto Ricans were recruited from New York, while Mexicans and Central Americans were recruited in Los Angeles and Minnesota. Thus their characteristics may not be representative of the whole country. It is also possible that some Hispanics born in the U.S. may have parents from different countries of origin. However, the majority of MESA participants were born outside the U.S. or were generation one immigrants.”
In addition, we did not account for other possible factors that may differ across Hispanic groups like acculturation43, access to health care, discrimination, and poverty.44 We used only one spot measure of albuminuria rather a 24 hour collection which may lead to some misclassification, but studies have shown that one spot ratio is highly associated with 24 hour excretion.7, 45
In summary, we found that Hispanic subgroups differ significantly in their genetic ancestral components, as well as in their risk factor profile by country of origin. We also found that levels of albuminuria vary significantly by country of origin among Hispanics and when compared to whites. Moreover, genetic ancestry may explain, at least in part, differences observed between Hispanics and whites in albuminuria. Our findings have important implications for future epidemiologic and genetic studies which should take into account Hispanic country of origin. In addition, albuminuria may be a phenotype amenable to admixture mapping among Hispanics. se in each ancestral component in nested models as above. We also conducted separate analyses with African ancestry because it is unknown, a priori, which ancestral component will be most associated with the outcome, and there is a potential for the associations to vary by Hispanic subgroup.
We investigated whether Hispanic subgroups (identified by country of origin) differ in both genetic component and risk for albumin in the urine, which is an important risk factor for cardiovascular events and kidney disease. We observed that Hispanics differ in their genetic ancestral component by country of origin. In addition, their risk of albuminuria differs by country of origin when compared with whites. Our findings suggest that country of origin should be included as a covariate when studying differences between Hispanics and non-Hispanic whites for select cardiovascular risk factors.
Acknowledgments
Funding Sources: This work was supported by contracts [N01-HC-95159 through N01-HC-95165] and [N01-HC-95169] from the National Heart, Lung, and Blood Institute. The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. This work was also funded by the NIDDK (1K23DK082793-01 to C.P.)
Footnotes
Disclosures: None
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.System USRD . USRDS 2007 Annual Data Report. National Institutes of Health; 2007. [Google Scholar]
- 2.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS. Prevalence of chronic kidney disease in the United States. Jama. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS. Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol. 2006;17:2892–2899. doi: 10.1681/ASN.2005101122. [DOI] [PubMed] [Google Scholar]
- 5.Burchard E Gonzalez, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR, Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-Stable EJ, Ziv E, Risch N. Latino populations: a unique opportunity for the study of race, genetics, and social environment in epidemiological research. Am J Public Health. 2005;95:2161–2168. doi: 10.2105/AJPH.2005.068668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanis CL, Hewett-Emmett D, Bertin TK, Schull WJ. Origins of U.S. Hispanics. Implications for diabetes. Diabetes Care. 1991;14:618–627. doi: 10.2337/diacare.14.7.618. [DOI] [PubMed] [Google Scholar]
- 7.Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 8.Jones CA, Francis ME, Eberhardt MS, Chavers B, Coresh J, Engelgau M, Kusek JW, Byrd-Holt D, Narayan KM, Herman WH, Jones CP, Salive M, Agodoa LY. Microalbuminuria in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2002;39:445–459. doi: 10.1053/ajkd.2002.31388. [DOI] [PubMed] [Google Scholar]
- 9.Bryson CL, Ross HJ, Boyko EJ, Young BA. Racial and ethnic variations in albuminuria in the US Third National Health and Nutrition Examination Survey (NHANES III) population: associations with diabetes and level of CKD. Am J Kidney Dis. 2006;48:720–726. doi: 10.1053/j.ajkd.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 10.Kramer H, Jacobs DR, Jr., Bild D, Post W, Saad MF, Detrano R, Tracy R, Cooper R, Liu K. Urine albumin excretion and subclinical cardiovascular disease. The Multi-Ethnic Study of Atherosclerosis. Hypertension. 2005;46:38–43. doi: 10.1161/01.HYP.0000171189.48911.18. [DOI] [PubMed] [Google Scholar]
- 11.Price AL, Patterson N, Yu F, Cox DR, Waliszewska A, McDonald GJ, Tandon A, Schirmer C, Neubauer J, Bedoya G, Duque C, Villegas A, Bortolini MC, Salzano FM, Gallo C, Mazzotti G, Tello-Ruiz M, Riba L, Aguilar-Salinas CA, Canizales-Quinteros S, Menjivar M, Klitz W, Henderson B, Haiman CA, Winkler C, Tusie-Luna T, Ruiz-Linares A, Reich D. A genomewide admixture map for Latino populations. Am J Hum Genet. 2007;80:1024–1036. doi: 10.1086/518313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchard EG, Avila PC, Nazario S, Casal J, Torres A, Rodriguez-Santana JR, Toscano M, Sylvia JS, Alioto M, Salazar M, Gomez I, Fagan JK, Salas J, Lilly C, Matallana H, Ziv E, Castro R, Selman M, Chapela R, Sheppard D, Weiss ST, Ford JG, Boushey HA, Rodriguez-Cintron W, Drazen JM, Silverman EK. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. Am J Respir Crit Care Med. 2004;169:386–392. doi: 10.1164/rccm.200309-1293OC. [DOI] [PubMed] [Google Scholar]
- 13.Bonilla C, Parra EJ, Pfaff CL, Dios S, Marshall JA, Hamman RF, Ferrell RE, Hoggart CL, McKeigue PM, Shriver MD. Admixture in the Hispanics of the San Luis Valley, Colorado, and its implications for complex trait gene mapping. Ann Hum Genet. 2004;68:139–153. doi: 10.1046/j.1529-8817.2003.00084.x. [DOI] [PubMed] [Google Scholar]
- 14.Chakraborty R, Weiss KM. Admixture as a tool for finding linked genes and detecting that difference from allelic association between loci. Proc Natl Acad Sci U S A. 1988;85:9119–9123. doi: 10.1073/pnas.85.23.9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins-Schramm HE, Phillips CM, Operario DJ, Lee JS, Weber JL, Hanson RL, Knowler WC, Cooper R, Li H, Seldin MF. Ethnic-difference markers for use in mapping by admixture linkage disequilibrium. Am J Hum Genet. 2002;70:737–750. doi: 10.1086/339368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKeigue PM. Mapping genes that underlie ethnic differences in disease risk: methods for detecting linkage in admixed populations, by conditioning on parental admixture. Am J Hum Genet. 1998;63:241–251. doi: 10.1086/301908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang H, Peng J, Wang P, Risch NJ. Estimation of individual admixture: analytical and study design considerations. Genet Epidemiol. 2005;28:289–301. doi: 10.1002/gepi.20064. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg NA, Li LM, Ward R, Pritchard JK. Informativeness of genetic markers for inference of ancestry. Am J Hum Genet. 2003;73(6):1402–1422. doi: 10.1086/380416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 20.Kosoy R, Nassir R, Tian C, White PA, Butler LM, Silva G, Kittles R, Alarcon-Riquelme ME, Gregersen PK, Belmont JW, De La Vega FM, Seldin MF. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, Detrano R, Roux AV Diez, Folsom AR, Greenland P, Jacob DR, Jr., Kronmal R, Liu K, Nelson JC, O’Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 24.Wassel CPJ, Peralta CA, Choudhry S, Seldin MF, Arnett DK. Genetic Ancestry is Associated with Subclinical Cardiovascular Disease in African Americans and Hispanics from The Multi-Ethnic Study of Atherosclerosis (MESA) Circ Cardiovasc Genet. 2009:629–36. doi: 10.1161/CIRCGENETICS.109.876243. Epub 2009 Sep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choudhry S, Coyle NE, Tang H, Salari K, Lind D, Clark SL, Tsai HJ, Naqvi M, Phong A, Ung N, Matallana H, Avila PC, Casal J, Torres A, Nazario S, Castro R, Battle NC, Perez-Stable EJ, Kwok PY, Sheppard D, Shriver MD, Rodriguez-Cintron W, Risch N, Ziv E, Burchard EG. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006;118:652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 26.Seldin MF, Tian C, Shigeta R, Scherbarth HR, Silva G, Belmont JW, Kittles R, Gamron S, Allevi A, Palatnik SA, Alvarellos A, Paira S, Caprarulo C, Guilleron C, Catoggio LJ, Prigione C, Berbotto GA, Garcia MA, Perandones CE, Pons-Estel BA, Alarcon-Riquelme ME. Argentine population genetic structure: large variance in Amerindian contribution. Am J Phys Anthropol. 2007;132:455–462. doi: 10.1002/ajpa.20534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 30.Cusumano AM, Bedat MC Gonzalez. Chronic kidney disease in Latin America: time to improve screening and detection. Clin J Am Soc Nephrol. 2008;3:594–600. doi: 10.2215/CJN.03420807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salari K, Choudhry S, Tang H, Naqvi M, Lind D, Avila PC, Coyle NE, Ung N, Nazario S, Casal J, Torres-Palacios A, Clark S, Phong A, Gomez I, Matallana H, Perez-Stable EJ, Shriver MD, Kwok PY, Sheppard D, Rodriguez-Cintron W, Risch NJ, Burchard EG, Ziv E. Genetic admixture and asthma-related phenotypes in Mexican American and Puerto Rican asthmatics. Genet Epidemiol. 2005;29:76–86. doi: 10.1002/gepi.20079. [DOI] [PubMed] [Google Scholar]
- 32.Yang N, Li H, Criswell LA, Gregersen PK, Alarcon-Riquelme ME, Kittles R, Shigeta R, Silva G, Patel PI, Belmont JW, Seldin MF. Examination of ancestry and ethnic affiliation using highly informative diallelic DNA markers: application to diverse and admixed populations and implications for clinical epidemiology and forensic medicine. Hum Genet. 2005;118:382–392. doi: 10.1007/s00439-005-0012-1. [DOI] [PubMed] [Google Scholar]
- 33.Lai CQ, Tucker KL, Choudhry S, Parnell LD, Mattei J, Garcia-Bailo B, Beckman K, Burchard EG, Ordovas JM. Population admixture associated with disease prevalence in the Boston Puerto Rican health study. Hum Genet. 2009;125:199–209. doi: 10.1007/s00439-008-0612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young BA, Katon WJ, Von Korff M, Simon GE, Lin EH, Ciechanowski PS, Bush T, Oliver M, Ludman EJ, Boyko EJ. Racial and ethnic differences in microalbuminuria prevalence in a diabetes population: the pathways study. J Am Soc Nephrol. 2005;16:219–228. doi: 10.1681/ASN.2004030162. [DOI] [PubMed] [Google Scholar]
- 35.Alarcon GS, Bastian HM, Beasley TM, Roseman JM, Tan FK, Fessler BJ, Vila LM, McGwin G., Jr. Systemic lupus erythematosus in a multi-ethnic cohort (LUMINA) XXXII: [corrected] contributions of admixture and socioeconomic status to renal involvement. Lupus. 2006;15:26–31. doi: 10.1191/0961203306lu2260oa. [DOI] [PubMed] [Google Scholar]
- 36.Lee ET, Howard BV, Wang W, Welty TK, Galloway JM, Best LG, Fabsitz RR, Zhang Y, Yeh J, Devereux RB. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113:2897–2905. doi: 10.1161/CIRCULATIONAHA.105.593178. [DOI] [PubMed] [Google Scholar]
- 37.Arar N, Nath S, Thameem F, Bauer R, Voruganti S, Comuzzie A, Cole S, Blangero J, MacCluer J, Abboud H. Genome-wide scans for microalbuminuria in Mexican Americans: the San Antonio Family Heart Study. Genet Med. 2007;9:80–87. doi: 10.1097/gim.0b013e31803068ec. [DOI] [PubMed] [Google Scholar]
- 38.Arar NH, Voruganti VS, Nath SD, Thameem F, Bauer R, Cole SA, Blangero J, MacCluer JW, Comuzzie AG, Abboud HE. A genome-wide search for linkage to chronic kidney disease in a community-based sample: the SAFHS. Nephrol Dial Transplant. 2008;23:3184–3191. doi: 10.1093/ndt/gfn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thameem F, Voruganti VS, He X, Nath SD, Blangero J, MacCluer JW, Comuzzie AG, Abboud HE, Arar NH. Genetic variants in the renin-angiotensin system genes are associated with cardiovascular-renal-related risk factors in Mexican Americans. Hum Genet. 2008;124:557–559. doi: 10.1007/s00439-008-0581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freedman BI, Kopp JB, Winkler CA, Nelson GW, Rao DC, Eckfeldt JH, Leppert MF, Hicks PJ, Divers J, Langefeld CD, Hunt SC. Polymorphisms in the Nonmuscle Myosin Heavy Chain 9 Gene (MYH9) Are Associated with Albuminuria in Hypertensive African Americans: The HyperGEN Study. Am J Nephrol. 2009;29:626–632. doi: 10.1159/000194791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collins-Schramm HE, Chima B, Morii T, Wah K, Figueroa Y, Criswell LA, Hanson RL, Knowler WC, Silva G, Belmont JW, Seldin MF. Mexican American ancestry-informative markers: examination of population structure and marker characteristics in European Americans, Mexican Americans, Amerindians and Asians. Hum Genet. 2004;114:263–271. doi: 10.1007/s00439-003-1058-6. [DOI] [PubMed] [Google Scholar]
- 42.Tsai HJ, Choudhry S, Naqvi M, Rodriguez-Cintron W, Burchard EG, Ziv E. Comparison of three methods to estimate genetic ancestry and control for stratification in genetic association studies among admixed populations. Hum Genet. 2005;118:424–433. doi: 10.1007/s00439-005-0067-z. [DOI] [PubMed] [Google Scholar]
- 43.Kandula NR, Diez-Roux AV, Chan C, Daviglus ML, Jackson SA, Ni H, Schreiner PJ. Association of acculturation levels and prevalence of diabetes in the multi-ethnic study of atherosclerosis (MESA) Diabetes Care. 2008;31:1621–1628. doi: 10.2337/dc07-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martins D, Tareen N, Zadshir A, Pan D, Vargas R, Nissenson A, Norris K. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;47:965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xin G, Wang M, Jiao LL, Xu GB, Wang HY. Protein-to-creatinine ratio in spot urine samples as a predictor of quantitation of proteinuria. Clin Chim Acta. 2004;350:35–39. doi: 10.1016/j.cccn.2004.06.019. [DOI] [PubMed] [Google Scholar]