Abstract
Under stress, men tend to withdraw socially while women seek social support. The current functional magnetic resonance imaging study indicates that stress also affects brain activity while viewing emotional faces differently for men and women. Fusiform face area (FFA) response to faces was diminished by acute stress in males but increased by stress in females. Furthermore, among stressed males viewing angry faces, brain regions involved in interpreting and understanding others' emotions (the insula, temporal pole and inferior frontal gyrus) showed reduced coordination with the FFA and the amygdala, whereas the functional connectivity among these regions increased with stress for females. These findings suggest that stress influences emotional perception differently for males and females.
Keywords: stress, sex differences, fusiform face area, amygdala, temporal pole, insula, inferior frontal gyrus, angry faces, functional connectivity, testosterone
Introduction
Stress can affect males and females quite differently [e.g., 1]. Sex differences in the effects of stress are particularly evident in social behavior: stress leads males to withdraw socially but leads females to seek social support [2]. Little is known about which brain regions mediate these sex differences in the effects of stress, but previous research suggests that the amygdala plays a role. The amygdala is activated by stress and helps regulate stress responses [3] and is more sexually dimorphic than most other brain regions [4]. Furthermore, animal research reveals that the amygdala responds differently to stress in males and females [5–7] and research with humans reveals sex differences in how the amygdala responds to emotionally arousing stimuli [4].
Of particular relevance for social behavior, the amygdala is involved in processing emotional faces, especially angry or fearful faces. For instance, whereas healthy individuals show increased fusiform and occipital cortex activity when viewing fearful faces, patients with amygdala lesions do not show this increased visual processing of emotional faces [8].
The current study builds on previous findings that stress increases affiliative behavior in response to stress for females but decreases it for males [2]. The specific hypothesis is that, for males observing other's emotions, stress will decrease interactions between the amygdala and brain regions such as the insula and temporal pole that help people understand others' state of mind and simulate others' emotions [9, 10], whereas for females, stress will increase interactions among these regions. In addition, it is hypothesized that, for males, stress will decrease coordination between a brain region engaged in basic visual processing of faces (the fusiform face area or FFA) [11] and regions engaged in simulating and interpreting facial emotions (again, the temporal pole and insula), whereas, for females, stress will increase coordinated activity among these regions.
Methods
Subjects
Forty-seven right-handed non-smoker young adults (age range 18–33, mean = 22.2 years) were included in the study (one additional female participated but did not complete the face scan session). Each of the four groups (male/female × stress/control) had 12 participants except the female control group, which had 11 participants. To reduce cortisol level variability, all participants were scanned between 2 and 5 pm and refrained from eating, exercising and caffeine for at least an hour and avoided sleeping for at least two hours before arriving for the study. None were on hormone birth control, corticosteroid medications or beta-adrenergic agonists. There were no differences by sex or stress group in age, years of education, hours of sleep the night before, or baseline measures of stress, affect or depression.
Experiment procedure
After participants gave informed consent for the session, they were asked to drink 8 oz. of water. After a delay of at least 10 minutes filled with scan instructions and questionnaires, they provided a 1-ml baseline saliva sample by drooling into a tube. Next, participants assigned to the stress group were asked to hold their hand in ice water (0–5° C) for as long as possible up to three minutes (control participants held their hand in 37–40° C water). To increase the strength of the stress manipulation, participants were also told they might need to repeat the hand immersion procedure (with the same temperature water) near the end of the session. For about the next 15 minutes, participants received instructions and practice trials for a decision task and entered the scanner. They first lay quietly during a prescan (2 min), a structural scan (5 min) and then were scanned while doing a 9-min decision task unrelated to the current study (without any social or pictorial stimuli). Approximately 35 minutesi after the stress manipulation onset, while still in the scanner, they gave a saliva sample using two small sponges placed inside their mouth. Immediately after this saliva sample, participants were scanned while they viewed eight blocks of 20 faces each. Half of the blocks had neutral faces and half had angry faces and block order was counterbalanced across participants. Face blocks were interspersed with 16-s fixation blocks. Each face appeared for 1.5 ms and participants indicated whether it was male or female.
Salivary biomarkers
After experimental sessions, samples were stored in a laboratory freezer at −30°C. At the end of the study, samples were transported frozen to analytical laboratories (Salimetrics, LLC, State College, PA) where duplicate assays for cortisol were conducted for each sample and for testosterone and estrogen for the baseline drool sample (the mean of the duplicate assays was analyzed).
Face localizer
After the angry/neutral face scan, a separate functional localizer scan identified the FFA for each participant by alternating four 18-s blocks of neutral faces with four 18-s blocks of intermixed non-face objects and scenes. Sixteen images were shown in each block and participants were asked to indicate whether each image was repeated. Contrasts of the face and non-face blocks during the localizer scan revealed the most significantly activated voxel in response to faces for each participant within a structurally defined mask of the right fusiform gyrus. Significantly activated voxels within an 8-mm radius sphere around this voxel were used as the FFA region of interest (ROI) for each participant.
Recognition memory
At the end of the experiment session, after exiting the scanner, participants completed a yes/no recognition test with 80 faces from the main face task and 40 new faces (half of each type were angry and half were neutral; old and new faces were counterbalanced across participants).
Scan parameters
Data were acquired on a Siemens 3T scanner using a T2*-sensitive echo-planar imaging sequence (slice thickness = 3.5 mm, TR = 2 s, TE = 25 ms and FOV = 92 mm). T1-weighted anatomical images were acquired using a 3D-MPRAGE sequence.
fMRI Analyses
FEAT Version 5.98, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl) was used. Data were skull stripped with BET [12], motion corrected with MCFLIRT [13], smoothed with a Gaussian kernel (5 mm FWHM) and registered with FLIRT. In the GLM, the models included the regressors of the angry and neutral face blocks as well as the temporal derivates. Temporal filtering was also applied [14]. Noise components were identified using MELODIC ICA [15] and removed.
Featquery was used to extract mean percent signal change values for the FFA during the main face task. In addition, to assess functional connectivity during emotional face viewing, three psychophysiological interaction (PPI) analyses [16] were conducted. The first used the functionally defined FFA for each participant as the seed region and the other two used the left and right amygdala (structurally defined using the FSL Harvard-Oxford cortical atlas) as seed regions. For each PPI analysis, a regressor was created that convolved: 1) Hemodynamic-response-function-convolved task regressor for the angry - neutral contrast and 2) the time-course of the seed ROI. This regressor was entered in a lower-level FEAT analysis. On the higher-level analysis, a 2×2 ANOVA design tested the effects of sex and stress in a mixed effects analysis. Both on the lower-level and higher-level analyses, Z (Gaussianised T/F) statistic images were thresholded at the whole-brain level using clusters determined with Z>2.3 voxel-wise thresholding and a family-wise error-corrected cluster significance threshold of p=0.05 [17].
Results
Cold pressor stress increased cortisol levels
The stress manipulation increased salivary cortisol levels (cortisol change in ug/dL from baseline to just before the angry vs. neutral faces task about 35 min later: Mstress=.15, SE=.03, Mcontrol= −.01, SE=.03, t(45)=3.95, p<.001). There were no significant sex differences in baseline cortisol or cortisol change; the stress effect on cortisol change was significant for both males (p<.01) and females (p=.01).
Encoding task and memory test accuracy were not significantly affected by stress
Gender judgments were highly accurate (M = 96% correct) with no significant differences by stress condition or sex group. Likewise, there were no significant effects for recognition memory accuracy (See Table, Supplemental Digital Content 1, http://links.lww.com/WNR/A78).
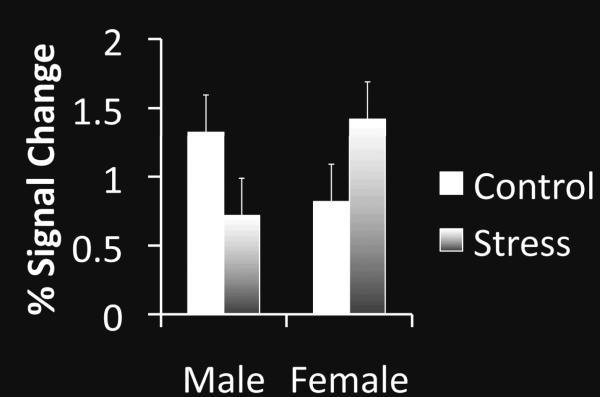
Stress had opposite effects on FFA response for males and females
A 2 (stress vs. control condition) × 2 (male vs. female) × 2 (neutral vs. angry facial expression) ANOVA on the mean percent signal change in the FFA during face viewing revealed a significant interaction of participant sex and stress, F(1,43)=5.84, p<.05, ηp2=.12. Stress increased FFA activity in response to faces for females but decreased it for males (Fig. 1). There were no other significant effects (all p>.2).
Figure 1.
Percent signal change in the fusiform face area (FFA) defined functionally for each participant. Error bars indicate standard error of the mean.
Across male and female participants, higher baseline testosterone levels predicted higher FFA activity in response to faces in the control condition, r(23)=.60, p<.01, but predicted lower FFA activity in the stress condition, r(23)=−.49, p<.05ii. Baseline estrogen did not correlate significantly with FFA face activation in either condition.
There were no sex differences in overall amygdala activity
Analyses of the mean percent signal change within the right and left amygdala structural ROIs revealed no significant sex effects or interactions.
For males versus females, stress had opposite effects on functional connectivity of regions involved in processing facial emotion
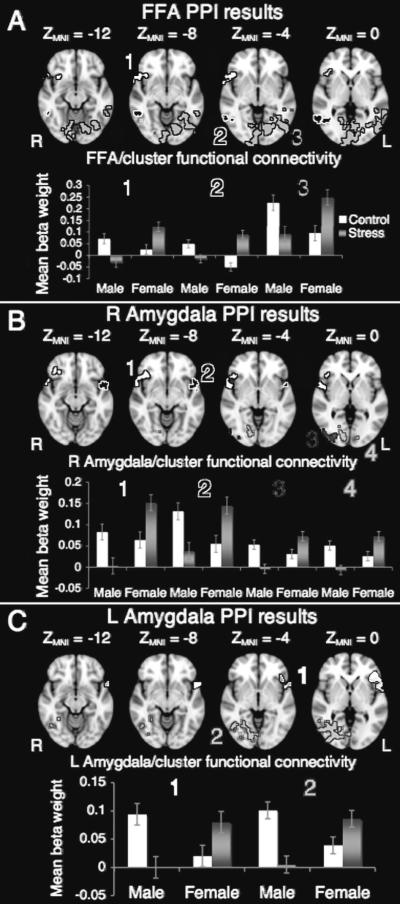
All three PPI analyses revealed that stress increased functional connectivity with clusters overlapping the right temporal pole, insula and inferior frontal gyrus for females but decreased functional connectivity with these regions for males (See Fig. 2, Table Supplemental Digital Content 2, http://links.lww.com/WNR/A79 and ROIs and color clusters in Supplemental Digital Content 3, http://links.lww.com/WNR/A80). In addition, similar sex-by-stress functional connectivity interactions were seen in posterior visual regions.
Figure 2.
A) A psychophysiological interaction (PPI) analysis with functionally defined FFA as the seed region and face expression (angry versus neutral) as a modulatory variable, revealed a sex by stress interaction in functional connectivity of the FFA with a cluster spanning portions of the temporal pole, inferior frontal cortex and insula (cluster 1) as well as with occipital cortex regions (clusters 2 and 3);
B) The same PPI analysis substituting the structurally defined right amygdala as the seed region revealed a sex by stress interaction in functional connectivity of the right amygdala with extended temporal pole clusters on both the right (cluster 1) and left (cluster 2) as well as with occipital fusiform cortex and other extrastriate regions (clusters 3 and 4);
C) Repeating the PPI analysis with the left amygdala as a seed region revealed a sex by stress interaction in functional connectivity of the left amygdala and a cluster overlapping left temporal pole, inferior frontal cortex and insula (cluster 1) as well as with occipital fusiform cortex and other extrastriate regions (cluster 2).
Across stress and control, functional connectivity during viewing angry faces was greater for females than for males
Although the main purpose of this study was to examine sex-by-stress interactions, in all three PPI analyses, females showed overall greater functional connectivity with the insula and adjacent brain regions than males did (See Table in Supplemental Digital Content 4, http://links.lww.com/WNR/A81 and Fig. in Supplemental Digital Content 5, http://links.lww.com/WNR/A82). There were no regions for which males showed greater functional connectivity to the amygdala and FFA than females did. Furthermore, the only main effect of stress was that, for the right amygdala, the control group showed greater functional connectivity with the middle frontal gyrus during viewing the angry faces than the stress group did. Thus, most effects of stress on functional connectivity with the amygdala were sex-specific.
Discussion
This study reveals that acute stress affects face perception in opposite ways for males and females. Activity in a visual region specialized for face processing (the FFA) showed an interaction effect such that FFA activity was greater under stress for females but diminished under stress for males, a relationship that was correlated with baseline testosterone but not estrogen levels.
In addition, there were sex differences in how stress affected functional connectivity among brain regions involved in socioemotional processing of faces. In order to interpret emotional expressions, people recruit a broader network of brain regions than just the FFA [18]. For instance, the temporal pole is important for face processing and understanding others' state of mind and emotions [9]. The insula contributes to empathy and social understanding because it helps simulate the experiences of others [10]. Together, the temporal pole, insula and nearby inferior frontal regions—along with the amygdala—are part of an action representation circuit that helps people internally simulate others' emotions [19].
Across the stress and control conditions in this study, females showed greater functional connectivity between the insula and the FFA and amygdala when viewing angry faces than males did, which is consistent with findings of greater emotion empathy among females than males [20, 21]. Furthermore, stress had opposite effects on males and females, reducing functional connectivity between regions involved in understanding and simulating others' facial emotions (the temporal pole, insula and inferior frontal cortex) and the FFA in males but increasing functional connectivity in those same networks in females. These findings cannot be explained by a failure to look at the faces among the stressed males, as they later remembered the faces as well as the other groups and rated the face gender as effectively. Instead, it appears that coordination of basic face processing by the FFA and interpretation and simulation of emotional expressions by the extended temporal pole region increased under stress for females but decreased under stress for males. This pattern is consistent with behavioral findings that stress promotes social affiliation for females but disrupts it for males [2]. However, these are the first findings to indicate that sex differences in the effects of stress on social behavior extend to one of the most basic social transactions—processing someone else's facial expression.
Previous studies examining amygdala activity during rest reveal sex differences in whether the right or left amygdala shows greater functional connectivity with other brain regions [22–25]. Unlike these previous resting-state studies, the present study examined how functional connectivity increased while viewing angry faces rather than neutral faces. There were some main effects of sex, such that, in general, females showed greater functional connectivity between the amygdala and other regions during viewing of angry faces, with this sex difference being strongest for the right amygdala.
However, the current findings indicate that such sex differences can reverse under stress, with consistent effects in the right and left amygdala. For females, stress increased connectivity between the amygdala and clusters overlapping the temporal pole, insula and inferior frontal cortex, whereas, for males, stress decreased connectivity between the amygdala and this extended temporal pole region during viewing of angry faces. Furthermore, stress affected amygdala functional connectivity with the right fusiform cortex and other extrastriate visual regions, suggesting that stress reduces the influence of the amygdala on males' visual processing of angry faces whereas stress increases the influence of the amygdala on females' visual processing of angry faces.
Conclusion
This study indicates that experiencing an acute stressor affects subsequent activity and interactions in brain regions involved in decoding and interpreting others' facial expressions in opposite ways for males and females. These findings contribute to a growing literature showing that stress affects males and females differently [1, 4–7].
Supplementary Material
Acknowledgement
This work was supported by National Institute on Aging Grant R21AG030758.
Footnotes
M=35.5 minutes, SD = 4.6, minimum=28 min, maximum=46 min. There were no significant differences in time from the stress manipulation onset to the pre-task saliva sample by condition or sex and no significant interaction of sex and condition.
For the FFA-testosterone correlations, one outlier with testosterone more than 3 standard deviations above the mean was excluded.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the Balloon Analogue Risk Task. PLoS ONE. 2009;4:e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor SE. Social support. In: Friedman HS, Siliver RC, editors. Foundations of Health Psychology. Oxford University Press; New York: 2007. pp. 145–171. [Google Scholar]
- 3.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10(6):397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cahill L. Why sex matters for neuroscience. Nature Reviews Neuroscience. 2006;7(6):477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki-Sekino A, et al. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34(2):226–237. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Mitsushima D, et al. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. European Journal of Neuroscience. 2006;24(11):3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang Z, et al. Sex differences in functional brain activation during noxious visceral stimulation in rats. Pain. 2009;145(1–2):120–128. doi: 10.1016/j.pain.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vuilleumier P, et al. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7(11):1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 9.Olson IR, Ploaker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- 10.Singer T, Lamm C. The social neuroscience of empathy. Year in Cognitive Neuroscience 2009. 2009:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- 11.Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkinson M, et al. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 14.Woolrich MW, et al. Temporal autocorrelation in univariate linear modelling of fMRI data. NeuroImage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 15.Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Transactions on Medical Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- 16.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 17.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; New York: 2001. [Google Scholar]
- 18.Adolphs R. The social brain: Neural basis of social knowledge. Annual Review of Psychology. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carr L, et al. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(9):5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derntl B, et al. Multidimensional assessment of empathic abilities: Neural correlates and gender differences. Psychoneuroendocrinology. 2010;35(1):67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Schulte-Ruther M, et al. Gender differences in brain networks supporting empathy. NeuroImage. 2008;42(1):393–403. doi: 10.1016/j.neuroimage.2008.04.180. [DOI] [PubMed] [Google Scholar]
- 22.Ciumas C, Hirschberg AL, Savic I. High fetal testosterone and sexually dimorphic cerebral networks in females. Cerebral Cortex. 2009;19(5):1167–1174. doi: 10.1093/cercor/bhn160. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick LA, et al. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- 24.Savic I, Lindstrom P. PET and MRI show differences in cerebral asymmetry and functional connectivity between homo- and heterosexual subjects. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(27):9403–9408. doi: 10.1073/pnas.0801566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, et al. Gender effect on functional networks in resting brain. In: Gao XH, et al., editors. Medical Imaging and Informatics. 2008. pp. 160–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.