Abstract
BACKGROUND:
Otitis media is the main reason young children receive antibiotics and is the leading reason for physician visits.
OBJECTIVE:
To characterize the incidence, recurrence and risk factors for otitis media in a population-based birth cohort.
METHODS:
All children born in southwestern British Columbia during 1999 to 2000 were followed until the age of three years. Otitis media was defined using The International Classification of Diseases, Ninth Revision coding of physician visits, and linked with antibiotic prescription data. Information on sex, birth weight, gestational age, Aboriginal status, maternal age, older siblings, maternal smoking during pregnancy, breastfeeding initiation, neighbourhood income, female education and rural residence were obtained from vital statistics, birth hospitalizations, perinatal registry and census data.
RESULTS:
Complete risk factor information was available for 50,474 children (86% of all births). Nearly one-half of the children (48.6%) had one or more physician visits for otitis media during follow-up, and 3952 children (7.8%) met the definition for recurrent otitis media. Of the children with at least three visits during follow-up (n=7571), 73% had their initial visit during the first year of life. Aboriginal status, maternal age younger than 20 years, male sex and older siblings were the strongest risk factors identified in the adjusted conditional logistic regression models.
DISCUSSION:
The present study established a population-based birth cohort by linking multiple administrative databases to characterize the incidence of and risk factors for otitis media. Although the incidence of otitis media is generally low in southwestern British Columbia, important risk factors continue to be young maternal age, mothers who smoke during pregnancy and children with Aboriginal ancestry.
Keywords: Birth cohort, Incidence, Otitis media, Risk factors
Abstract
HISTORIQUE :
L’otite moyenne est la principale raison pour laquelle les jeunes enfants reçoivent des antibiotiques et la première raison de rendre visite à un médecin.
OBJECTIF :
Caractériser l’incidence, la récurrence et les facteurs de risque de l’otite moyenne dans une cohorte de naissance en population.
MÉTHODOLOGIE :
Tous les enfants nés dans le sud-ouest de la Colombie-Britannique entre 1999 et 2000 ont été suivis jusqu’à l’âge de trois ans. Les chercheurs ont défini l’otite moyenne au moyen de la codification des consultations chez le médecin de The International Classification of Diseases, Ninth Revision, qu’ils ont liée aux données d’ordonnances d’antibiotiques. Ils ont obtenu l’information sur le sexe, le poids de naissance, l’âge gestationnel, le statut d’autochtone, l’âge de la mère, la fratrie plus âgée, le tabagisme de la mère pendant la grossesse, l’initiation de l’allaitement, le revenu du quartier, l’éducation de la mère et la résidence en milieu rural au moyen des statistiques de l’état civil, des hospitalisations pour accoucher, des registres périnatals et des données de recensement.
RÉSULTATS :
Les chercheurs ont obtenu l’information complète de 50 474 enfants (86 % de toutes les naissances) à l’égard des facteurs de risque. Près de la moitié des enfants (48,6 %) avaient rendu visite à un médecin au moins une fois à cause d’une otite moyenne pendant le suivi, et 3 952 enfants (7,8 %) respectaient la définition d’otite moyenne récurrente. Chez les enfants qui avaient rendu au moins trois visites au médecin pendant le suivi (n=7 571), 73 % avaient fait leur première visite pendant leur première année de vie. Le statut d’autochtone, avoir une mère de moins de 20 ans, le sexe masculin et une fratrie plus âgée constituaient les facteurs de risque les plus solides repérés dans les modèles de régression logistique conditionnelle rajustés.
EXPOSÉ :
La présente étude a établi une cohorte de naissance en population en liant de multiples bases de données administratives pour caractériser l’incidence et les facteurs de risque de l’otite moyenne. Même si l’incidence de l’otite moyenne est généralement faible au sud-ouest de la Colombie-Britannique, les facteurs de risque importants continuent d’être le jeune âge de la mère, le tabagisme de la mère pendant la grossesse et l’ascendance autochtone.
Otitis media is the most common reason young children are prescribed antibiotics and is the leading reason for physician visits (1,2). Because of the high incidence, treatment costs, potential for long-term learning disability and increasing antibiotic resistance, the accurate characterization of incidence trends and associated risk factors of otitis media is essential (3). Unfortunately, there have been few studies (4,5) that have followed children through the first years of life. Most studies have been cross-sectional (6,7) or have examined a population from a particular hospital, clinic or physician office.
The incidence of otitis media peaks at six to 18 months of age and exhibits the same seasonal trend as upper respiratory tract infections, which often precede or occur with otitis (8). Male sex, Aboriginal status, lack of breastfeeding, older siblings, daycare, passive smoke exposure and low socioeconomic status have consistently been identified as risk factors during early childhood (9–13). Birth weight, gestational age and season of birth have also been studied, but with less consistent findings (14,15).
As part of an ongoing study (16), we had the opportunity to longitudinally examine otitis media during the first three years of life in a large population-based birth cohort from British Columbia (BC). The aims of the present study were to characterize the population incidence, recurrence and risk factors for otitis media, and to evaluate the utility of linking administrative data for population-based epidemiological studies.
METHODS
Population
Vital statistics birth certificate information was collected from the BC Linked Health Database, which is maintained by the Centre for Health Services and Policy Research. (The BC Ministry of Health was the original source for the BC Linked Health Database extract.) Every singleton birth in southwestern BC from January 1, 1999, to December 31, 2000, was identified based on maternal postal code and date of birth. Children who died during follow-up or those with incomplete risk factor information were excluded.
Outcome measure
The BC Linked Health Database also provided information on all outpatient physician visits during the first three years of life based on children’s unique personal health number (PHN) and date of encounter. Otitis media physician visits were identified using The International Classification of Diseases, Ninth Revision (ICD-9) coding: 381, nonsuppurative otitis media and eustachian tube disorders; 381.0, acute nonsuppurative otitis media; 382, suppurative and unspecified otitis media; 382.0, acute suppurative otitis media; and 382.9, unspecified otitis media. Any visit occurring within two weeks of a previous visit was assumed to be a follow-up or treatment failure and was, therefore, excluded. Recurrent otitis media (ROM) was defined as four or more visits during a 12-month window, or as three or more visits during a six-month window (8).
Prescription records for antibiotics used to treat acute otitis media (17) were obtained from the College of Pharmacists of BC (Vancouver, BC), and data were linked to otitis media physician visits based on PHN and allowing a zero- to four-day lag between physician and pharmacy visits. Linked data were used for sensitivity analyses.
Risk factors
Sex and month of birth were obtained from birth certificates. Aboriginal status, as defined by federal billing data, was ascertained from birth hospitalization files. Information on older siblings, maternal smoking during pregnancy, breastfeeding initiation, birth weight, gestational age and maternal age were obtained from the BC Reproductive Care Program Perinatal Database Registry. This registry includes all women who receive prenatal care in the province of BC (approximately 90% of all births) (unpublished data). These data were linked to physician and prescription data by PHN. Neighbourhood income, female education and rural residence at the time of birth were obtained from the 2001 Canadian census. These data were linked to the cohort based on census dissemination area (approximately four city blocks in an urban area).
The University of British Columbia (Vancouver) Behavioural Research Ethics Board (B05-0123) approved the present study. The Centre for Health Services and Policy Research at the University of British Columbia completed all data linkages by either PHN or dissemination area. All personal identifiable information (full date of birth, postal code and PHN) was removed from the linked data set before release.
Statistical analysis
Maternal age, birth weight, gestational age and female education were converted into categorical variables for analysis. Age and month of first visit were calculated from birth certificate and physician visit data. Independent t tests and χ2 statistics were used to examine univariate differences between children who had at least one physician visit or ROM, and those who did not. Conditional logistic regression was used to model the association between risk factors and the likelihood of one physician visit and ROM separately. Cases and controls were matched (1:1) on month and year of birth due to the strong influence of season and age on otitis media incidence. Risk factors with P<0.05 in univariate models were included in multivariate models to obtain adjusted ORs. If two variables were highly correlated (r>0.50), only one was chosen for the adjusted model based on the log-likelihood ratio test and the Wald statistic. All of the statistical analyses were completed using SAS 9.1 (SAS Institute Inc, USA).
RESULTS
Population
There were 58,705 singleton births during the study period, and physician visit data were available for every child. Children who had missing or erroneous risk factor information (n=8158), or who died (n=73) were excluded. The final cohort included 50,474 children (86% of all births). There were no significant differences in outcome or available risk factor distribution between the original and final cohort (data not shown).
Outcome measure
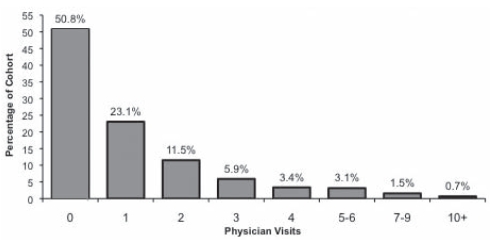
The 14-day exclusion removed 23% of the otitis media physician visits, leaving 57,695 visits during the first three years of life. The total number of visits per child ranged from zero to 20. The greatest rate of visits occurred during the first (21 per 100 children) and second (20 per 100 children) year of life. Overall, there were 38 physician visits per 100 child-years and the mean (± SD) number of visits per child was 1.14±1.82. The total number of physician visits by cohort percentage is shown in Figure 1.
Figure 1).
Total number of physician visits by cohort percentage
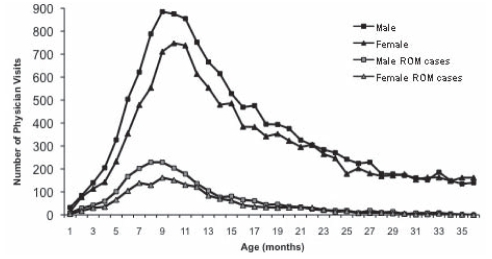
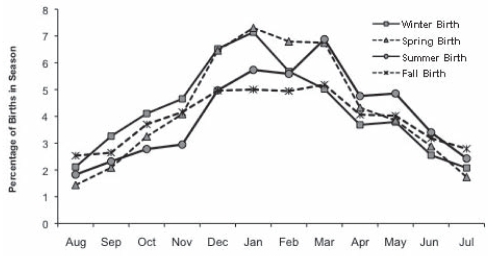
There were 24,551 children (48.6%) with at least one visit for otitis media: 12,045 during the first year, 8573 during the second year and 3933 during the third year of life. There were 3952 ROM cases (7.8%) identified from the 7571 children (15%) who had at least three visits during follow-up. Among those defined as ROM cases, 72% had their first visit before 12 months of life. The age at first visit is shown in Figure 2 for children with at least one visit and for those children who became ROM cases. The mean age of first visit was 16 months for ROM controls and 10 months for ROM cases. Male infants consistently had more visits during the first 20 months of life. Figure 3 illustrates the month of first physician visit by birth season.
Figure 2).
Age at first physician visit for otitis media. ROM Recurrent otitis media
Figure 3).
Month of first physician visit for otitis media
Risk factors
Maternal age ranged from 14 to 51 years (mean 31±5.4 years), birth weight ranged from 450 g to 6072 g (mean 3451±353 g) and gestational age ranged from 22 weeks to 43 weeks (mean 38.9±1.8 weeks). Additional information on the risk factor distribution is provided in Table 1.
TABLE 1.
Conditional logistic results for initial and recurrent otitis media
| Cohort, % |
Initial otitis media, OR (95% CI) |
Recurrent otitis media, OR (95% CI) |
|||
|---|---|---|---|---|---|
| Univariate | Multivariate* | Univariate | Multivariate* | ||
| Total | 100 | ||||
| Sex | |||||
| Male | 51.5 | 1.24 (1.20–1.28) | 1.23 (1.19–1.27) | 1.34 (1.26–1.43) | 1.32 (1.24–1.41) |
| Female | 48.5 | Reference | Reference | Reference | Reference |
| Older siblings | |||||
| Yes | 53.9 | 1.14 (1.10–1.18) | 1.18 (1.14–1.22) | 1.28 (1.20–1.36) | 1.32 (1.23–1.41) |
| No | 46.1 | Reference | Reference | Reference | Reference |
| Maternal smoking during pregnancy | |||||
| Yes | 10.4 | 1.24 (1.17–1.31) | 1.16 (1.09–1.23) | 1.17 (1.07–1.29) | 1.09 (0.98–1.20) |
| No | 89.6 | Reference | Reference | Reference | Reference |
| Initiation of breastfeeding | |||||
| No | 8.7 | 0.87 (0.81–0.92) | 0.83 (0.78–0.89) | 1.04 (0.93–1.16) | 0.97 (0.87–1.09) |
| Yes | 91.3 | Reference | Reference | Reference | Reference |
| Aboriginal status | |||||
| Yes | 2.4 | 1.71 (1.52–1.92) | 1.47 (1.30–1.65) | 1.70 (1.43–2.02) | 1.45 (1.21–1.73) |
| No | 97.6 | Reference | Reference | Reference | Reference |
| Birth weight, g | |||||
| >4000 | 13.5 | 1.23 (1.17–1.30) | 1.18 (1.12–1.24) | 1.24 (1.14–1.36) | 1.16 (1.07–1.27) |
| 2500–4000 | 81.7 | Reference | Reference | Reference | Reference |
| <2500 | 4.8 | 0.89 (0.82–0.97) | 0.95 (0.88–1.03) | 1.05 (0.91–1.22) | 1.10 (0.95–1.28) |
| Gestational age, weeks | |||||
| >41 | 1.6 | 0.98 (0.85–1.12) | 0.82 (0.63–1.08) | ||
| 37–41 | 90.5 | Reference | Reference | ||
| 30–36 | 6.3 | 0.99 (0.93–1.06) | 1.15 (1.02–1.29) | ||
| <30 | 0.4 | 1.07 (0.87–1.31) | 1.57 (1.14–2.15) | ||
| Maternal age, years | |||||
| <20 | 3.1 | 1.36 (1.22–1.51) | 1.34 (1.20–1.49) | 1.49 (1.26–1.76) | 1.53 (1.28–1.82) |
| 20–24 | 13.0 | 1.10 (1.04–1.17) | 1.09 (1.03–1.15) | 1.09 (0.99–1.22) | 1.09 (0.98–1.22) |
| 25–29 | 28.7 | Reference | Reference | Reference | Reference |
| 30–34 | 33.8 | 0.93 (0.89–0.98) | 0.93 (0.89–0.97) | 1.07 (0.98–1.16) | 1.05 (0.97–1.14) |
| 35–39 | 18.1 | 0.87 (0.83–0.92) | 0.86 (0.82–0.91) | 0.93 (0.85–1.03) | 0.90 (0.82–0.99) |
| >39 | 3.3 | 0.75 (0.68–0.83) | 0.74 (0.66–0.81) | 0.80 (0.65–0.98) | 0.76 (0.62–0.93) |
| Neighbourhood income level | |||||
| 1 (lowest) | 20.2 | 0.96 (0.90–1.02) | 0.83 (0.78–0.88) | 0.98 (0.88–1.09) | 0.85 (0.76–0.95) |
| 2 | 21.3 | 0.92 (0.87–0.97) | 0.82 (0.77–0.87) | 0.96 (0.87–1.07) | 0.86 (0.77–0.96) |
| 3 | 21.0 | 1.00 (0.95–1.06) | 0.92 (0.87–0.97) | 1.02 (0.92–1.13) | 0.94 (0.85–1.05) |
| 4 | 20.4 | 0.98 (0.92–1.03) | 0.93 (0.88–0.98) | 0.96 (0.87–1.07) | 0.92 (0.83–1.02) |
| 5 (highest) | 17.1 | Reference | Reference | Reference | Reference |
| Neighbourhood female postsecondary education | |||||
| 1 (lowest) | 25.3 | 1.27 (1.21–1.34) | 1.30 (1.23–1.37) | 1.29 (1.18–1.41) | 1.27 (1.15–1.41) |
| 2 | 25.1 | 1.20 (1.14–1.25) | 1.20 (1.15–1.27) | 1.18 (1.08–1.29) | 1.17 (1.06–1.28) |
| 3 | 24.7 | 1.10 (1.05–1.16) | 1.10 (1.05–1.16) | 1.10 (1.00–1.20) | 1.09 (0.99–1.19) |
| 4 (highest) | 24.9 | Reference | Reference | Reference | Reference |
| Residence indicator | |||||
| Rural | 10.9 | 1.18 (1.12–1.25) | 1.16 (1.10–1.23) | 1.12 (1.02–1.24) | 1.10 (1.00–1.21) |
| Urban | 89.1 | Reference | Reference | Reference | Reference |
Multivariate models are adjusted for sex, siblings, maternal smoking, breastfeeding, Aboriginal status, birth weight, maternal age, neighbourhood income, neighbourhood female education and residence
Regression analyses
In univariate analyses (Table 1), the greatest odds for one or more visit were for Aboriginal status, maternal age younger than 20 years, living in neighbourhoods with the lowest female education, male sex, maternal smoking during pregnancy and birth weight of greater than 4000 g. The results for ROM were similar to those for one or more visits.
In multivariate analyses (Table 1), male Aboriginal children living with older siblings in rural areas and whose mothers smoked during pregnancy had the greatest odds of otitis media. Surprisingly, the noninitiation of breastfeeding was associated with lower odds in both adjusted models, although this effect was nonsignificant for ROM. Birth weight of more than 4000 g was associated with greater odds of otitis media. Gestational age was not included in the adjusted models. ORs decreased with increasing maternal age. Relative to children born in the highest income and female education neighbourhoods, the odds were lower for children born in low-income neighbourhoods but were higher for those born in low female education neighbourhoods. ORs for male sex, older siblings, birth weight and maternal age younger than 20 years were stronger in the adjusted ROM model versus the model of one or more visits.
There were 15,712 children who filled an antibiotic prescription within four days of their otitis media physician visit (64% of those with a physician visit for otitis media). These children were considered to be acute otitis media cases because they would have received the prescription at the initial physician visit or at a subsequent follow-up visit if watchful waiting was used for the first 48 h to 72 h. Multivariate modelling using these data (results not shown) confirmed the results presented in Table 1, with the exception of the ORs for breastfeeding initiation and rural residence, which both became nonsignificant. Because antibiotic data could only be linked to 64% of the otitis media visits, it is likely that some disease misclassification occurred. The outcome definition may have captured Eustachian tube disorders and chronic otitis media with effusion by including the broad three-digit ICD-9 codes. A subanalysis using visits coded specifically for acute otitis media (ICD-9 codes 381.0 and 382.0) was completed, but the results (not shown) were generally not statistically significant (only 7% of all visits used four-digit ICD-9 coding). Exceptions were the estimates for breastfeeding initiation and rural residence, which both changed direction and became statistically significant.
Separate unmatched analyses examined birth season (Table 2). Children born during the spring and winter seasons had higher odds for at least one otitis media visit during the first year of life, but this association became negligible during the latter years.
TABLE 2.
Adjusted* logistic regression results for initial otitis media visit
| Season of birth | Cohort, % | Entire follow-up, OR (95% CI) | First year of life, OR (95% CI) | Second year of life, OR (95% CI) |
|---|---|---|---|---|
| Winter | 21.7 | 1.15 (1.09–1.21) | 1.35 (1.26–1.44) | 0.94 (0.86–1.02) |
| Spring | 27.3 | 1.15 (1.10–1.21) | 1.42 (1.33–1.51) | 0.89 (0.83–0.97) |
| Summer | 26.6 | 1.05 (1.00–1.10) | 1.12 (1.05–1.19) | 0.99 (0.92–1.07) |
| Fall | 24.3 | Reference | Reference | Reference |
Models are adjusted for sex, siblings, maternal smoking, breastfeeding, Aboriginal status, birth weight, maternal age, neighbourhood income, neighbourhood female education and residence
DISCUSSION
To accurately assess the population-level incidence and risk factors for otitis media in BC, we used Canada’s universal health care databases and linked multiple administrative data. We identified every child born in our study region during a two-year period. The availability of comprehensive data enabled us to follow each child through the first three years of life and to evaluate many otitis media risk factors.
Otitis media incidence and recurrence
The present study found otitis media incidence during the first year of life to be at the lower bound, compared with other studies (22% versus 21% to 64%), but the median age of first visit (eight to 10 months) concurs with those studies (18–21). The data here are more recent than previous studies and may reflect changes in physician practices and regional differences. Regardless, otitis media continues to be the main reason young children receive antibiotics in BC (2).
Seventy-three per cent of the children with at least three visits during follow-up (n=7571) had their initial visit during the first year of life. Additionally, children with a visit for otitis media before their first birthday were seven times more likely to become ROM cases by their third birthday than children who had their visit in the latter years. This observation suggests that children who initially present earlier, do so as a result of an underlying vulnerability.
Otitis media risk factors
The present study found spring and winter birth to be positively associated with otitis media during the first year of life. This is contrary to previous findings that found spring birth to be protective (22) and fall birth to be a risk factor (11) for otitis media. These children are probably at greater risk because those born in the spring reach eight to 10 months of age during the seasonal peak following their birth. More importantly, although children may be at increased risk partly due to the timing of their birth, this effect is only observed during the first year of life.
The estimates for sex and older siblings were slightly lower than those found in the US, but concur with studies from northern Europe (10,23–27). Interestingly, the present study found that the sex difference levelled off at approximately 20 months. It is possible that sex differences in respiratory maturity or immune system development exist at birth but become less important as children develop (14).
In the present study, it was not possible to identify mothers who quit smoking during their pregnancy or to identify the presence of other smokers in the home. This probably resulted in some misclassification in which children in our ‘no’ category were actually exposed. This type of bias would attenuate the estimated ORs toward the null and may explain the difference between the adjusted ROM estimate here (OR=1.16) and those of two meta-analyses (23,28) that examined parental smoking (OR=1.38 and OR=1.76).
This same type of bias may be present for the Aboriginal status variable because it required children to be registered with the federal government. Thus, although the largest ORs were for Aboriginal children, these may be biased toward the null. While unmeasured socioeconomic factors could be responsible, genetics could also play a role in making these infants more susceptible (12,29,30). Although Aboriginal children were more often born overweight versus their non-Aboriginal counterparts, the numbers were too small to have influenced the findings for high birth weight.
The present study joined several others in not finding a protective role for breastfeeding with respect to infection (23,31). However, the variable used (ie, ‘breastfeeding initiated at time of discharge from hospital’) did not measure duration or exclusivity of breastfeeding (as used in previous studies [32]), which are better indicators of the protective capacity of breastfeeding. Provincial data found that 69% of new mothers breastfed for at least three months in 1999, suggesting that approximately 20% of our cohort were breastfed for some duration less than three months (33).
Maternal age and female education had an inverse relationship with otitis media, while the opposite relationship was found with neighbourhood income. Canada’s universal health care system ensures that every child has access to a physician; thus, it is unlikely that children of low-income neighbourhoods have less access to physicians. It is more probable that children living in high-income neighbourhoods are more likely to attend daycare. Rural residence was associated with otitis media, but this may be partially explained by rural families having more children because the present study adjusted for parity but not the number of older siblings. While neighbourhood income, female education and rural residence data were linked to this cohort based on dissemination area, it is important to note that they were sufficiently robust in the multivariate models to adjust for individual-level risk factors.
Study limitations
Information on daycare attendance, duration of breast-feeding and non-Aboriginal ethnic groups was not available at the population level and, therefore, could not be considered in the present study. Our study could not include emergency room visits, but it is standard practice for emergency room physicians to recommend that children follow up with their family physician. A physician visit could result in more than one diagnosis, and otitis media diagnoses may not be recorded during visits in which a more serious disease was also diagnosed. It is also possible that caregivers will not seek medical attention for every episode of otitis media, especially if physicians are unlikely to prescribe antibiotics.
During the study period, there was a growing tendency for physicians to use the ‘wait and see’ approach for the management of otitis media (34). However, during the study period, it was standard for physicians to treat children younger than two years of age with antibiotics if acute otitis media was suspected, and more than 85% of the physician visits were for these children (17).
Our study is the largest Canadian population-based study of otitis media and its associated risk factors. The present study demonstrated the utility of linking multiple administrative databases to examine childhood illness. For example, by following the methodology described here, future research could examine the impact of interventions or other changes in clinical practice and disease management. Otitis media incidence is comparatively low in southwestern BC, but it remains the most common reason young children receive antibiotics. The greatest estimates were for male Aboriginal children with young mothers who live in neighbourhoods with low female education.
Acknowledgments
Additional support was provided by the Center for Health and Environment Research at the University of British Columbia, funded by the Michael Smith Foundation for Health Research (MSFHR). The Canadian Institutes of Health Research and MSFHR supported Elaina MacIntyre through a University of British Columbia Bridge Strategic Training Fellowship, Senior Graduate Studentship, and Frederick Banting and Charles Best Canada Graduate Scholarship. Mieke Koehoorn was supported in part by an MSFHR Scholar Award.
Footnotes
SUPPORT: This research was supported by the BC Centre for Disease Control via an agreement with Health Canada under the Border Air Quality Strategy (GEH0402).
REFERENCES
- 1.Forgie S, Zhanel G, Robinson J, Canadian Paediatric Society, Infectious Diseases and Immunization Committee Antibiotic management of acute otitis media Paediatr Child Health 19983265–7.20401260 [Google Scholar]
- 2.Marra F, Patrick DM, Chong M, Bowie WR. Antibiotic use among children in British Columbia, Canada. J Antimicrob Chemother. 2006;58:830–9. doi: 10.1093/jac/dkl275. [DOI] [PubMed] [Google Scholar]
- 3.Monobe H, Ishibashi T, Nomura Y, Shinogami M, Yano J. Role of respiratory viruses in children with acute otitis media. Int J Pediatr Otorhinolaryngol. 2003;67:801–6. doi: 10.1016/s0165-5876(03)00124-1. [DOI] [PubMed] [Google Scholar]
- 4.Bennett KE, Haggard MP. Accumulation of factors influencing children’s middle ear disease: Risk factor modelling on a large population cohort. J Epidemiol Community Health. 1998;52:786–93. doi: 10.1136/jech.52.12.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett KE, Haggard MP, Silva PA, Stewart IA. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child. 2001;85:91–5. doi: 10.1136/adc.85.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eldeirawi K, Persky VW. History of ear infections and prevalence of asthma in a national sample of children aged 2 to 11 years: The Third National Health and Nutrition Examination Survey, 1988 to 1994. Chest. 2004;125:1685–92. doi: 10.1378/chest.125.5.1685. [DOI] [PubMed] [Google Scholar]
- 7.Lieu JE, Feinstein AR. Effect of gestational and passive smoke exposure on ear infections in children. Arch Pediatr Adolesc Med. 2002;156:147–54. doi: 10.1001/archpedi.156.2.147. [DOI] [PubMed] [Google Scholar]
- 8.Bluestone C, Klein J. Otitis Media in Infants and Children. 4th edn. Philadelphia: WB Saunders Company; 2007. [Google Scholar]
- 9.Paradise JL, Rockette HE, Colborn DK, et al. Otitis media in 2253 Pittsburgh-area infants: Prevalence and risk factors during the first two years of life. Pediatrics. 1997;99:318–33. doi: 10.1542/peds.99.3.318. [DOI] [PubMed] [Google Scholar]
- 10.Ey JL, Holberg CJ, Aldous MB, Wright AL, Martinez FD, Taussig LM. Passive smoke exposure and otitis media in the first year of life. Group Health Medical Associates. Pediatrics. 1995;95:670–7. [PubMed] [Google Scholar]
- 11.Pettigrew MM, Gent JF, Triche EW, Belanger KD, Bracken MB, Leaderer BP. Infant otitis media and the use of secondary heating sources. Epidemiology. 2004;15:13–20. doi: 10.1097/01.ede.0000101292.41006.2e. [DOI] [PubMed] [Google Scholar]
- 12.Beery QC, Doyle WJ, Cantekin EI, Bluestone CD, Wiet RJ. Eustachian tube function in an American Indian population. Ann Otol Rhinol Laryngol Suppl. 1980;89:28–33. doi: 10.1177/00034894800890s310. [DOI] [PubMed] [Google Scholar]
- 13.Teele DW, Klein JO, Chase C, Menyuk P, Rosner BA. Otitis media in infancy and intellectual ability, school achievement, speech, and language at age 7 years. Greater Boston Otitis Media Study Group. J Infect Dis. 1990;162:685–94. doi: 10.1093/infdis/162.3.685. [DOI] [PubMed] [Google Scholar]
- 14.Engel J, Anteunis L, Volovics A, Hendriks J, Marres E. Prevalence rates of otitis media with effusion from 0 to 2 years of age: Healthy-born versus high-risk-born infants. Int J Pediatr Otorhinolaryngol. 1999;47:243–51. doi: 10.1016/s0165-5876(98)00185-2. [DOI] [PubMed] [Google Scholar]
- 15.Engel JA, Straetemans M, Zielhuis GA. Birth characteristics and recurrent otitis media with effusion in young children. Int J Pediatr Otorhinolaryngol. 2005;69:533–40. doi: 10.1016/j.ijporl.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 16.Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. A cohort study of traffic-related air pollution impacts on birth outcomes. Environ Health Perspect. 2008;116:680–6. doi: 10.1289/ehp.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.British Columbia Medical Association Guidelines & Protocols: Acute Otitis Media (AOM) < www.healthservices.gov.bc.ca/msp/protoguides/gps/otitaom.pdf> (Accessed on October 4, 2004).
- 18.Homoe P, Christensen RB, Bretlau P. Acute otitis media and age at onset among children in Greenland. Acta Otolaryngol. 1999;119:65–71. doi: 10.1080/00016489950181963. [DOI] [PubMed] [Google Scholar]
- 19.Sipila M, Pukander J, Karma P. Incidence of acute otitis media up to the age of 1 1/2 years in urban infants. Acta Otolaryngol. 1987;104:138–45. doi: 10.3109/00016488709109059. [DOI] [PubMed] [Google Scholar]
- 20.Baraibar R. Incidence and risk factors of acute otitis media in children. Clin Microbiol Infect. 1997;3(Suppl 3):S13–22. [PubMed] [Google Scholar]
- 21.Kvaerner KJ, Nafstad P, Hagen JA, Mair IW, Jaakkola JJ. Recurrent acute otitis media: The significance of age at onset. Acta Otolaryngol. 1997;117:578–84. doi: 10.3109/00016489709113441. [DOI] [PubMed] [Google Scholar]
- 22.Caceres Udina MJ, Alvarez Martinez JA, Argente del Castillo J, et al. [Incidence, air pollution and risk factors of acute otitis media in the first year of life: A prospective study.] An Pediatr (Barc) 2004;60:133–8. doi: 10.1016/s1695-4033(04)78233-8. [DOI] [PubMed] [Google Scholar]
- 23.Uhari M, Mantysaari K, Niemela M. A meta-analytic review of the risk factors for acute otitis media. Clin Infect Dis. 1996;22:1079–83. doi: 10.1093/clinids/22.6.1079. [DOI] [PubMed] [Google Scholar]
- 24.Alho OP, Kilkku O, Oja H, Koivu M, Sorri M. Control of the temporal aspect when considering risk factors for acute otitis media. Arch Otolaryngol Head Neck Surg. 1993;119:444–9. doi: 10.1001/archotol.1993.01880160092014. [DOI] [PubMed] [Google Scholar]
- 25.Kero P, Piekkala P. Factors affecting the occurrence of acute otitis media during the first year of life. Acta Paediatr Scand. 1987;76:618–23. doi: 10.1111/j.1651-2227.1987.tb10531.x. [DOI] [PubMed] [Google Scholar]
- 26.Daigler GE, Markello SJ, Cummings KM. The effect of indoor air pollutants on otitis media and asthma in children. Laryngoscope. 1991;101:293–6. doi: 10.1288/00005537-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Teele DW, Klein JO, Rosner B. Epidemiology of otitis media during the first seven years of life in children in greater Boston: A prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 28.Strachan DP, Cook DG. Health effects of passive smoking. 4. Parental smoking, middle ear disease and adenotonsillectomy in children. Thorax. 1998;53:50–6. doi: 10.1136/thx.53.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boswell JB, Nienhuys TG. Onset of otitis media in the first eight weeks of life in aboriginal and non-aboriginal Australian infants. Ann Otol Rhinol Laryngol. 1995;104:542–9. doi: 10.1177/000348949510400708. [DOI] [PubMed] [Google Scholar]
- 30.Spivey GH, Hirschhorn N. A migrant study of adopted Apache children. Johns Hopkins Med J. 1977;140:43–6. [PubMed] [Google Scholar]
- 31.Bauchner H, Leventhal JM, Shapiro ED. Studies of breastfeeding and infection: How good is the evidence? JAMA. 1986;256:557–92. [PubMed] [Google Scholar]
- 32.Celedon JC, Litonjua AA, Weiss ST, Gold DR. Day care attendance in the first year of life and illnesses of the upper and lower respiratory tract in children with a familial history of atopy. Pediatrics. 1999;104:495–500. doi: 10.1542/peds.104.3.495. [DOI] [PubMed] [Google Scholar]
- 33.Canadian Perinatal Surveillance System Canadian Perinatal Health Report 2003 Minister of Public Works and Government Services Canada. Ottawa: Health Canada; 2003. [Google Scholar]
- 34.Corbeel L. The “wait and see” approach of acute otitis media. Eur J Pediatr. 2005;164:1–2. doi: 10.1007/s00431-004-1565-z. [DOI] [PubMed] [Google Scholar]