SUMMARY
Neurexins are multi-domain synaptic cell-adhesion proteins that associate with multiple partnering proteins. Genetic evidence indicates that neurexins may contribute to autism, schizophrenia and nicotine dependence. Using analytical ultracentrifugation, single particle electron microscopy, and solution X-ray scattering, we obtained a three-dimensional structural model of the entire extracellular domain of neurexin-1α. This protein adopts a dimensionally asymmetric conformation that is monomeric in solution with a maximum dimension of ~170Å. The extracellular domain of α-neurexin maintains a characteristic “Y” shape, whereby LNS domains 1–4 form an extended base of the “Y” and LNS5–6 the shorter arms. Moreover, two major regions of flexibility are present: one between EGF1 and LNS2, corresponding to splice site 1, another between LNS5 and 6. We thus provide the first structural insights into the architecture of the extracellular region of neurexin-1α, show how the protein may fit in the synaptic cleft, and how partnering proteins could bind simultaneously.
INTRODUCTION
Genes encoding neuronal cell-adhesion proteins are essential for development and maintenance of connectivity in the nervous system. Synaptic cell-adhesion proteins constitute a principal pathway contributing to genetic susceptibility of autism spectrum disorders (ADS) (Geschwind and Levitt, 2007). Emerging evidence indicates that variations in copy number and other rare variants within the genes encoding neurexin-1 and 3 (NRXN1 and NRXN3) contribute to ASD susceptibility and mental retardation (Feng et al., 2006; The Autism Genome Project Consortium, 2007; Kim et al., 2008; Yan et al., 2008; Glessner et al., 2009; Zahir et al., 2009; Zweier et al., 2009).
Neurexin-1α (αNRXN1) is a neuronal cell surface receptor that was originally identified as a high affinity receptor for the spider toxin α-latrotoxin, whereas NRXN2 and 3 were subsequently identified from DNA sequence similarity with αNRXN1. Within each NRXN gene the presence of two promoters, α and β, enables the expression of a longer α and a shorter βNRXN, yielding a total of six NRXN proteins (Missler and Südhof, 1998). Extensive independent alternative splicing of the encoded proteins (Ullrich et al., 1995) could specify a code of interactions between NRXNs and their ligands in different classes of synapses. Moreover, alternative splicing in NRXN3 creates a large diversity of secreted gene products including those encoding multiple variants with in-frame stop codons (Ushkaryov and Südhof, 1993; Ullrich et al., 1995). Similar to the construct used in this study, all secreted splice variants end between the sixth Laminin, Neurexin, Sex-hormone-binding globulin (LNS) domain and various positions before the beginning of the transmembrane domain.
Currently, four groups of endogenous ligands for α and βNRXNs have been identified: neuroligins (NLGN) (Ichtchenko et al., 1995), neurexophilins (Missler et al., 1998), dystroglycan (Sugita et al., 2001), and leucine-rich repeat transmembrane proteins (LRRTM2) (de Wit et al., 2009, Ko et al., 2009). NRXNs and NLGNs are thought to form a trans-synaptic complex meeting near the center of the synaptic cleft, with the C-terminal sequences of either protein extending in opposite directions, tethering them to the pre- and post-synaptic membranes respectively (Comoletti et al., 2007, Fabrichny et al., 2007, Araç et al., 2007, Chen et al., 2008). Early studies of cell association with neurons in cell culture suggested that NLGNs and NRXNs are sufficient to induce formation of new synapses (Scheiffele st al., 2000, Graf et al., 2004). In particular, all three αNRXNs induce clustering of the GABAergic postsynaptic scaffolding protein gephyrin and NLGN2, but not of the glutamatergic postsynaptic scaffolding protein PSD-95 or NLGN 1/3/4 in an artificial synapse-formation assay (Kang et al., 2008). This suggests that αNRXNs may be mediators of GABAergic synaptic protein recruitment and stabilization. Earlier studies of α NRXN-knockout mice revealed only mild variations in synaptic density and ultrastructure (Dudanova et al., 2007). More recently, a study of the αNRXN-1 knockout showed severe impairment of excitatory neurotransmission pathways (Etherton et al., 2009). αNRXNs appear to play a functional role at the synapse, including mediating Ca2+-triggered neurotransmitter release (Missler et al., 2003), but do not seem to participate in synapse formation. These findings suggest that the αNRXNs are a group of trans-synaptic cell-adhesion molecules that participate in a modular organization of presynaptic terminals by mediating the localized activation of Ca2+ channels.
Structurally, αNRXN1 is a large (~150kDa) multi-domain protein composed of several discernable regions. A cleavable N-terminal signal peptide is responsible for trafficking the protein to the cell membrane. The mature protein contains three homologous repeats, each motif composed of a central epidermal growth factor (EGF) domain flanked upstream and downstream by two LNS domains sharing limited protein identity (Figure 1A). These three repeats, formed by nine independently folded domains that span ~90% of the protein sequence, are followed by a single stalk domain that is likely to be partially rigidified through extensive O-linked glycosylation. The stalk domain connects to a single transmembrane domain and a short cytoplasmic tail containing a classical PDZ recognition motif that appears to target NRXN to the presynaptic region (Fairless et al., 2008). Although the crystal structures of the isolated second, fourth, and sixth LNS domains of αNRXN1 have been solved (Rudenko et al., 1999; Sheckler et al., 2006; Shen et al., 2008), the overall domain complexity of the intact protein has been an impediment to examining structural organization beyond individual LNS domains.
Figure 1. Schematic Diagram of the αNRXN1 Constructs Used and Hydrodynamic Characterization of the Purified Extracellular Domain of αNRXN_1–6.

A) Top, full length protein. N and C, N-and C-termini of the wild type, full-length protein. Stalk, O-linked glycosylation domain, E1 to E3, EGF domains. The intracellular domain is on the right side of the cell membrane. Potential N-linked glycosylation sites are reported using single letter code. Open arrowheads, sites of alternative splicing. L31, E280, and T1309 refer to amino acid number in the protein sequence. Dotted line, potential center of symmetry. B) Size exclusion chromatography trace of purified αNRXN_1–6. Inset, Coomassie blue staining of a sample composed of the main peak. C) G(s) distribution plots from the enhanced van Holde-Weischet analysis of sedimentation velocity experiments of two concentrations of αNRXN_1–6. D) 2-dimensional spectrum/Monte Carlo analysis of the 20 nM velocity experiment. The distribution shows a single, monomeric species with a molecular weight of ~140 kDa. The gray gradient indicates partial concentration. E) Concentration histogram of the globally fitted fixed molecular weight distribution analysis of the sedimentation equilibrium data.
See also Figure S1.
Using a set of complementary biophysical techniques, we describe a three-dimensional structural model of the entire extracellular domain of αNRXN1 in solution. Although some flexibility at the extremities of the extracellular domain is detected, the overall architecture of αNRXN1 is consistent with a semi-elongated protein with a stable shape resembling the letter “Y”. The results reported here represent, to our knowledge, the first three-dimensional structural models of the extracellular domain of αNRXN super-family that include neurexin (1 to 3) and Caspr (Contactin associated-like protein) 1 to 5. Together, these results should facilitate the understanding how αNRXN might be arranged in the limited space of the synaptic cleft and how this protein may associate with multiple transmembrane and soluble synaptic proteins.
RESULTS
Characterization of the purified extracellular domain of αNRXN
Protein expression and N-terminal sequencing
Two constructs from the extracellular domain of αNRXN1 were expressed as soluble entities in the cell culture medium of HEK293 GnTI- cells: one starting at position Leu31 and encompassing sites of alternative splicing #1 and #3 (αNRXN_1–6) and an N-terminal deletion devoid of the first LNS and EGF domains to yield a protein starting at position Glu280 (αNRXN_2–6) (Figure 1A). By size exclusion chromatography both constructs elute as single peaks (Figure S1B and S1A) indicating the presence of homogeneous monomeric species. In SDS-PAGE followed by Coomassie blue staining, αNRXN_1–6 and αNRXN_2–6 appear as single bands of ~140kDa and ~110kDa (insets of Figure S1B and S1A), consistent with the calculated molecular weight of the peptides. As we express αNRXN protein with its native leader peptide (mgtallqrggcfllclsllllgcwaelgsgLEFPG), Edman degradation of the first 5 residues of the mature protein was performed to assign the initial amino acids after the cleavage of the leader sequence. The unambiguously determined sequence Leu-Glu-Phe-Pro-Gly indicated that the mature protein starts at Leu31, consistent with previous findings (Missler et al., 1998) and sequence predictions.
Mass spectrometric analysis of αNRXN
Both αNRXN1 constructs were expressed in the culture medium of HEK293 GnTI- cells. These cells lack N-acetylglucosaminyltransferase I (GnTI) activity, and consequently glycosylation remains restricted to a homogeneous seven-residue oligosaccharide (Reeves et al., 2002), thus simplifying structural analyses. αNRXN1 contains four potential N-linked glycosylation sites at positions N125, N190, N790, and N1223 (Figure 1). Whereas the peptidic mass of the expressed protein is calculated to be 140,619 Da, MALDI-TOF indicated a MW value of 145,896 Da (data not shown) with a difference of 5,278 Da between the two values. As GnTI- cells only add to each N-linked glycosylation site a Man5GlcNAc2 (mass = 1361 Da) the estimated occupancy of the potential N-linked sugars in 3.87 units per molecule, a value consistent with conjugation by oligosaccharde at all four N-linked glycosylation sites. Functionally, αNRXN_1–6 is fully active in binding neuroligin-1 (Figure S1B and Boucard et al. 2005) and LRRTM2 (de Wit et al. 2009).
Analytical ultracentrifugation analyses of the extracellular domain of αNRXN1
Sedimentation velocity and equilibrium measurements provide complementary information useful in determining the dimensional asymmetry and oligomerization state of the extracellular domain of αNRXN1 in solution. To determine if αNRXN_1–6 forms reversibly self-associating oligomers, we compared sedimentation coefficient distributions extracted using the enhanced van Holde-Weischet analysis (Demeler & van Holde, 2004) of two different loading concentrations. This analysis shows identical monodisperse species for both concentrations (Figure 1C), suggesting an absence of oligomerization at these concentrations. The same data were then analyzed by Monte Carlo analysis (Demeler & Brookes, 2008) together with a 2-dimensional spectrum analysis (Brookes et al., 2010) and genetic algorithm analysis (Brookes and Demeler, 2007) to establish a molecular weight (Figure 1D). These analyses indicated that αNRXN_1–6 has a sedimentation coefficient of 6.36s (6.28s, 6.39s) and a molecular weight of 141 kDa (136.5 kDa, 151.4 kDa), with a frictional ratio of 1.54 (1.51, 1.64), consistent with the monomeric mass of αNRXN and indicative of an elongated particle (values in parenthesis are 95% confidence intervals from the Monte Carlo analysis) (Demeler 2009). The monomeric molecular weight was confirmed by sedimentation equilibrium data and a fixed molecular weight distribution analysis that gave a peak molecular weight of ~140 kDa (Figure 1E). Both sedimentation velocity and equilibrium experiments were in excellent agreement with the expected molecular weight based on amino acid sequence and mass spectrometry analyses.
Single Particle Electron Microscopy
αNRXN_1–6
To obtain structural information on the extracellular domain of αNRXN1, the purified protein was negative stained and imaged using a transmission EM. αNRXN_1–6 particles were monodisperse and homogeneous in size, although individual particles adopted a variety of conformations (Figure 2A). Approximately 6,000 particles were analyzed using multivariate statistics, image classification and averaging. In the majority of the class averages, only 5 globular domains were clearly visible. We presumed that only 5 of the 6 LNS domains were uniformly aligned due to extensive conformational heterogeneity intrinsic to the particles (figure 2B). Analysis of the SDS-PAGE profiles (not shown) indicated that the protein was intact and careful inspection of the raw particle images indicate that all six domains were always detectable but appear faintly only in few class averages (figure 2C). We concluded that the missing domain was poorly averaged due to its extensive flexibility. These observations provide direct evidence that the LNS1-EGF1 tandem is extremely mobile, due to the flexible linkage of the LNS1 domain respect to the more rigid bulk of the molecule. Molecular labeling was conducted to deduce the N- to C- terminal orientation of the protein. We introduced a FLAG tag at the N-terminus and an HA tag at the C-terminus and labeled the purified protein with the antigen-binding fragment (Fab) against each of the epitope tags separately. The images of the HA Fab tagged α NRXN_1–6 particles identified the C-terminus (LNS6) in the more structured triangular region (figure 2D), whereas the FLAG Fab tagged α NRXN_1–6 identify first LNS domain in the more elongated, and flexible region (figure 2D). Overall, single particle EM reveals that the extracellular domain of αNRXN adopts a semi-elongated and asymmetric structure with a shape reminiscent of the letter Y. Although this labeling does not allow a positive identification of each LNS domain, by their sequence in the protein we infer that LNS domains 1–4 form the longer base of the "Y" and LNS5–6 the two arms.
Figure 2. Single Particle Electron Microscopy Characterization of αNRXN_1–6 and α NRXN_2–6.

6,090 particles were aligned and grouped into 100 class averages. A) Raw data images of the particles before alignment (Left panel). Scale bar 20 nm. Boxed particle, scale bar 10 nm. B) Selection of the highest represented class averages which show only five visibly distinct domains. The panels show the breadth of flexibility of the particles. Scale bar, 10 nm. C) Several averages with low representation indicate the presence of a sixth domain. Scale bar, 10 nm. D) Labeling of αNRXN-1–6 with Fab fragments against a C-terminal HA tag, and an N-terminal FLAG tag. Raw data images are above and a schematic representation with arrows pointing to the identified Fab fragment is below. Scale bar, 10 nm. E) Left panel, raw data of αNRXN_2–6 particles. 17,321 particles were aligned and grouped into 150 class averages. Scale bar, 20 nm. F) Six of the highest represented averages show various conformations.
Scale bar, 10 nm.
See also Figure S2.
αNRXN_2–6
To reduce the large conformational flexibility in the molecule conferred by the first LNS domain, we removed the N-terminal portion of the protein that included the first LNS and EGF domains, and the flexible linker region containing the alternative splice site 1 (Figure 1A) generating a protein beginning at Glu280 (αNRXN_2–6). Approximately 17,000 particles of αNRXN_2–6 were analyzed and 150 class averages were generated. The most common class averages clearly define all five LNS domains in αNRXN_2–6. By removing LNS1, our identification of the N- and C-termini was also confirmed: the particle alignment was greatly improved by the N-terminal truncation and the structured, triangular region was maintained (figure 2E&F). The difference in particle shapes detectable in the class averages show that, although some flexibility remains at the junctions between LNS domains, the general Y shape is conserved. The class averages shown in figure 2F depict the range of conformational variability. Whereas little variation is present at the N-terminal of the protein, more flexibility is adopted at the C-terminus where the Y shape can be disrupted by LNS6 being removed from LNS4. The complete set of class averages shown in Figure S2 yields a more comprehensive view of the conformational heterogeneity of the extracellular domain of αNRXN1. Probably due to their small size, densities corresponding to the three EGF domains remain unresolved. Overall, it appears that αNRXN1 has a tightly packed core composed of LNS2–4 and contains flexible regions that allow the extremities of the protein to extend and retract freely. From a biological perspective this mobility could be relevant for allowing the domain to fit within the dimensions of the synaptic cleft and interact simultaneously with multiple binding partners. Having established the basic two-dimensional conformation of the extracellular domain of αNRXN1, we proceeded to reconstruct the three-dimensional structure using solution scattering methods.
Small Angle X-Ray Scattering Analysis of the Extracellular Domain of αNRXN1
Reproducible, high quality scattering data for the entire extracellular domain of αNRXN_1–6 were collected from three independent sample preparations as well as from monodisperse solutions of lysozyme used as a secondary standard for calibration of scattering intensity (Krigbaum and Kugler 1970). Single particle EM results show that the first LNS-EGF pair is significantly flexible. Therefore, to simplify our structural analyses and to strengthen our interpretation of the small angle X-ray scattering (SAXS) data, experiments on the truncated αNRXN_2–6 construct were also performed. The forward scattering intensities (I(0)) and radius of gyration (Rg) of each of the αNRXN_1–6 and αNRXN_2–6 samples were derived from the scattering data using Guinier analysis. As expected for monodisperse particles in solution, excellent linear correlations were observed in the Guinier plots for both constructs (Figure S3, top panels) and no significant concentration dependent change in the Rg or I(0) values were observed (Figure S3, lower panels). Using the known relationship I(0)/c ∝ MW (with c in units of mg/mL) and comparing data with that of lysozyme scattering, estimates of the molecular weight of the scattering particles were 164–174 kDa for αNRXN_1–6, and 109–118 kDa for αNRXN_2–6 consistent with hydrodynamic and mass spectrometric measurements (for detailed tabulated results of I(0), Rg and molecular weight estimates, see Table S1).
Indirect Fourier transformation yields the probable inter-atomic distance distribution P(r) within the scattering molecule, providing an estimate of the maximum dimension of the particle and its shape. The P(r) profiles for αNRXN_1–6 and α NRXN_2–6 (figure 3), calculated using the program GNOM (Svergun, 1992), indicate that both proteins (Figure 3B&D respectively) are extended particles in solution (structurally anisotropic) as noted by the skewed distribution of vector lengths. The maximum dimension, Dmax, of the full-length construct is ~170 Å with an average Rg of 53.0 ± 0.3 Å. As expected, the truncated variant is significantly smaller, with a Dmax of ~145Å and an average Rg of 44.2 ± 0.6 Å. Removing the LNS-EGF pair shortens the maximum dimension of the protein by ~25 Å and decreases the radius of gyration of ~9 Å without altering the general shape of the P(r) profile. Despite inherent segmental flexibility, the significantly shorter maximum dimension of the deletion mutant αNRXN_2–6 indicates that the domains within the LNS_2–6 region of the protein retain their extended configuration upon removal of the LNS1-EGF1 domain pair. Taken together, these data show that the extracellular domain of α NRXN is monomeric in solution and free of aggregation or interparticle interference. Thus, the scattering data fulfill the requirements necessary for extracting accurate shape information from which reliable three-dimensional structural models of αNRXN_1–6 and αNRXN_2–6 can be constructed.
Figure 3. Scattering Intensity and P(r) Functions of the αNRXN_1–6 and αNRXN_2–6.

A & C Scattering profiles of the highest concentrations of αNRXN_1–6 and 2–6 and P(r) fits. B & D - P(r) functions of αNRXN_1–6 and 2–6 proteins, indicating the maximum dimension of the particle. Statistical quality of the data can be assessed by the error bars; some estimated errors are smaller than the symbols.
See also Table S1 and Figure S3.
Three-dimensional Reconstruction of the Extracellular Domain of αNRXN1
Using a combination of high-resolution structures and homology models of the individual LNS and EGF subunits, rigid-body modeling of the SAXS data enabled us to obtain independent three-dimensional structural models of αNRXN that closely resemble the shapes obtained with single particle EM. The crystal structures of LNS 2, 4 and 6 are available (Rudenko et al., 1999; Sheckler et al., 2006; Shen et al., 2008), and homology models of the remaining individual subunits (LNS1, 3, and 5; EGF1, 2, and 3) were built using various high resolution templates as specified in the Experimental Procedures. Eight sequences, ranging between 4 and 26 residues, linking various LNS and EGF domains did not have a suitable three-dimensional template and thus were initially omitted from the calculations performed with the program SASREF (Petoukhov & Svergun 2005). Distance constraints were imposed between the nine individual rigid bodies to ensure that the N-and C-termini of the individual domains remain within reasonable distances during refinement (details in Table S2 and S3). The SASREF refinements were run multiple times against scattering intensity data from αNRXN_1–6 and αNRXN_2–6. The majority (~80%) of solutions converged toward a single class of Y-shaped molecule with LNS1/LNS2 forming the base of the Y and LNS5/LNS6 forming the arms (Figure 4), in excellent agreement with the EM images (Figure 2).
Figure 4. Rigid Body Modeling of the αNRXN_1–6 and 2–6 with the SAXS Data and αNRXN_2–6 SAXS Models Overlay on Selected EM particles.

A - Best fit models of αNRXN_1–6 and α NRXN_2–6 derived from BUNCH rigid-body refinement and their respective fits to the data. The EGF domains are colored blue (EGF3 is occluded in αNRXN_1–6). B - Structural ensembles represented as semi-transparent green surfaces and ribbons of both αNRXN constructs generated using SASREF and BUNCH rigid body modeling. Brackets indicate different domains of the ensemble. For clarity, brackets and domain labels have been omitted for some ensembles. C- Green and red are two different SASREF reconstructions manually superimposed to two similar class averages to show their degree of identity. Atomic models were made using PyMol (http://www.pymol.org)
See also Tables S2 and S3.
Approximately 11% of the mass of αNRXN_1–6 (primarily in the linkers connecting LNS1/EGF1 and EGF1/LNS2) was not included in the SASREF modeling, while only ~4% of the mass was missing for αNRXN_2–6. We therefore used the program BUNCH that, while similar to SASREF, can additionally account for contributions from regions of the model with unknown structure. Initial BUNCH refinements were performed using similar distance constrains as those used in SASREF against both αNRXN_1–6 and αNRXN_2–6 datasets and the fit to the data improved for both constructs (Figure 4A) (further details on BUNCH refinement strategy are available in the supplemental material). As expected, all of the refined BUNCH models maintain the characteristic Y-shape obtained by the other methods. In addition, they show the likely average positioning of the linkers of unknown structure.
Because of the inherent flexibility of the multidomain architecture, while we present the ‘best-fit’ SASREF and BUNCH models for both constructs (Figure 4B), it is most pertinent to view the results in terms of ensembles of structures that share a common Y-shaped topology (figure 4A&2). For example, the arms of the Y (LNS5 or LNS6) can be spatially swapped without greatly affecting the general Y-shape of the model. Furthermore, upon comparing each member across the SASREF and BUNCH ensembles, the individual domains can undergo a limited localized tilting or rotation relative to their domain neighbors without affecting the fits to the data. Consequently although the general architecture of the protein is maintained, the RMSD Cα across the ensembles is broad, ranging from ~7–25 Å. Although this variability could be due inherent limitations in modeling a complex, multi-domain shape, it is also consistent with the electron microscopy data that indicate individual domains can reorient via flexible inter-domain linkers.
DISCUSSION
The small volumes and spanning dimensions of synaptic clefts are critical for rapidity and fidelity of synaptic transmission. With a typical span of ~24nm between the pre- and post-synaptic membrane, it is unclear how large, multi-domain proteins such αNRXN (~1400 amino acids in its entire extracellular domain) L1CAM (~1300 amino acids), protocadherin (~1100 amino acids), along with other large synaptic receptors and channels, are structured to coexist within the cleft and maintain synaptic structure and function. Furthermore, the extracellular domains of these large proteins are generally composed of a sequential arrangement of several individually folded domains connected by flexible linkers, and they associate with multiple partnering proteins. Whether these proteins are completely flexible or have a restricted inter-domain segmental motion is unknown. The αNRXNs are not only bulky, multi-domain molecules, but between the sixth LNS and transmembrane domains they contain a sequence of ~100 residues that is relatively rich in Ser and Thr and shown to be O-linked glycosylated (Ushkaryov et al., 1992). The presence of oligosaccharides, combined with a relative abundance of Pro residues, presumably rigidifies and elongates the peptide chain, as demonstrated in neuroligin-1 and other cell surface receptors (Li et al., 1996; Merry et al., 2003; Comoletti et al., 2007). The length of this single chain tether may be advantageous to extend the sixth LNS domain so that it can approach the center of the synaptic space, permitting association with post-synaptic proteins such as neuroligin or LRRTM2 (Comoletti et al., 2007, de Wit et al., 2009, Ko et al., 2009). The presence of the stalk domain, however, extends the overall length of αNRXN beyond what a semi-elongated amino acid sequence would predict.
Sedimentation velocity and equilibrium analyses unambiguously show that the extracellular domain of αNRXN is a semi-elongated monomer. Although analytical ultracentrifugation experiments employ low protein concentration (between 10nM and 7µM equivalent to 0.0014 and 1mg/mL, respectively) that could favor dissociation to the monomeric species, SAXS experiments were conducted at concentrations up to ~6mg/mL (~40µM). Under these conditions, higher order oligomers were not detected, indicating that the extracellular domain of αNRXN, similar to β-neurexin (Comoletti et al., 2006), does not self associate. Thus, unless oligomerization occurs through the short intracellular domain, αNRXN is likely present as a monomer on the cell surface.
Single particle EM shows that the LNS1-EGF1 pair of αNRXN_1–6 has extensive inter-domain linkages flexibility respect to the rest of the protein. This flexibility is likely due to the length of the linker (27 amino acids, S256–Y282) that contains splice insert 1. Conversely, the excellent averaging of the particles for αNRXN_2–6 indicates that the rest of the molecule maintains a more stable conformation (Figure 2). The central region of the protein shows LNS2 to 4 in a linear arrangement whereas LNS5 lies at various angles in relation to the previous domains. LNS6 normally folds back on the protein, making a triangular or Y shaped arrangement with LNS4, but displays large degree of flexibility.
Building on the crystal structures of LNS domains 2, 4 and 6 (Rudenko et al., 1999; Sheckler et al., 2006; Shen et al., 2008) and from homology models for the remaining domains, we constructed three dimensional structures of the entire extracellular domain of αNRXN1, including the linker regions, and optimized them against X-ray solution scattering data. Consistent with hydrodynamic and electron microscopy findings, the three dimensional best-fit models show that αNRXN maintains a semi-elongated structure, with LNS domains 1–4 arranged linearly and LNS 4, 5 and 6 adopting a triangular ‘clover leaf’ conformation (Figure 4). Scattering data provide a time and ensemble average of the molecules present in the solution rather that a ‘snap shot’ of a single molecule on a grid as it is observed by EM. The rotational averaging inherent to the solution scattering experiment reduces the information content to one-dimension and thus, when interpreting three-dimensional models, some basic starting assumptions must be satisfied. First, on comparing the P(r) profiles of αNRXN_1–6 and αNRXN_2–6 and from a direct overlay of the scattering data, a level of structural preservation must be maintained within the LNS2-LNS6 region of the protein that is not affected by the removal of the LNS1-EGF1 domain pair. Second, in the jelly roll fold of the LNS domain the metal-binding pocket, where neuroligins bind, is located at the rim of the β sheet sandwich opposite the N and C termini (Rudenko et al., 1999) that reside close together. Consequently when one LNS domain is connected to the next (or to an EGF domain) by relatively short ~5–7 amino acid linkers, as in the case of the LNS4–6, these domains will be constrained toward a ‘clover leaf’ spatial arrangement as opposed to a linear ‘beads on a string’ conformation (Tisi et al., 2000; Carafoli et al., 2009). Third, although the extracellular domain of αNRXN_1–6 could be considered symmetrical with EGF2 at the center of a 2-fold symmetry (Figure 1A), the N- and C-termini identification with Fab tagging and the deletion construct αNRXN_2–6 yields a precise orientation of the protein. Together, the three-dimensional reconstructions and electron microscopy micrographs show extensive interaction between domains 2, 3 and 4, thus explaining the relative rigidity of this part of the molecule. Consistent with the higher degree of flexibility evident in the raw particle images in the EM micrographs, fewer contacts appear between LNS1 and 2 and between LNS5 and 6. Together, these results indicate that the protein maintains a stable core architecture that likely exists in an extracellular milieu and anchors its biological functions (Figure 5).
Figure 5. Schematic Model of the complex Between αNRXN and its Ligands in the Context of the Synapse.
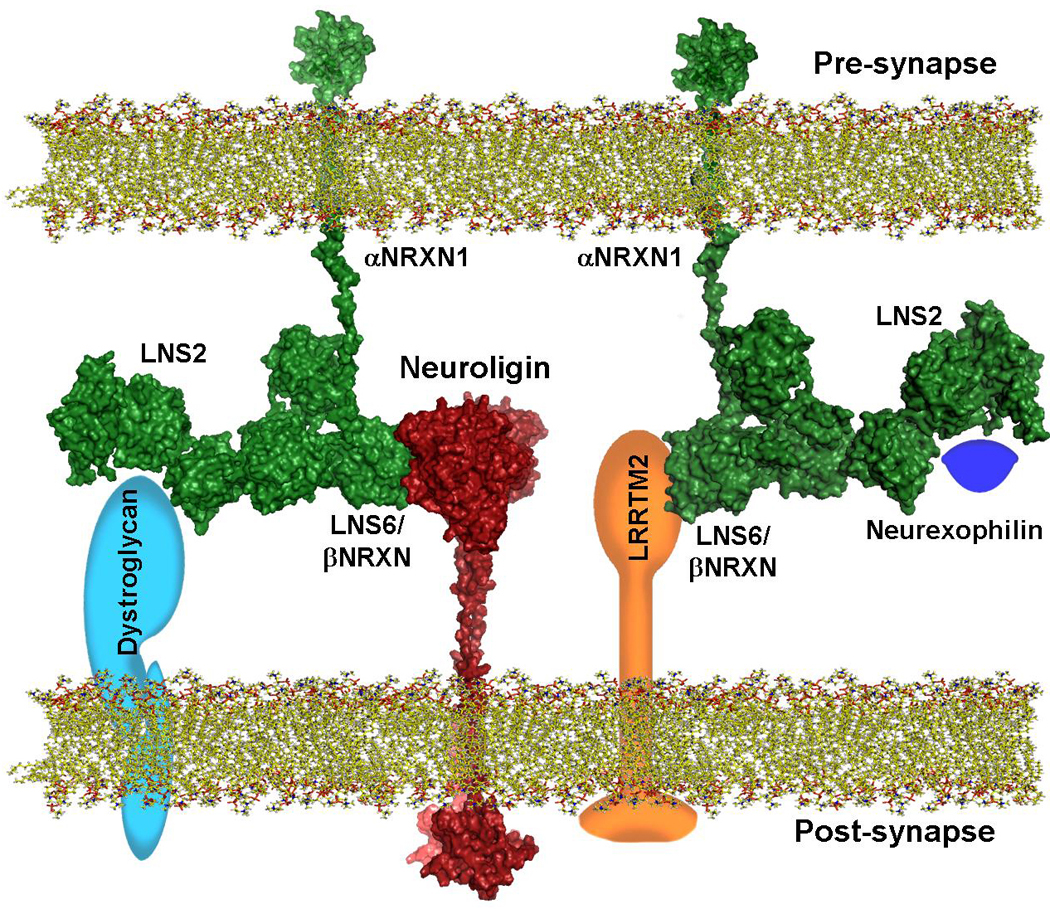
The αNRXN stalk domain connects the pre-synaptic membrane to LNS6 of one of the structural models obtained with SASREF. Information on the contact surface between NLGN and LNS6 of αNRXN was taken from available crystal structure. Stalk domains are drawn extended because of their likely semi-rigid structure. Intracellular domains of both NLGN and NRXN have no conformational assignment. Schematic models of the other known αNRXN ligands (dystroglycan, neurexophilin and LRRTM2) are added to show how multiple ligands can associate simultaneously to their respective LNS domain. Structural models have depth cue visual information. Approximate distances and dimensions are to scale (Pre- and post-synaptic gap is maintained at ~22nm).
Proteins comprising a large number of independently folded domains, such as the αNRXN, laminin-G, and others are normally flexible because inter-domain motions are likely linked to their biological activities. The high resolution structure of LNS 1–3 of laminin α2 (Carafoli et al., 2009) shows that inter-LNS domain linkers maybe extended and flexible. In the case of αNRXN, multiple interacting proteins have been isolated and characterized. In particular, neurexophilin appears to bind to the second LNS domain of αNRXN (Missler et al., 1998) whereas the neuroligins and LRRTM2 associate with the sixth LNS domain (Ichtchenko et al., 1995, Boucard at al 2005; de Wit at al.., 2009; Ko et al., 2009) and dystroglycan associate with both the second and the sixth LNS domains (Sugita et al., 2001). The mobility of LNS1 provides greater surface accessibility to the second LNS domain. LNS6, belonging to the arms of the “Y” shape, tends to fold back towards LNS4 creating a more compact structure, potentially limiting the accessibility to binding partners (Reissner et al., 2008). However, single particle EM data show that LNS6 retains some flexibility, and the clover-leaf arrangement suggests that the metal binding rim of each LNS domain is likely solvent exposed rather than being confined to interdomain stabilization. Regarding the decreased affinity that αNRXN shows with the neuroligins (Boucard at al., 2005) it is possible that the extensive flexibility and segmental motion of LNS6 may require that optimal binding is achieved only after a conformational change, thus acting as a factor limiting NLGN binding.
The three-dimensional structural models of αNRXN we present here are, to our knowledge, the first experimentally derived models of the entire extracellular domain of the αNRXN super-family or other LNS modular multi-domain proteins. Taking advantage of the multiple crystal structures available of the complex between neuroligin 1 and 4 (Fabrichny et al., 2007, Araç et al., 2007, Chen et al., 2008) with αNRXN, and using our previous findings on the structure of the stalk domain of the neuroligins, we assembled a model of the complex between one of the αNRXN_1–6 models and neuroligin 1 in the context of the synaptic cleft. In the case of αNRXN, flexibility, partial elongation, and the clover-leaf arrangement of LNS4–6 domains are ideal for binding multiple ligands because each LNS subunit can associate with partnering proteins independent of the neighboring domains. As shown in the model of the complex (figure 5), the presence of the stalk region and the elongated nature of this model explain how multiple ligands can bind simultaneously to the extracellular domain of αNRXN, and how it may orient in the synaptic cleft.
As hypothesized in an earlier work (Tabuchi & Südhof 2002), mutations in NRXN genes likely confer subtle phenotype changes that may result in widespread dysfunction during the development of a complex nervous system, particularly in a polygenic disorder involving other proteins affecting neuronal system development. In fact, new evidence indicates that variations in copy number and rare variants within the genes encoding neurexin-1 and 3 (NRXN1 and NRXN3) contribute to ASD susceptibility, mental retardation (Feng et al., 2006; The Autism Genome Project Consortium, 2007; Kim et al., 2008; Yan et al., 2008; Glessner et al., 2009, schizophrenia (Rujescu et al., 2009), and in altering addiction and reward behaviors to nicotine (Nussbaum et al., 2008).
EXPERIMENTAL PROCEDURES
Expression of αNRXN1
The construct encoding the secreted, soluble extracellular domain of αNRXN1 IgG fusion protein (Ig-N1α-1) (Boucard et al., 2005) was a kind gift of Dr. Thomas Südhof (Stanford, CA). We adapted this construct by introducing a 3C protease cleavage site (LEVLFQ/GP) between residue T1309 of αNRXN and the beginning of the hIgG sequence. HEK293 GnTI- cells were transfected with the appropriate plasmids and selected by growth in G418 (Geneticin, Sigma) (Comoletti et al., 2003). For protein expression, cells were maintained at 37°C and 10% CO2 in Dulbecco’s modified Eagle’s medium containing up to 2% fetal bovine serum.
Analytical ultracentrifugation
All analytical ultracentrifugation experiments were performed in a Beckman/Coulter XL-I ultracentrifuge using An60Ti and An50Ti rotors at the Center for Analytical Ultracentrifugation of Macromolecular Assemblies, San Antonio, TX. Sedimentation equilibrium and velocity experiments were analyzed with the UltraScan software, version 9.9, release 847 (Demeler, 2009). All samples were run in 10 mM sodium phosphate buffer, pH 7.4, with 137 mM NaCl and 2.7 mM KCl, at 4°C. Hydrodynamic corrections were made according to Laue et al. (1992) as implemented in UltraScan.
Negative-stain single particle electron microscopy
Purified αNRXN1 (0.05–0.1 mg/ml) in 10mM HEPES pH 7.4 and 150mM NaCl solution was applied to glow-discharged carbon-coated grids and negatively stained with 0.75% uranyl formate as described earlier (Ohi et al. 2004). EM images for class averages were collected using an FEI 200KV Sphera microscope equipped with an LaB6 electron filament using low-dose procedures on Kodak film at a magnification of 52,000× and nominal defocus of −1.5µm. Micrographs were digitized with a Nikon scanner, and particles were selected interactively using the WEB display program. A second pass selection of properly centered particles was done interactively, and particles were aligned and classified by reference-based alignment and the K-means classification (100–150 classes) using the SPIDER suite (Frank et al., 1996).
Small angle X-ray scattering data acquisition of αNRXN1
Data were collected from the proteins and their solvent blanks (ultrafiltrate buffers for the αNRXN and last step dialysate for lysozyme) at 20°C, using an Anton Paar SAXSess line collimation instrument at the University of Utah on 2D position-sensitive image plates (10 mm slit and integration width) as described in Jeffries et al., 2008.
Structure Modeling from the Scattering Data
Rigid body modeling was performed using the programs SASREF and BUNCH (Petoukhov & Svergun, 2005). Both techniques refine the domain positions within the protein against the scattering data using calculated partial scattering amplitudes derived from the atomic structures of the individual component domains.
More information on some of the methods can be found in the supplemental information.
HIGHLIGHTS
The extracellular domain of αNRXN1 is monomeric in solution
αNRXN1 maintains a characteristic “Y” shape in solution
Extensive flexibility is present between LNS1 and 2 and between LNS5 and 6
A three-dimensional structural model of the αNRXN1 is presented and discussed
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by: USPHS Grant R37 GM-18360 and NIEHS P42ES10337 to PT; U. S. Department of Energy Grant (DE-FG02-05ER64026) to J.T. ; Autism Speaks 2617 to DC; John Merck Fund and Hellman Foundation to TN. We acknowledge the use of the UCSD Cryo-Electron Microscopy Facility which was supported by NIH grants 1S10RR20016 and GM033050 to Dr. Timothy S. Baker and a gift from the Agouron Institute to UCSD. AUC supercomputer analyses were supported by NSF Teragrid allocation TG-MCB070038 (BD). UltraScan development is supported by NIH-RR022000 (BD). Calculations on Lonestar were supported by NSF TeraGrid allocation TG-MCB070038 (BD). We thank Dr. Dennis Winge (University of Utah, UT) for quantitative amino acid analysis, and Majid Ghassemian, Department of Chemistry and Biochemistry, for Maldi-TOF analysis. Dr. AG Porter of the National University of Singapore for the kind gift of the 3C protease plasmid. We thank Dr. Michael Baker (Protein Data Bank) for helpful discussion on homology modeling and Greg Fuchs for excellent technical help during the preparation of the cleavable αNRXN construct.
Abbreviations
- SAXS
small angle X-ray scattering
- EM
electron microscopy
- AUC
analytical ultracentrifugation
- αNRXN
neurexin α
- NLGN
neuroligin
- LNS
laminin, neurexin, sex-hormone-binding globulin
- EGF
epidermal growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Araç D, Boucard AA, Ozkan E, Strop P, Newell E, Südhof TC, Brunger AT. Structures of neuroligin-1 and the neuroligin-1/neurexin-1 beta complex reveal specific protein-protein and protein-Ca2+ interactions. Neuron. 2007;56:992–1003. doi: 10.1016/j.neuron.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Südhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–236. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Brookes E, Cao W, Demeler B. A two-dimensional spectrum analysis for sedimentation velocity experiments of mixtures with heterogeneity in molecular weight and shape. Eur Biophys J. 2010;39:405–414. doi: 10.1007/s00249-009-0413-5. [DOI] [PubMed] [Google Scholar]
- Brookes E, Demeler B. Parsimonious Regularization using Genetic Algorithms Applied to the Analysis of Analytical Ultracentrifugation Experiments. GECCO Proceedings ACM. 2007;978 978-1-59593-697-4/07/0007. [Google Scholar]
- Chen X, Liu H, Shim AH, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2008;15:50–56. doi: 10.1038/nsmb1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comoletti D, Flynn R, Jennings LL, Chubykin A, Matsumura T, Hasegawa H, Südhof TC, Taylor P. Characterization of the interaction of a recombinant soluble neuroligin-1 with neurexin-1beta. J Biol Chem. 2003;278:50497–50505. doi: 10.1074/jbc.M306803200. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Flynn AA, Boucard B, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Südhof TC, Taylor P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry. 2006;45:12816–12827. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Grishaev A, Whitten AE, Tsigelny I, Taylor P, Trewhella J. Synaptic arrangement of the neuroligin/beta-neurexin complex revealed by X-ray and neutron scattering. Structure. 2007;15:693–705. doi: 10.1016/j.str.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svergun D, Barberato C, Koch MHJ. CRYSOL – a Program to Evaluate X-ray Solution Scattering of Biological Macromolecules from Atomic Coordinates. Journal of Applied Crystallography. 1995;28:768–773. [Google Scholar]
- Demeler B. UltraScan version 9.9, release 847. Dept. of Biochemistry; Analytical Ultracentrifugation Data Analysis software. The University of Texas Health Science Center at San Antonio. 2009 http://www.ultrascan.uthscsa.edu.
- Demeler B, van Holde KE. Sedimentation velocity analysis of highly heterogeneous systems. Anal. Biochem. 2004;335:279–288. doi: 10.1016/j.ab.2004.08.039. [DOI] [PubMed] [Google Scholar]
- Demeler B, Brookes E. Monte Carlo Analysis of Sedimentation Experiments. Prog. Colloid. Polym. Sci. 2008;286:129–137. [Google Scholar]
- de Wit J, Sylwestrak E, O'Sullivan ML, Otto S, Tiglio K, Savas JN, Yates JR, 3rd, Comoletti D, Taylor P, Ghosh A. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudanova I, Tabuchi K, Rohlmann A, Südhof TC, Missler M. Deletion of alphaneurexins does not cause a major impairment of axonal pathfinding or synapse formation. J Comp Neurol. 2007;502:261–274. doi: 10.1002/cne.21305. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Südhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairless R, Masius H, Rohlmann A, Heupel K, Ahmad M, Reissner C, Dresbach T, Missler M. Polarized targeting of neurexins to synapses is regulated by their C-terminal sequences. J Neurosci. 2008;28:12969–12981. doi: 10.1523/JNEUROSCI.5294-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Schroer R, Yan J, Song W, Yang C, Bockholt A, Cook EH, Jr, Skinner C, Schwartz CE, Sommer SS. High frequency of neurexin 1beta signal peptide structural variants in patients with autism. Neurosci Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Frank J, Radermacher M, Penczek P, Zhu J, Li Y, Ladjadj M, Leith A. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190–199. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: developmental disconnection syndromes. Curr. Opin. Neurobiol. 2007;17:103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, Zhang H, Estes A, Brune CW, Bradfield JP, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Südhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Jeffries CM, Whitten AE, Harris SP, Trewhella J. Small-angle X-ray scattering reveals the N-terminal domain organization of cardiac myosin binding protein C. J Mol Biol. 2008;377:1186–1199. doi: 10.1016/j.jmb.2008.01.080. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhang X, Dobie F, Wu H, Craig AM. Induction of GABAergic postsynaptic differentiation by alpha-neurexins. J Biol Chem. 2008;283:2323–2334. doi: 10.1074/jbc.M703957200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, Lally E, Weiss LA, Najm J, Kutsche K, Descartes M, Holt L, Braddock S, Troxell R, Kaplan L, Volkmar F, Klin A, Tsatsanis K, Harris DJ, Noens I, Pauls DL, Daly MJ, MacDonald ME, Morton CC, Quade BJ, Gusella JF. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko J, Fuccillo MV, Malenka RC, Südhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev PV, Volkov VV, Sokolova AV, Koch MHJ, Svergun DI. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Cryst. 2003;36:1277–1282. [Google Scholar]
- Krigbaum WR, Kugler FR. Molecular conformation of egg-white lysozyme and bovine alpha-lactalbumin in solution. Biochemistry. 1970;9:1216–1223. doi: 10.1021/bi00807a024. [DOI] [PubMed] [Google Scholar]
- Laue TM, Shah BD, Ridgeway TM, Pelletier SL. In: Analytical Ultracentrifugation in Biochemistry and Polymer Science. Harding SE, Rowe AJ, Horton JC, editors. Cambridge: Royal Society of Chemistry; 1992. pp. 90–125. [Google Scholar]
- Li F, Erickson HP, James JA, Moore KL, Cummings RD, McEver RP. Visualization of P-selectin glycoprotein ligand-1 as a highly extended molecule and mapping of protein epitopes for monoclonal antibodies. J. Biol. Chem. 1996;271:6342–6348. doi: 10.1074/jbc.271.11.6342. [DOI] [PubMed] [Google Scholar]
- Merry AH, Gilbert RJ, Shore DA, Royle L, Miroshnychenko O, Vuong M, Wormald MR, Harvey DJ, Dwek RA, Classon BJ, et al. O-glycan sialylation and the structure of the stalk-like region of the T cell co-receptor CD8. J Biol Chem. 2003;278:27119–27128. doi: 10.1074/jbc.M213056200. [DOI] [PubMed] [Google Scholar]
- Missler M, Hammer RE, Südhof TC. Neurexophilin binding to alpha-neurexins. A single LNS domain functions as an independently folding ligand-binding unit. J Biol Chem. 1998;273:34716–34723. doi: 10.1074/jbc.273.52.34716. [DOI] [PubMed] [Google Scholar]
- Missler M, Südhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–26. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–948. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Nussbaum J, Xu Q, Payne TJ, Ma JZ, Huang W, Gelernter J, Li MD. Significant association of the neurexin-1 gene (NRXN1) with nicotine dependence in European- and African-American smokers. Hum Mol Genet. 2008;17:1569–1577. doi: 10.1093/hmg/ddn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohi M, Li Y, Cheng Y, Walz T. Negative staining and image classification - powerful tools in modern electron microscopy. Biol. Proced. Online. 2004;6:23–34. doi: 10.1251/bpo70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoukhov MV, Svergun DI. "Global rigid body modeling of macromolecular complexes against small-angle scattering data.". Biophys J. 2005;89:1237–1250. doi: 10.1529/biophysj.105.064154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner C, Klose M, Fairless R, Missler M. Mutational analysis of the neurexinneuroligin complex reveals essential and regulatory components. Proc Natl Acad Sci U S A. 2008;105:15124–15129. doi: 10.1073/pnas.0801639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko G, Nguyen T, Chelliah Y, Südhof TC, Deisenhofer J. The structure of the ligand-binding domain of neurexin Ibeta: regulation of LNS domain function by alternative splicing. Cell. 1999;99:93–101. doi: 10.1016/s0092-8674(00)80065-3. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Ingason A, Cichon S, Pietiläinen OP, Barnes MR, Toulopoulou T, Picchioni M, et al. Disruption of the neurexin 1 gene is associated with schizophrenia. Hum Mol Genet. 2009;18:988–996. doi: 10.1093/hmg/ddn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shanks NF, Maruo T, Farina An, Ellisman MH, Nakagawa T. Contribution of the global subunit structure and stargazin on the maturation of AMPA receptors”. Journal of Neuroscience. 2010;30:2728–2740. doi: 10.1523/JNEUROSCI.5146-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheckler LR, Henry L, Sugita S, Südhof TC, Rudenko G. Crystal structure of the second LNS/LG domain from neurexin 1alpha: Ca2+ binding and the effects of alternative splicing. J Biol Chem. 2006;281:22896–22905. doi: 10.1074/jbc.M603464200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KC, Kuczynska DA, Wu IJ, Murray BH, Sheckler LR, Rudenko G. Regulation of neurexin 1beta tertiary structure and ligand binding through alternative splicing. Structure. 2008;16:422–431. doi: 10.1016/j.str.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Südhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–445. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svergun DI. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Cryst. 1992;25:495–503. [Google Scholar]
- Tabuchi K, Südhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–859. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- The Autism Genome Project Consortium. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nature Genetics. 2007;39:319–328. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisi D, Talts JF, Timpl R, Hohenester E. Structure of the C-terminal laminin G-like domain pair of the laminin alpha2 chain harbouring binding sites for alpha-dystroglycan and heparin. EMBO J. 2000;19:1432–1440. doi: 10.1093/emboj/19.7.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Südhof TC. Neurexin III alpha: extensive alternative splicing generates membrane-bound and soluble forms. Proc Natl Acad Sci U S A. 1993;90:6410–6414. doi: 10.1073/pnas.90.14.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Südhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Yan J, Noltner K, Feng J, Li W, Schroer R, Skinner C, Zeng W, Schwartz CE, Sommer SS. Neurexin 1alpha structural variants associated with autism. Neurosci Lett. 2008;438:368–370. doi: 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- Zahir FR, Baross A, Delaney AD, Eydoux P, Fernandes ND, Pugh T, Marra MA, Friedman JM. A patient with vertebral, cognitive and behavioural abnormalities and a de novo deletion of NRXN1alpha. J Med Genet. 2008;45:239–243. doi: 10.1136/jmg.2007.054437. [DOI] [PubMed] [Google Scholar]
- Zweier C, de Jong EK, Zweier M, Orrico A, Ousager LB, Collins AL, Bijlsma EK, Oortveld MA, Ekici AB, Reis A, Schenck A, Rauch A. CNTNAP2 and NRXN1 are mutated in autosomal-recessive Pitt-Hopkins-like mental retardation and determine the level of a common synaptic protein in Drosophila. Am J Hum Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


