Abstract
Navigation requires real-time heading estimation based-on self-movement cues from optic flow and object motion. We presented a simulated heading discrimination task to young, middle-aged and older adult, normal, control subjects and to patients with mild cognitive impairment or Alzheimer’s disease. Age-related decline and neurodegenerative disease effects were evident on a battery of neuropsychological and visual motion psychophysical measures. All subject groups made more accurate heading judgements when using optic flow patterns than when using simulated movement past earth-fixed objects. When both optic flow and congruent object were presented together, heading judgements showed intermediate accuracy. In separate trials, we combined optic flow with non-congruent object motion, simulating an independently moving object. In the case of non-congruent objects, almost all of our subjects shifted their perceived self-movement to heading in the direction of the moving object. However, patients with Alzheimer’s disease uniquely indicated that perceived self-movement was straight-ahead, in the direction of visual fixation. The tendency to be confused by objects that appear to move independently in the simulated visual scene corresponded to the difficulty patients with Alzheimer’s disease encountered in real-world navigation through the hospital lobby (R2 = 0.87). This was not the case in older normal controls (R2 = 0.09). We conclude that perceptual factors limit safe, autonomous navigation in early Alzheimer’s disease. In particular, the presence of independently moving objects in naturalistic environments limits the capacity of patients with Alzheimer’s disease to judge their heading of self-movement.
Keywords: Alzheimer’s disease, mild cognitive impairment, visual cognition, cerebral cortex, cognitive neurology
Introduction
Self-movement perception relies on the integration and segregation of visual cues. Cues about a moving observer’s heading direction are imbedded in the radial pattern of visual motion in optic flow. The heading cues in optic flow are supplemented by the relative visual movement of earth-fixed landmark objects, which move in a manner that is congruent with the optic flow field. In contrast, independently moving objects violate the radial pattern of optic flow and provide robust cues for detecting threats and following targets that are themselves moving through the visual environment.
The human integration of visual cues regarding self-movement is associated with the activation of posterior cortical areas by optic flow and object motion stimuli (Aguirre and D'Esposito, 1997). Functional imaging reveals selective activation by optic flow in dorsal stream parietotemporal visual processing areas (Morrone et al., 2000; Goossens et al., 2006) with object motion resulting in selective activation in overlapping (Shulman et al., 1999) or adjacent temporal areas (Epstein et al., 1999). The cortical pathology of Alzheimer’s disease has a great impact on these areas (Arnold et al., 1991; Armstrong 1996), often as the first focal pathology in patients particularly affected by visuospatial processing impairments (Tang-Wai et al., 2004).
This posterior cortical localization of visual cue integration for self-movement perception is consistent with the identification of macaque monkey cortical neuronal populations specialized for combining these optic flow and object motion cues (Logan and Duffy, 2006). In these studies, recordings of medial superior temporal neurons have shown a gating of object motion influence on neuronal responses to optic flow; depending on whether the object moves congruently with superimposed optic flow, or simulating an independently-moving object, incongruently in violation of the motion pattern in the optic flow. The effects of congruent and incongruent object motion on optic flow-based heading estimation have been well documented in psychophysical studies of normal human subjects. These studies have reported disruptions of heading estimation, particularly when the heading in optic flow was obscured by an incongruently moving object (Royden and Hildreth, 1999), in a manner that is consistent with computational models of medial superior temporal neuronal function (Zemel and Sejnowski, 1998; Royden, 2002).
We have previously shown that ageing and Alzheimer’s disease are associated with successively greater difficulty in the perceptual processing of object form and visual motion related to self-movement (Tetewsky and Duffy, 1999; O'Brien et al., 2001). These deficits create a spectrum of impairment that has several apparently independent components in the domains of visual processing and cognitive function (O'Brien et al., 2001; Mapstone et al., 2003). These impairments are linked to navigational disabilities (Cushman and Duffy, 2007) and neurophysiological dysfunction (Kavcic et al., 2006). When integrated into functional assessments of perceptual capacity for heading estimation, they reveal an important distinction between older adults and patients with Alzheimer’s disease (Mapstone et al., 2006): older adults combine optic flow and congruent landmark object motion cues to support accurate heading estimation, whereas in contrast, patients with Alzheimer’s disease are confounded by congruent object motion, such that it disrupts heading estimation from optic flow. This may cause patients with Alzheimer’s disease to have greater difficulty in self-movement heading estimation in natural environments that include optic flow and landmark objects.
In the current study, we superimposed non-congruent object motion, simulating independently moving objects, on optic flow stimuli. Our goal was to test the hypothesis that Alzheimer’s disease results in deficits of perceptual integration that might explain further the behavioural limiting navigational impairment associated with Alzheimer’s disease. We found evidence for a unique and robust adverse impact of independently moving objects on heading estimation in Alzheimer’s disease and that these factors greatly contribute to navigational impairment in those patients.
Materials and methods
Subjects
A total of 132 individuals participated in this study. Thirteen patients with early Alzheimer’s disease and 20 patients with mild cognitive impairment (MCI) were referred to the study by a dementia neurologist or psychiatrist affiliated with the clinical programs at the University of Rochester Medical Centre. All patients with early Alzheimer’s disease met National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association (NINCDS–ADRDA) criteria for probable Alzheimer’s disease (McKhann et al., 1984). All participants with MCI met criteria for amnestic MCI (Petersen, 1996). Ninety-nine participants served as normal control subjects. The normal control participants were divided into three groups in order to examine the effects of ageing. Thirty-nine older adult normal control subjects were between the ages of 60 and 84 years, 27 middle-aged normal control subjects were between the ages of 40 and 59 years, and 33 younger adult normal control subjects were between the ages of 20 and 39 years. The older normal control group included volunteers from the community and many were spouses or caregivers of participants with early Alzheimer’s disease or MCI. The middle-aged normal control group were volunteers from the community and employees of the University of Rochester or Strong Memorial Hospital. The younger normal control group were students or staff at the University of Rochester. All participants were free from neurological and psychiatric illness with the exception of Alzheimer’s disease in the early Alzheimer’s disease group and MCI in the MCI group. Thirty percent of the early Alzheimer’s disease group and 40% of the MCI group were female, while 50% were female in the older normal control group, 70% were female in the middle-aged normal control group and 51% were female in the younger normal control group. All participants had corrected binocular visual acuity of at least 20/40 and were free from ophthalmic illness. All subjects in this study were native speakers of English. As defined, the younger, middle-aged and older normal control groups differed in age (P < 0.001), but the older normal controls, MCI and early Alzheimer’s disease groups did not (P > 0.05) (Table 1). We previously reported a separate set of experiments that included a subset of this sample (Mapstone et al., 2006). All patients with early Alzheimer’s disease, 30 older normal controls, 17 middle-aged normal controls and 18 younger normal controls in the present study were part of the previously published study. In the present study, we report on a new experiment, add additional subjects in the three normal control groups and include the new patient group of MCI subjects to examine more closely perceptual changes that occur during the transition from normal ageing to Alzheimer’s disease.
Table 1.
Summary statistics for the five subject groups showing demographic and neuropsychological test results
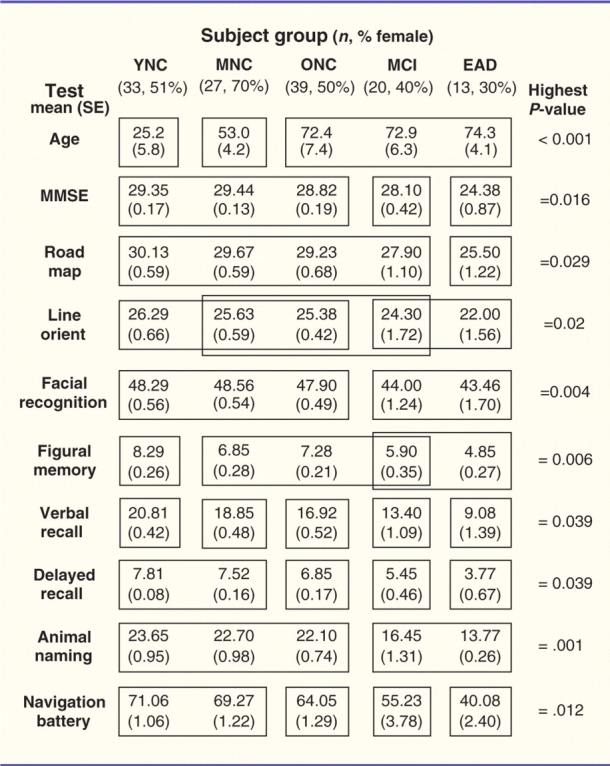 |
A two-way ANOVA showed that all of the tests yielded significant between group effects (P-values on right). Post hoc tests of group differences were performed using Tukey's honestly significant differences (P < 0.05). The results of Tukey's honestly significant differences that do not significant group differences are included within the same box frame; those that are significantly different are in separate frames.
YNC = younger normal controls; MNC = middle-aged normal controls; ONC = older normal controls; EAD = early Alzheimer's disease; MMSE = Mini-Mental State Examination.
Procedure
All testing was completed in the Visual Orientation Laboratory at the University of Rochester Medical Centre in three, 1 h sessions. The protocol was explained to all participants in advance and written consent was obtained. All participants completed the same experimental protocol. The first visit consisted of pencil and paper cognitive tests, the second visit consisted of the Spatial Navigation Test Battery and the third visit consisted of visual motion coherence threshold testing and three visual motion based pointing tasks, two of which have been reported previously (Mapstone et al., 2006). The University of Rochester Institutional Review Board approved all protocols used.
Pencil and paper cognitive tests
A battery of cognitive tests was administered to corroborate the diagnosis of Alzheimer’s disease in the early Alzheimer’s disease group and MCI in the MCI group. The tests also were used to rule out specific cognitive impairments in the normal control groups. The Mini-Mental State Examination was used as a measure of global cognitive ability. Mean Mini-Mental State Examination (Folstein et al., 1975) score for the early Alzheimer’s disease group was (24.38 ± 0.87) suggesting that these participants were in the earliest detectable stage of the disease (Table 1). Verbal immediate and delayed memory were assessed using the Wechsler Memory Scale-Revised Paired Association Learning Test (Wechsler, 1987) in which participants learn novel associations between eight unrelated word pairs. Nonverbal immediate memory was assessed using the Wechsler Memory Scale-Revised Figural Memory Test (Wechsler, 1987) in which participants study novel geometric designs and perform an immediate three-alternative forced choice recognition task for the design. Language was assessed using a category fluency test in which participants name as many animals as possible in 1 min. Three measures of visuospatial function were administered including the Judgement of Line Orientation task (Benton et al., 1983), which assesses the ability to judge spatial relationships between two lines. In this task participants are shown two lines that create an angle and must identify which two lines create the same angle as the sample from among thirteen lines arranged in a fan-shaped array. We also administered the Benton Facial Recognition Test in which participants match pictures of unfamiliar faces from different perspectives and under different lighting conditions (Benton et al., 1983). We also administered the Money Road Map Test (Money, 1976), which assesses route-following and topographic orientation. In this test, participants visually follow a marked route on a top-down perspective city map and indicate left or right turns at each intersection.
Spatial Navigation Test Battery
The Spatial Navigation Test Battery is a unique set of spatial navigation tasks we developed to quantify real-world navigation. This task has been described previously (Monacelli et al., 2003). Briefly, we used the lobby of Strong Memorial Hospital at the University of Rochester as an experimental environment in a 90 min test that began with an experimenter-directed tour of the lobby on a fixed path with subjects seated in a wheelchair. We pointed out how the subjects’ perspective changed during self-movement with straight ahead gaze and reinforced this point as the subjects were introduced to the simulation. Although independent ambulatory exploration would be more naturalistic, we did not use that approach because of the potential for confounding effects of the wide variability of mobility in older adults. Subjects were instructed to concentrate on the route, as they would later be asked to retrace it and undergo testing related to it. The subject and wheelchair were then pushed along the 1000 feet path over ∼4 min. On completion of the route, eight sub-tests were administered to assess navigational capacity; each sub-test consisted of 10 questions. The Route Learning subtest was presented first and required subject-directed repetition of the route. The remaining tests were presented in a rotationally counter-balanced sequence. The Free Recall subtest consisted of naming objects along the route. The Self Orientation subtest required the subject to point toward unseen landmarks encountered on the route. The Route Drawing subtest required the subject to trace the route on a map of the lobby. In the Landmark Recall subtest, subjects named objects used to navigate the route. The Photo Recognition subtest required subjects to identify photos as being from the route or not. Finally, the Photo Location and Video Location subtests required subjects to match photos or video clips to labelled sites on a map of the route. The total score on the Spatial Navigation Battery was 80 points with higher scores indicating better performance.
Experimental setup
Apparatus
All subjects completed the visual motion coherence threshold and visual motion-based pointing tasks in the same experimental environment that has been described previously (Mapstone et al., 2006). Briefly, subjects sat near the centre of a darkened 2.4 × 2.4 × 1.8 m enclosure, the front wall of which was a 2.4 × 1.8 m rear-projection tangent screen. The display covered the central 90° × 60° of the subject’s visual field while they sat in a fixed orientation facing the screen. Participants maintained fixation on the centre of the screen in all tasks with central fixation monitored by infrared oculography (ASL, Inc., Bedford, MA). All visual stimuli were generated on a personal computer using proprietary software and projected onto the screen by a TV projector (Electrohome, Inc., Ontario, Canada). Participants turned a steering wheel left or right or pushed one of two buttons in order to respond to each stimulus (depending on the task). Neither the steering wheel nor the button box obstructed vision of the screen. All stimulus parameters, gaze and response cursor position were recorded in real time using the Real-Time Experimental (REX) control system (Hays et al., 1982).
Visual motion stimuli
The same visual motion stimuli were used for both the visual motion threshold testing and the visual motion-based pointing tasks and have been described previously (Mapstone et al., 2006). Briefly, the stimuli simulated observer self-movement into the plane of the projection screen. Observer heading was indicated by an optic flow field or a moving object. The optic flow stimuli consisted of animated sequences of 1000 white dots (2.69 cd/m2) presented on a dark background at a 60 Hz frame rate (O'Brien et al., 2001). The object stimulus was a wire frame, 3D representation of a standard four-drawer file cabinet. The object stimulus moved on the screen with constant geometric transformation simulating a stable observer perspective relative to the cabinet. Prior to the experimental tasks, each subject was pushed in a wheeled desk chair with normal room illumination to illustrate the visual motion in optic flow. They were also moved past a four-drawer file cabinet with luminous taped edges in an otherwise dark room to demonstrate how movement past an earth-fixed object can provide heading cues. All subjects indicated understanding of how these self-movement cues provided heading information and expressed their readiness to begin testing after a few practice trials in each task.
Visual motion thresholds
Our method of obtaining optic flow and object visual motion coherence thresholds has been described previously (O'Brien et al., 2001; Mapstone et al., 2006). Briefly, to obtain optic flow coherence thresholds we presented optic flow fields on the tangent screen with a focus of expansion 30° to the right or left of the centre, on the horizontal midline of the screen. Random dot motion was mixed in with these coherent motion patterns. The percentage of randomly and coherently moving dots varied between trials in order to determine the motion coherence thresholds. At each frame, dots were randomly assigned to the coherent and random groups with the appropriate proportions (Fig. 1A). All stimuli had the same luminance, contrast and density. The speed of the coherent dots in the optic flow varied with simulated heading direction to simulate a constant speed of observer self-movement.
Figure 1.
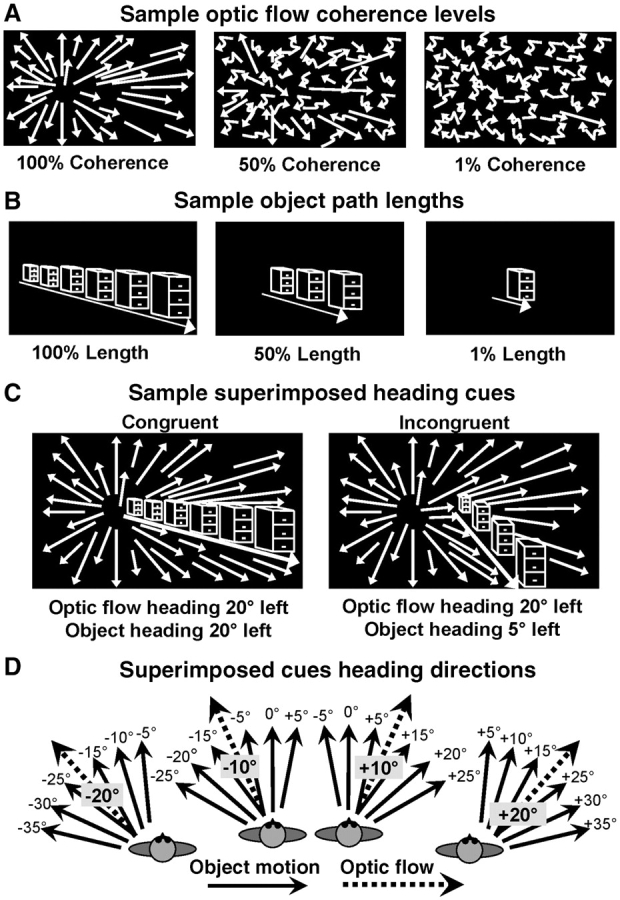
Visual motion stimuli used in the cue conflict heading discrimination tasks. (A) Outward radial optic flow having a 30° left or right sided focus of expansion was formed by a randomly distributed pattern of white dots presented on a black background. A selected percentage of the dots were drawn from the pattern to undergo randomly directed motion to alter the strength of the simulated heading direction cue. The parameter estimation (PEST) algorithm controlled the percentage of randomly moving dots to determine each subject’s motion coherence thresholds. (B) Looming object motion simulated a filing cabinet viewed during the observer’s straight line path of approaching self-movement. The percentage of the full path length presented was selected to alter simulated heading direction signal strength and determine each subject’s path length thresholds. (C) Optic flow and object motion stimuli were superimposed to create two conditions with respect to a moving observer: Two cues simulating the same heading direction in congruent combination (left) as with an earth-fixed object. Two cues simulating different heading directions in incongruent combination (right) as with an independently moving animate object. (D) Top-down diagrammatic representation of the 24 incongruent stimulus conditions tested by combining four optic flow headings (±10° and ±20°) with six relative object motion headings (±5°, ±10° and ±15°).
Visual motion thresholds for object motion were determined by varying the exposure duty cycle of the stimulus as the percentage of the cabinet’s simulated path, which was presented during a left/right (±30°) heading discrimination trial (Fig. 1B). At every exposure duty cycle level the object was always present on the screen: at a low exposure duty cycle it repeatedly covered only the middle segment of its potential path, at a high exposure duty cycle it repeatedly covered a greater portion of its potential path.
In a second condition, we further disrupted the heading cue in optic flow and object motion stimuli by manipulating the simulated heading direction, in addition to the strength of the perceptual signal. The addition of heading jitter simulated the irregular movement of heading direction that can occur with ambulatory or vehicular self-movement, especially at an uneven pace or across an uneven surface. In this condition, the heading location represented by the optic flow or object motion stimulus varied across frames within one trial. This heading jitter randomly displaced the simulated heading direction ±5° along the horizontal and vertical axes in each frame. The result was the superposition of both variability in simulated heading direction as well as algorithmic changes in the coherence of optic flow stimuli or the path length of object motion stimuli. The magnitude of these effects exceed (∼3×) those seen during running in young subjects with intact vestibulo-ocular reflexes (Grossman, 1989). Visual motion thresholds for optic flow and object headings were determined using a one interval, two-alternative forced-choice left/right identification task. Each trial began with the subject centring a cursor, using the steering wheel, over a central fixation square on the screen for 0.5 s while fixating in the centre. Each trial started after central fixation was maintained for 0.5 s. Subjects indicated whether the simulated heading was on the left or right of the central fixation square by moving the steering wheel. Once each trial ended, a large letter ‘L’ and letter ‘R’ were visible on the screen and the subject moved the steering wheel towards the ‘L’ if the heading was to the left of the central fixation or to the ‘R’ if the heading was to the right. The next trial began after the computer recorded each response. Subjects were encouraged to guess if they were unsure of the answer. Each subject completed 50 trials for optic flow and object headings in both the coherence and coherence plus jitter conditions for a total of four blocks of visual motion threshold testing. The tasks were presented with a short break between blocks.
Motion coherence and exposure duty cycles were selected for each trial using threshold parameter estimation by sequential testing (Pentland, 1980; Harvey 1997). The resulting direction heading discrimination threshold for both optic flow and the moving object was the coherence or exposure duty cycle, yielding 82.5% correct left/right discriminations.
Visual motion based pointing task
In our previous paper (Mapstone et al., 2006) we reported the results of our subject groups (excluding the MCI group included in the current article) on tasks of heading localization (pointing) and heading control (steering) in three conditions: with the optic flow stimulus alone, the object alone and the optic flow and object together indicating the same heading. In this article we report the findings of the pointing task in a fourth condition: both the optic flow and object together, but with offset headings such as that experienced when a moving observer encounters an independently moving object in the environment (Fig. 1C, right). We conducted the congruent stimulus studies before presenting incongruent conditions, reasoning that experience with the objects as incongruent cues would predispose our subjects to assume that they might not be earth-fixed and prevent them from considering them as such even in congruent heading stimuli. We present relevant updated data from the previous paper in this article to provide context for the current findings.
Pointing at the heading direction
Experimental details of the pointing task conditions of optic flow alone, object alone and congruent optic flow and object can be found in our previously published report of these data (Mapstone et al., 2006). For the incongruent optic flow and object pointing task, an optic flow field indicating observer self movement to one of four heading directions either 10° or 20° to the left or right of central fixation was presented on the screen (Fig. 1D). In each trial, an incongruent object heading (Fig. 1C, right) was simultaneously presented at offsets of 5°, 10° or 15° to the left or right of the optic flow heading, simulating an independently moving object in the environment (Fig. 1D). The stimuli remained on the screen for 1.5 s while subjects maintained centred gaze (±5°). The heading stimulus was then extinguished and subjects were free to shift their gaze. Within 1 s after the stimulus was extinguished, subjects were required to rotate the steering wheel to move the cursor (clockwise for rightward displacement) to indicate their perceived heading direction simulated in the preceding stimulus. The trial ended after 8 s or when the subject indicated a final position by holding the cursor in a 1° area for 1 s. The final position of the cursor was recorded at the end of the trial and pointing error in visual angle from the heading direction simulated by the stimulus was determined. We determined pointing error relative to the target location [(–) medial to the target, (+) lateral to the target] to measure response undershoot (–) or overshoot (+) of the subject’s moving the cursor from the centre starting position to the eccentric heading direction. Subjects completed three randomly presented trials at each of the six object offsets for each optic flow heading directions for a total of 72 trials.
Controlling for perceptual differences
To minimize the impact of group and individual motion perceptual differences we presented the optic flow and object motion stimuli at each subject’s previously determined coherence and exposure duty cycle (but not jitter) threshold plus two times their 95% confidence interval.
Controlling for visuomotor coordination
As in the previous report (Mapstone et al., 2006), we attempted to minimize the impact of group and individual differences in visuomotor coordination by subtracting the error made on an elementary visuomotor control task. In that task, subjects pointed to the location of a previously flashed square placed along the horizontal meridian (Mapstone et al., 2006). In that control study we found relatively small effects of eccentricity and subject group with typical values of 2°–3° at the eccentricities used in the current study.
In order to compare previously reported data that utilized 10 different heading locations with the current experiment, which utilized only four primary heading locations, we extracted only the data from the 10° and 20° heading locations either left or right of the central fixation from the larger data set for use in this analysis.
Data analysis
A multivariate analysis of variance (MANOVA) was used to test for group differences on the pencil and paper cognitive tests and the Spatial Navigation Battery. A nested, repeated measures analysis of variance (ANOVA) with subject Group (younger, middle-aged and older normal controls, MCI, early Alzheimer’s disease) as the between-subjects factor and visual motion threshold Stimulus (optic flow, object) and visual motion threshold Noise type (coherence/exposure duty cycle, coherence/exposure duty cycle plus jitter) as the within-subjects factors was used to test for group differences on the visual motion perceptual thresholds.
As in our previous study, preliminary analyses of hemi-spatial lateralization effects revealed no difference in performance for targets on the left side of the screen compared to the right side of the screen, so we folded the left and right hemi-fields along the vertical meridian such that pointing error at each eccentricity reflects the average error at that eccentricity for both left and right hemi-fields. This reduced the number of target locations to two and doubled the number of trials at each eccentricity. Thus, for the pointing task, we analysed pointing error in the larger group of subjects in each of the normal control and early Alzheimer’s disease groups in addition to the new MCI group by way of a comprehensive four-way ANOVA examining effects of Group (5), optic Flow heading (two levels: 10°, 20°), object relative Direction (two levels: medial, lateral) and object relative Heading eccentricity (three levels: 5°, 10°, 15°). Significant main effects were followed up with one-way ANOVA and post hoc tests using Tukey’s honestly significant differences, as indicated.
Finally, we computed mean error across all heading eccentricities by Stimulus type (optic flow, object, combined) and Task (pointing, steering) for each individual subject. We used step-wise multiple linear regression analyses to examine relationships between performance on the incongruent pointing task and performance on the pencil and paper cognitive tests, the Spatial Navigation Battery and perceptual thresholds for visual motion stimuli. Alpha levels for statistical significance on all tests were set at P < 0.05. All statistical analyses were run using Statistical Package for the Social Sciences software (SPSS Inc. 2005).
Results
We studied 132 subjects from five groups. Basic heading estimation studies were conducted on younger, middle-aged and older normal controls, as well as patients with early Alzheimer’s disease. An additional MCI group was included in more detailed studies of heading estimation with incongruent stimuli. The older normal control, MCI and early Alzheimer’s disease groups did not have significantly different ages; there were more women in the MCI and early Alzheimer’s disease groups (Table 1).
Neuropsychological and perceptual performance
Neuropsychological testing showed the largest group effects on category fluency and facial recognition, in both cases attributable mainly to differences between the younger, middle-aged and older normal control groups versus the MCI and early Alzheimer’s disease groups. All other neuropsychological tests confirmed the classification of the subject groups highlighting ageing effects in some cognitive domains and the mnemonic, language and visuospatial deficits that characterize the definitional identification of the MCI and early Alzheimer’s disease clinical syndromes (Table 1).
We derived left/right heading discrimination thresholds for optic flow and object motion stimuli in our four main subject groups. Optic flow thresholds were obtained by varying the proportion of moving dots allocated between radial pattern motion and random motion. The early Alzheimer’s disease group showed significantly elevated optic flow thresholds relative to all other groups; all but an exclusively disease based effect. Object motion thresholds were obtained by varying the length of the simulated path of movement past the object. These studies revealed a regular increase in object motion thresholds across all subject groups, suggesting a substantial effect of ageing as well as an additional effect of Alzheimer’s disease (Fig. 2A).
Figure 2.
Perceptual thresholds obtained in our four subject groups using optic flow and object motion stimuli. (A) Optic flow evoked similar motion coherence thresholds in younger, middle-aged and older normal controls subjects; whereas patients with early Alzheimer’s disease had thresholds that were roughly double those of the other groups. Object motion evoked steadily increasing path length thresholds across the four groups, the lowest thresholds in younger normal control subjects and the highest in patients with early Alzheimer’s disease. (B) Optic flow and object motion evoked perceptual thresholds were also obtained with the addition of heading direction jitter as frame-by-frame variation in the simulated heading direction. Heading jitter had a negligible effect on optic flow thresholds in all groups, but a substantial impact on object motion thresholds. Jitter effects on object motion threshold effects were larger in younger subjects and proportionately smaller in the older groups. YNC = younger normal controls; MNC = middle-aged normal controls; ONC = older normal controls; EAD = early Alzheimer’s disease. *indicates P < 0.05.
We considered whether an alternative naturalistic degradation of the stimuli might alter the pattern of group differences in heading perception thresholds by adding random displacement of the simulated heading to all stimuli as frame-wise ±5° horizontal and vertical jitter. Our goal was to present a worst-case scenario that might parallel effects seen with the decline in vestibulo-ocular reflex gain in ageing (Paige, 1991c; Baloh, 1993).
We found that there was very little additional disruption of heading discrimination from optic flow. In contrast, there were substantial increases of heading discrimination thresholds from object motion in all (P < 0.05) but the early Alzheimer’s disease group (P = 0.97), the latter effect potentially reflecting the limitations of near maximal thresholds. Thus, object motion based heading perception appears to be more susceptible to the type of noise that may be encountered in many naturalistic circumstances (Fig. 2B).
Incongruent heading cues
Incongruently superimposed optic flow and object motion showed effects that distinguished the early Alzheimer’s disease group from all others. Younger, middle-aged and older normal control and MCI subjects shifted their heading estimates in the direction of the heading in the object motion: laterally incongruent objects resulted in a lateral shift of heading estimates, whereas medially incongruent objects resulted in a medial shift of heading estimates. Patients with early Alzheimer’s disease were less responsive to incongruent objects, showing medial offset of heading estimates with both lateral and medially incongruent objects (Fig. 3).
Figure 3.
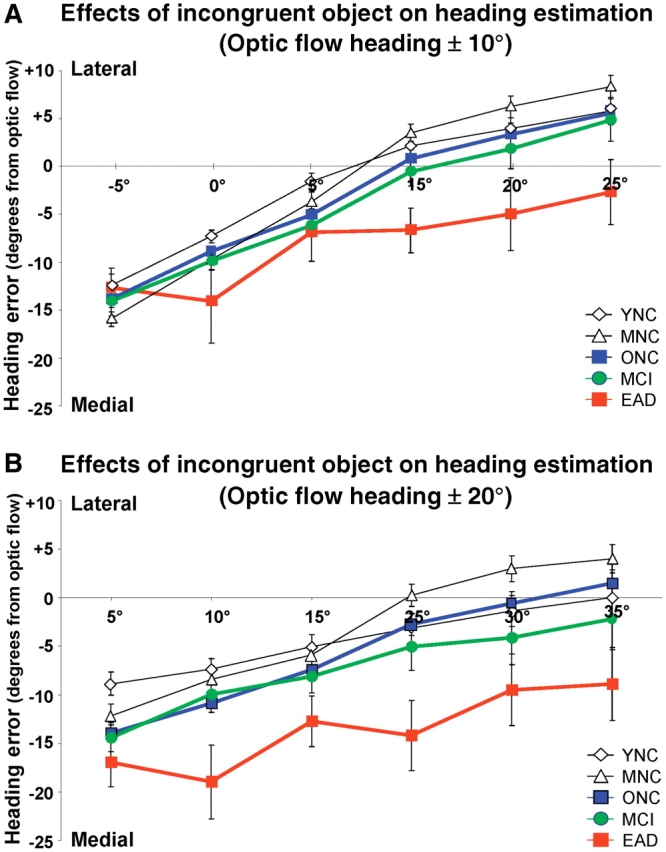
The effects of incongruent object motion on heading estimate errors relative to the heading direction simulated in optic flow having a ±10° heading (A) or ±20° heading (B) as recorded in five subject groups. All subject groups showed more lateral deflections of their perceived heading with more lateral object motion headings and more medial deflections with more medial object motion headings. All groups also showed a bias toward underestimating heading eccentricity that tended to be more substantial in older normal controls and MCI subjects and was more clearly seen in patients with in early Alzheimer’s disease. YNC = younger normal controls; MNC = middle-aged normal controls; ONC = older normal controls; EAD = early Alzheimer’s disease.
We used a comprehensive four-way ANOVA to discern significant effects of Group (5), optic Flow heading (two levels: 10°, 20°), object relative Direction (2 levels: medial, lateral), and object relative Heading eccentricity (three levels: 5°, 10°, 15°). All four main variables had a significant impact on heading estimates. Central to our findings was the significant main effect of subject group [F(4,1524) = 38.46, P < 0.001]. Group differences included both ageing and disease effects, reflected in post hoc tests: younger normal controls = middle-aged normal controls < older normal controls < MCI < early Alzheimer’s disease. Thus, ageing effects were evident beyond middle age, with further disease effects evident in the MCI and early Alzheimer’s disease groups.
A significant effect of optic flow heading [F(1,1524) = 58.91, P < 0.001] indicated larger heading estimation errors with the more eccentric optic flow headings for all groups. The positive slopes of the heading error functions in all groups reflected the combination of significant effects of object direction (P < 0.008, lateral objects causing lateral heading error) and of object eccentricity (P < 0.001, more eccentric objects causing larger error). Finally, a second order interaction between group and object direction was significant (P < 0.001) reflecting the early Alzheimer’s disease subjects’ unique pattern of medially displaced heading estimates, regardless of the medial or lateral direction of incongruent object motion.
Heading estimation
We combined the current findings, focused on incongruent object motion, with the results of our previous study of congruent object motion (Mapstone et al., 2006). We have done so to increase understanding into heading direction estimation across our comprehensive set of five naturalistic stimulus conditions, constrained by the omission of MCI subjects from the previous study: (i) optic flow presented alone as in self-movement through a textured environment; (ii) object motion presented alone as in corresponds to relative movement, from observer or object motion, with respect to a discretely salient feature in the environment; (iii) optic flow and object motion that is congruently superimposed, representing the same heading direction, corresponds to movement through a textured environment that contains a discrete, earth-fixed landmark object; (iv) optic flow and object motion incongruently superimposed with the object having a more lateral heading then the optic flow, corresponds to an animate object moving on a more eccentric course than that maintained by the observer’s self-movement; and (v) optic flow and object motion incongruently superimposed, with the object having a more medial heading then the optic flow, corresponds to an animate object moving on a less eccentric course than the observer. In all cases in which optic flow is presented, the naturalistically most parsimonious interpretation of the stimulus is consistent with the heading in the optic flow (Fig. 1C).
We found significant differences between subject groups and across the five stimulus conditions (Fig. 4). Two-way ANOVAs for Group (4) × Condition (5) at the 10° optic flow heading eccentricity yielded significant main effects of subject Group [F(3,324) = 13.49, P < 0.001] and stimulus Condition [F(4,324) = 52.73, P < 0.001] with a Group × Condition interaction [F(12,324) = 2.15, P = 0.014]. Similarly, at the 20° optic flow heading eccentricity yielded significant main effects of subject Group [F(3,324) = 14.48, P < 0.001] and stimulus Condition [F(4,324) = 26.65, P < 0.001] with a Group × Condition interaction [F(12,324) = 2.03, P = 0.022]. In both the 10° and 20° heading eccentricities, subject group effects were uniquely attributable to the patients with early Alzheimer’s disease showing greater heading estimation errors then any other group, with no significant difference between other groups (Tukey’s honestly significant differences: younger normal controls = middle-aged normal controls = older normal controls < early Alzheimer’s disease; P < 0.001). The MCI group was not included in this analysis because an insufficient number of MCI subjects completed these tests.
Figure 4.
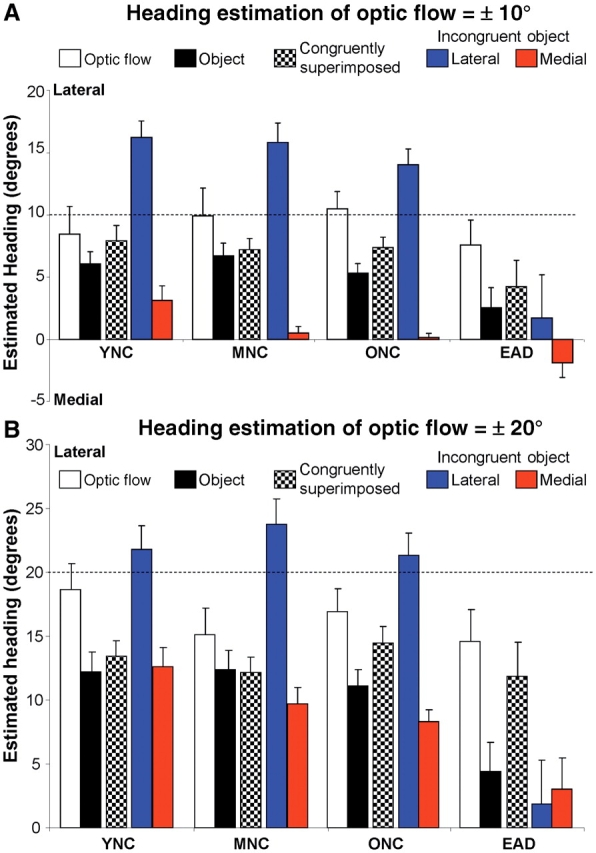
Heading estimation across five cue configurations and four subject groups. Heading estimation accuracy was determined by varying the simulated heading direction along the horizontal meridian in optic flow and object motion stimuli. Four optic flow or object motion headings were presented alone (±5°, ±10°, ±15°, ±20°, ±25°) or in a limited set of combination stimuli in which optic flow (±10° or ±20°) was combined with object motion either with matched headings (congruently superimposed) or with object motion heading displaced from the optic flow headings by ±5°, ±10° or ±15° (laterally or medially incongruently superimposed relative to the heading in optic flow). (A and B) Black and white bars depict results from optic flow and object motion alone or in congruent superimposition (Mapstone et al., 2006), included for comparison to results from incongruently superimposed cues (blue and red bars). Heading discrimination was most accurate using the optic flow stimuli alone compared to the object alone in all groups. Congruently combined optic flow and (simulated earth-fixed) objects allowed for heading discrimination which tended to be intermediate between the two single stimuli in all groups. With incongruent stimuli (simulating an independently moving object with respect to a moving observer) all normal control groups showed relatively uniform bias towards the object when it was more lateral to the simulated observer heading in optic flow. In contrast, the Alzheimer’s disease group did not show a bias toward the lateral object, but indicated self-movement heading was generally straight ahead in the direction of central visual fixation. On the other hand, a simulated object with a more medial heading than the observers’ simulated heading also led to bias toward the object, but with a pronounced ageing and disease effect Alzheimer’s disease > older normal controls > middle-aged normal controls > younger normal controls. YNC = younger normal controls; MNC = middle-aged normal controls; ONC = older normal controls; EAD = early Alzheimer’s disease.
There were substantial differences between the 10° and 20° optic flow heading eccentricities with respect to stimulus condition effects. At the 10° optic flow heading eccentricity, the flow alone condition yielded significantly smaller heading errors than all other conditions. At the 20° optic flow heading eccentricity, the flow alone and the congruently combined optic flow and object motion conditions yielded significantly smaller errors than those obtained with object motion alone, lateral incongruent objects, or medial incongruent objects. However, these condition effects are dominated by the significant Group × Condition interactions, with early Alzheimer’s disease subjects being the only group in which laterally incongruent objects great a substantial shift of perceived headings in the medial direction (toward centred gaze). In all other groups, laterally incongruent objects shift perceived heading laterally.
Linking perception and navigation
We assessed the naturalistic significance of heading estimation accuracy by studying our subjects with the battery of real-world navigational tasks described earlier (Monacelli et al., 2002). This test showed a significant decline in navigational capacity across the five subject groups tested [ANOVA F(4,119) = 39.61, P < 0.001]. Post hoc tests showed that this effect was attributable to disease effects (Tukey’s honestly significant difference: younger = middle-aged = older normal controls > MCI > Alzheimer’s disease; P < 0.001). All eight sub-tests played a significant role in contributing to the total navigation score (P < 0.001), most strongly for self-orientation (r2 = 0.84) and least for naming objects recalled from in the environment (r2 = 0.42). To understand the impact of disease on navigation, we used multiple linear regression to relate the older normal controls and Alzheimer’s disease groups’ navigation scores to their optic flow thresholds, object motion thresholds, and heading estimation errors.
The older normal control group showed a small but significant link between navigational test scores and heading estimates in the object alone and congruent stimulus conditions (R2 = 0.09, P < 0.001), although neither factor was itself significant in the regression model (object alone, P = 0.74; congruent stimuli, P = 0.53). The early Alzheimer’s disease group showed substantially stronger relations between the same set of stimulus parameters (R2 = 0.87, P < 0.001), with both factors contributing significantly to the regression model (object alone, P = 0.01; congruent stimuli, P < 0.001) (Fig. 5).
Figure 5.

Relationships between navigational performance and perceptual processing of self-movement cues. (A) Navigational performance was assessed in a real world navigational test battery implemented in the Strong Memorial Hospital lobby. Subjects were moved along a path (arrows) through the test environment and then presented with an 80 item test about the route and the environment. (B) Ageing and Alzheimer’s disease are linked to successive declines of navigational performance (ordinate, mean ± SEM). (C) Navigational test scores were not related to perceptual measures in younger groups, were weakly so in normal ageing but significantly so in patients with Alzheimer’s disease. Perceptual predictors of navigational performance included heading estimation with object motion alone and congruent optic flow and object. YNC = younger normal controls; MNC = middle-aged normal controls; ONC = older normal controls; EAD = early Alzheimer’s disease.
The link between heading estimation and navigational performance was strongest for specific heading and navigational sub-tests. Heading estimation in the incongruent cue conditions, both lateral and medial incongruent object headings, were significantly correlated (P < 0.001) with all but object naming: route learning (r2 = 0.53 and 0.52), self-orientation (r2 = 0.5 and 0.5), route drawing (r2 = 0.46 and 0.37), route learning (r2 = 0.33 and 0.54), photo location (r2 = 0.48 and 0.52), video location (r2 = 0.44 and 0.52) and free recall (r2 = 0.45 and 0.41). Heading estimation by optic flow alone, object motion alone or the congruent combination of those cues did not attain this level of significance. We conclude that the ability to accurately estimate simulated headings in the presence of incongruent object motion is linked to the decline in navigational capacity seen in early Alzheimer’s disease.
Discussion
Cue equivalence for naturalistic stimuli
Previous studies have described group differences in visual motion processing (Trick and Silverman, 1991; Gilmore et al., 1992; Tetewsky and Duffy, 1999). Our first goal was to assess independently our subjects’ ability to perceive our stimuli, and their ability to use these stimuli as cues regarding their simulated direction of self-movement. Based on our earlier work, we anticipated group differences in basic perception of motion stimuli. To address this, we attempted to create stimuli of equivalent perceptual salience for all subjects by first measuring perceptual thresholds, and then using those thresholds to modify the stimuli presented in the heading estimation task. We added incoherent random dot motion to degrade optic flow cues and varied the length of the stimulus path to degrade object motion cues (Mapstone et al., 2006).
Our discrimination threshold studies revealed a substantial ageing effect on heading perception from optic flow and from object motion. Our younger, middle-aged and older normal control subjects showed comparable optic flow motion coherence thresholds, but patients with Alzheimer’s disease showed a significant threshold increase relative to all other groups (Fig. 2A, left). In contrast, younger, middle-aged and older normal controls, and subjects with early Alzheimer’s disease showed successively increasing object motion path length thresholds with significant differences between younger and older normal control groups, and between patients with early Alzheimer’s disease and others (Fig. 2A, right). Thus, we see fundamental differences in the effects of ageing on optic flow and object motion perception, with object motion being more vulnerable to age-related degradation of perceptual capacities; but patients with early Alzheimer’s disease being impaired with both optic flow and object motion stimuli.
Our use of motion coherence and path length thresholds has some naturalistic relevance, in that similar sources of noise are present in the environment. An alternative approach is to add positional noise as might be created by the bouncing irregularity of jogging or driving. We added heading jitter to our optic flow and object motion stimuli and independently re-determined each subject’s motion coherence and path length thresholds. The added jitter had no significant effects on optic flow motion coherence thresholds with group difference being similar to those obtained without jitter (Fig. 2B, left). However, adding jitter to object motion significantly increased the mean object motion path length thresholds of all subject groups (Fig. 2B, right).
These findings suggest that heading jitter extended the range of path length thresholds in the normal control groups, but had less effect on patients with early Alzheimer’s disease. The relative decline in the magnitude of this effect in the patients with early Alzheimer’s disease may reflect a restricted range at the high end of the range of thresholds that is limited at 100%. Alternatively, this could reflect adaptation to jitter in older subjects who routinely experience such effects in ambulation because of age-related declines in vestibulo-ocular reflex gain (Paige, 1991c; Baloh, 1993). Such an effect might be thought of as a visual processing analogue to the compensatory gain in cervico-ocular reflexes in this population (Kelders, 2003).
Optic flow’s resilience to high frequency heading changes may reflect its derivation from the global pattern of motion in which any subset of moving elements specifies the instantaneous heading (Longuet-Higgins and Prazdny, 1980; Beusmans, 1993). In contrast, object motion supports heading by forming a discrete pattern of spatially coherent moving elements, which present a local motion signal that may be more responsive to successive positional perturbations (Cornilleau-Peres and Droulez, 1994; Zemel and Sejnowski, 1998). Alternatively, we might consider that simulated heading is an explicitly motion-defined point in optic flow, evident in every successive image (Burr and Santoro, 2001). In contrast, the perceptual derivation of the heading simulated by object motion might require a longer time-scale of temporal integration, and be more subject to interactions with our path length manipulation (Fredericksen et al., 1994). These views represent emphasis on the spatial and temporal aspects of the jitter effect, which cannot be distinguished in our studies.
Our findings suggest that we should avoid adding repeated heading changes during stimulus presentation by using stable straight line paths of self-movement to assess cue effects on heading estimation across subject groups. We must also consider that the differences between optic flow and object responses might be altered by using other configurations of object stimuli (e.g. multiple objects). Such changes could improve the contribution of objects to heading estimation but, at the limit, it obscures the distinction between objects and flow fields.
Optic flow and object motion cue interactions
In this study, all normal subject groups accurately detected heading directions simulated by optic flow alone, object motion alone, and with congruently combined optic flow and object motion. Performance with optic flow was better than with object motion alone in all subject groups, suggesting that ageing does not have a significant impact on the relative utility of optic flow, object motion or congruently combined cues. This conclusion is buttressed by the fact that the subjects’ behavioural task is the same in all heading estimation conditions. We should also note that our previous use of a flashed square target in the visuomotor control task showed relatively little distorting effects of target horizontal position, especially across groups (Mapstone et al., 2006). Nevertheless, some effect was evident and we must consider that the underlying neural mechanisms may be the same.
Congruent optic flow and object motion simulate self-movement in the presence of earth-fixed landmark objects. We have found that congruent cues allow all normal subjects to improve their heading estimation over performance with object motion alone. However, that performance does not meet the heading estimation accuracy achieved with optic flow alone. We might expect that the addition of congruent object motion to optic flow would improve heading estimation accuracy by providing an additional, demonstrably useful source of heading information. To the contrary, our data suggest that congruent object motion does not improve heading estimation from optic flow, but actually reduces the accuracy of heading estimation relative to that achieved with optic flow alone, making it more like the poorer performance achieved with object motion alone. Thus, congruent object motion appears to be, at best, redundant with optic flow. Surprisingly, congruently moving objects appear to be a dominant cue in heading estimation, even when it is a less accurate cue. The interpretation of this finding must be tempered by the fact that our simulation did not provide naturalistically rich objects that might include other cues (e.g. disparity, shadowing, etc.). Although we have found that virtual reality measures of navigational impairment in Alzheimer’s disease closely mirror effects in real world navigation (Cushman, 2008), the current stimuli are highly simplified virtual reality scenarios.
A very different picture emerged when we presented optic flow with an incongruently moving object. In that case, an ideal moving observer would base heading estimation exclusively on the optic flow, interpreting the object as undergoing independent, animate movement. However, in younger, middle-aged and older normal control subjects incongruent object motion shifts heading estimates in the direction of the object’s heading. This observation is consistent with earlier studies showing that incongruent object motion biases heading estimates in the direction of the object’s heading, particularly as the object crosses the heading point in the optic flow (Royden and Hildreth, 1996, 1999).
Our patients with early Alzheimer’s disease showed remarkably distinct effects of incongruent object motion. Both lateral and medially displaced incongruent object motion caused patients with early Alzheimer’s disease to see all of the optic flow simulated headings as being straight ahead, near the centre of the display screen (Fig. 4). This robust effect of incongruent object motion was not greatly affected by the simulated heading of the object (Fig. 5) or of the optic flow, and was not very different from the patients’ with early Alzheimer’s disease heading estimation based-on optic flow alone. Thus, we again see that object motion can be a perceptually dominant cue in heading estimation.
Animal models provide some insights into the underlying mechanism of interactions between object motion and optic flow. Single neurons in the posterior parietotemporal cortex of macaque monkeys respond to both optic flow and object motion stimuli with heading selective directional tuning (Logan and Duffy, 2006). These neurons show a greater effect of object motion when the objects are presented in incongruent combinations with optic flow. Such effects may create a mechanism for shifting heading estimation from an environmental reference frame to one centred on an animate object, especially as that object crosses through central vision. Such a mechanism might promote the avoidance of predators and the interception of prey.
Another possible interpretation of the dominance of object motion, especially incongruent object motion, is that the objects are interpreted as earth-fixed landmarks. Such landmarks may be of great navigational significance (Golledge, 1999) so that they capture the attention of the observer and interfere with the processing of the heading information in optic flow (Mapstone et al., 2001). In our studies of heading sensitive neurons in monkeys, we have seen robust effects of attention on responses to heading information, with selective shifting from responsiveness to small central areas of motion versus large peripheral patterns of motion (Dubin and Duffy, 2007; Page and Duffy, 2008). Profound attentional effects have also been seen in functional imaging of homologous areas of human posterior parietotemporal cortex (Haxby et al., 1991; O'Craven et al., 1997).
The unique deficits seen in our patients with early Alzheimer’s disease when confronted with incongruent object motion may reflect the combined breakdown of top-down attentional control of sensory processing, superimposed on the vulnerability of the bottom-up sensory processing seen in Alzheimer’s disease (O'Brien et al., 2001; Mapstone et al., 2003). These findings might also be interpreted as a loss of attentional control over the allocation of visual processing capacity to competing stimuli, as in dual-task interference (Pashler, 1993). Such an interpretation may be consistent with the loss of attentional control in ageing as a result of impaired frontal cortical control mechanisms (Greenwood, 2000; Mapstone et al., 2008).
Perception limits navigation in Alzheimer’s disease
We characterized our subject’s navigational capacities using a real-world test battery, replicating our previous findings of successive impairment in ageing and Alzheimer’s disease (Monacelli et al., 2003; Cushman et al., 2008). Multiple linear regression show a significant, but small relationship between navigation and perceptual measures, and a much stronger relationship in patients with early Alzheimer’s disease (Fig. 5). In both groups, the only significant factors were the heading estimation errors seen with congruent optic flow and object motion stimuli and with object motion alone.
The robust relationship between navigation and heading estimation with object motion in patients with early Alzheimer’s disease may reflect two factors. First, the salient characteristics of architectural environments, such as the hospital lobby used in our navigational test battery, contain a large number of recognizable objects that are fixed to the environment (e.g. structural features and furnishings) forming congruent self-movement cues. Thus, navigation through such an environment may depend on the processing of those cues. Second, patients with early Alzheimer’s disease may show more robust effects because of the large magnitude and greater group variability of their object heading estimation errors. Heading estimation errors with incongruent stimuli may not account for a significant degree of variance in navigational performance because incongruent objects reflect independent object motion and have little utility as navigational cues. We must be mindful that, during independent ambulation, the mobility problems of many older adults may obscure the relationships seen in this study.
The consistent conclusion of these studies is that patients with early Alzheimer’s disease uniquely exhibit great difficulty in interpreting object motion as a heading estimation cue. This finding contrasts with the graded impact of ageing and Alzheimer’s disease in the less naturalistic setting of two-alternative forced choice left-right heading discrimination, especially in comparison. The significance of these findings is highlighted by the context of the substantial, detrimental impact of Alzheimer’s disease on optic flow heading discrimination. The combined effect of being unable to process the radial motion patterns of optic flow (O'Brien et al., 2001; Mapstone et al., 2008), and being unable to interpret object motion as a heading cue, may account for devastating effects of Alzheimer’s disease on navigation and visuospatial orientation (Monacelli et al., 2003; Cushman et al., 2008).
This perspective differs from the more conventional view that relates navigational impairment in Alzheimer’s disease to the disease’s impact on hippocampal mechanisms related to cognitive mapping (Burgess et al., 2006; Laczo et al., 2009). Our findings support a systems perspective on navigational impairment in Alzheimer’s disease, recognizing a role for the hippocampus but also the important role of a distributed cortical and sub-cortical system for navigation (Duffy et al., 2010) including many foci of pathology in Alzheimer’s disease (Brun and Gustafon 1976; Rosenbaum et al., 2005).
We conclude that optic flow perceptual factors limit safe, autonomous navigation in Alzheimer’s disease, particularly the capacity to integrate the variety of potentially conflicting cues present in naturalistic environments. Our work suggests that many Alzheimer’s patients, even in the earliest stages of the disease, may have difficulty judging their own heading especially in the presence of an independently moving object. The results of this study highlight the importance of early screening for visual perceptual impairments in elderly drivers, which will become important as we face a projected increase in the prevalence of Alzheimer’s disease in the coming decades. Ultimately, we hope these findings can help guide novel strategies designed to enhance the visual environment and improve driving performance in the elderly.
Funding
NIA grants AG17596 and AG20647, NEI grant EY10287.
Conflict of interest: Dr Duffy is a principal of Cerebral Assessment Systems, Inc. from which he received that portion of his regular salary for time not devoted to this funded research.
Acknowledgements
The authors gratefully acknowledge the assistance of Mark Abroms, David Logan, Teresa Steffenella and William Vaughn in conducting these experiments.
Glossary
Abbreviations
- MCI
mild cognitive impairment
References
- Aguirre GK, D'Esposito M. Environmental knowledge is subserved by separable dorsal/ventral neural areas. J Neurosci. 1997;17:2512–8. doi: 10.1523/JNEUROSCI.17-07-02512.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA. Visual field defects in Alzheimer's disease patients may reflect differential pathology in the primary visual cortex. Optom Vis Sci. 1996;73:677–82. doi: 10.1097/00006324-199611000-00001. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991;1:103–16. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Baloh RW, Fife TD, Zwerling L, Socotch T, Jacobson K, Bell T, Beykirch K. Comparison of static and dynamic posturography in young and older normal people. J Am Geriatr Soc. 1994;42:405–12. doi: 10.1111/j.1532-5415.1994.tb07489.x. [DOI] [PubMed] [Google Scholar]
- Benton A, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment: a clinical manual. New York: Oxford University Press; 1983. [Google Scholar]
- Beusmans JM. Computing the direction of heading from affine image flow. Biol Cybern. 1993;70:123–36. doi: 10.1007/BF00200826. [DOI] [PubMed] [Google Scholar]
- Brun A, Gustafon L. Distribution of cerebral degeneration in alzheimer's disease. Arch Psychiat Nervenkr. 1976;223:15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- Burgess N, Trinkler I, King J, Kennedy A, Cipolotti L. Impaired allocentric spatial memory underlying topographical disorientation. Rev Neurosci. 2006;17:239–51. doi: 10.1515/revneuro.2006.17.1-2.239. [DOI] [PubMed] [Google Scholar]
- Burr DC, Santoro L. Temporal integration of optic flow, measured by contrast and coherence thresholds. Vis Res. 2001;41:1891–9. doi: 10.1016/s0042-6989(01)00072-4. [DOI] [PubMed] [Google Scholar]
- Cornilleau-Peres V, Droulez J. The visual perception of three-dimensional shape from self- motion and object-motion. Vis Res. 1994;34:2331–6. doi: 10.1016/0042-6989(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Cushman LA, Duffy CJ. The sex specificity of navigational strategies in Alzheimer disease. Alzheimer Dis Assoc Disord. 2007;21:122–9. doi: 10.1097/WAD.0b013e318047df2f. [DOI] [PubMed] [Google Scholar]
- Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–95. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin MJ, Duffy CJ. Behavioral influences on cortical neuronal responses to optic flow. Cereb Cortex. 2007;17:1722–32. doi: 10.1093/cercor/bhl083. [DOI] [PubMed] [Google Scholar]
- Duffy CJ, Logan DJ, Dubin MJ, Page WK. Neural mechanisms of self-movement perception for navigation and spatial orieintation. In: Jenkins M, Harris LR, editors. Cortical mechanisms of vision. New York: Cambridge University Press; 2010. pp. 119–46. [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Rese. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fredericksen RE, Verstraten FA, van de Grind WA. Spatial summation and its interaction with the temporal integration mechanism in human motion perception. Vis Res. 1994;34:3171–88. doi: 10.1016/0042-6989(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Gilmore GC, Wenk HE, Naylor LA, Stuve TA. Motion perception and aging. Psychol Aging. 1992;7:654–60. doi: 10.1037//0882-7974.7.4.654. [DOI] [PubMed] [Google Scholar]
- Golledge RG. Wayfinding behavior: cognitive mapping and other spatial processes. Baltimore: The Johns Hopkins University Press; 1999. [Google Scholar]
- Goossens J, Dukelow SP, Menon RS, Vilis T, van den Berg AV. Representation of head-centric flow in the human motion complex. J Neurosci. 2006;26:5616–27. doi: 10.1523/JNEUROSCI.0730-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood PM. The frontal aging hypothesis evaluated. J Int Neuropsychol Soc. 2000;6:705–26. doi: 10.1017/s1355617700666092. [DOI] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Perception of coherent motion, biological motion and form-from-motion under dim-light conditions. Vision Res. 1999;39:3721–7. doi: 10.1016/s0042-6989(99)00084-x. [DOI] [PubMed] [Google Scholar]
- Harvey LO. Efficient estimation of sensory thresholds with ML-PEST. Spatial Vision. 1997;11:121–8. doi: 10.1163/156856897x00159. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, et al. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci USA. 1991:1621–5. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conference Proceedings. 1982;2:1–10. [Google Scholar]
- Kavcic V, Fernandez R, Logan DJ, Duffy CJ. Neurophysiological and perceptual correlates of navigational impairment in Alzheimer's disease. Brain. 2006;129:736–46. doi: 10.1093/brain/awh727. [DOI] [PubMed] [Google Scholar]
- Kelders WP, Kleinrensink GJ, van der Geest JN, Feenstra L, de Zeeuw CI, Frens MA. Compensatory increase of the cervico-ocular reflex with age in healthy humans. J Physiol. 1993;553:311–7. doi: 10.1113/jphysiol.2003.049338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laczo J, Vlcek K, Vyhnalek M, Vajnerová O, Ort M, Holmerová I, et al. Spatial navigation testing discriminates two types of amnestic mild cognitive impairment. Behav Brain Res. 2009;202:252–9. doi: 10.1016/j.bbr.2009.03.041. [DOI] [PubMed] [Google Scholar]
- Logan DJ, Duffy CJ. Cortical area MSTd combines visual cues to represent 3-D self-movement. Cereb Cortex. 2006;16:1494–507. doi: 10.1093/cercor/bhj082. [DOI] [PubMed] [Google Scholar]
- Longuet-Higgins HC, Prazdny K. The interpretation of a moving retinal image. Pro R Soc Lond B. 1980;208:385–97. doi: 10.1098/rspb.1980.0057. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Dickerson K, Duffy CJ. Distinct mechanisms of impairment in cognitive ageing and Alzheimer's disease. Brain. 2008;131 doi: 10.1093/brain/awn064. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Logan D, Duffy CJ. Cue integration for the perception and control of self-movement in ageing and Alzheimer's disease. Brain. 2006;129:2931–44. doi: 10.1093/brain/awl201. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Rosler A, Hays A, Gitelman DR, Weintraub S. Dynamic allocation of attention in aging and Alzheimer disease: uncoupling of the eye and mind. Arch Neurol. 2001;58:1443–7. doi: 10.1001/archneur.58.9.1443. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Steffenella TM, Duffy CJ. A visuospatial variant of mild cognitive impairment: Getting lost between aging and AD. Neurology. 2003;60:802–8. doi: 10.1212/01.wnl.0000049471.76799.de. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS- ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Monacelli AM, Cushman LA, Kavcic V, Duffy CJ. Spatial disorientation in Alzheimer's disease: the remembrance of things passed. Neurology. 2003;61:1491–7. doi: 10.1212/wnl.61.11.1491. [DOI] [PubMed] [Google Scholar]
- Money J. A Standardized Road Map Test of Direction Sense. San Rafael, CA: Academic Therapy Publications; 1976. [Google Scholar]
- Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 2000;3:1322–8. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- O'Brien HL, Tetewsky S, Avery LM, Cushman LA, Makous W, Duffy CJ. Visual mechanisms of spatial disorientation in alzheimer's disease. Cereb Cortex. 2001;11:1083–92. doi: 10.1093/cercor/11.11.1083. [DOI] [PubMed] [Google Scholar]
- O'Craven KM, Rosen BR, Kwong KK, Treisman A, Savoy RL. Voluntary attention modulates fMRI activity in human MT-MST. Neuron. 1997;18:591–8. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- Page WK, Duffy CJ. Cortical neuronal responses to optic flow are shaped by visual strategies for steering. Cereb Cortex. 2008;18:727–39. doi: 10.1093/cercor/bhm109. [DOI] [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement response to linear head motion in the squirrel monkey: I. Basic characteristics. J Neurophysiol. 1991;65:1170–82. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Pashler H, O'Brien S. Dual-task interference and the cerebral hemispheres. J Exp Psychol Hum Percept Perform. 1993;19:315–30. [PubMed] [Google Scholar]
- Pentland A. Maximum likelihood estimation: the best PEST. Percept Psychophys. 1980;28:377–9. doi: 10.3758/bf03204398. [DOI] [PubMed] [Google Scholar]
- Rosenbaum RS, Gao F, Richards B, Black SE, Moscovitch M. “Where to?“ remote memory for spatial relations and landmark identity in former taxi drivers with Alzheimer's disease and encephalitis. J Cogn Neurosci. 2005;17:446–62. doi: 10.1162/0898929053279496. [DOI] [PubMed] [Google Scholar]
- Royden CS. Computing heading in the presence of moving objects: a model that uses motion-opponent operators. Vision Res. 2002;42:3043–58. doi: 10.1016/s0042-6989(02)00394-2. [DOI] [PubMed] [Google Scholar]
- Royden CS, Hildreth EC. Human heading judgments in the presence of moving objects. Percept Psychophys. 1996;58:836–56. doi: 10.3758/bf03205487. [DOI] [PubMed] [Google Scholar]
- Royden CS, Hildreth EC. Differential effects of shared attention on perception of heading and 3-D object motion. Percept Psychophys. 1999;61:120–33. doi: 10.3758/bf03211953. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Ollinger JM, Akbudak E, Conturo TE, Snyder AZ, Petersen SE, et al. Areas involved in encoding and applying directional expectations to moving objects. J Neurosci. 1999;19:9480–96. doi: 10.1523/JNEUROSCI.19-21-09480.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPSS Inc. SPSS 12.0. Upper Saddle River, NJ: Prentiss Hall, Inc.; 2005. [Google Scholar]
- Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, et al. Clinical, genetic, and neuropathological characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–74. doi: 10.1212/01.wnl.0000140289.18472.15. [DOI] [PubMed] [Google Scholar]
- Tetewsky S, Duffy CJ. Visual loss and getting lost in Alzheimer's disease. Neurology. 1999;52:958–65. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- Trick GL, Silverman SE. Visual sensitivity to motion: age-related changes and deficits in senile dementia of the Alzheimer type. Neurology. 1991;41:1437–40. doi: 10.1212/wnl.41.9.1437. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale, Revised Manual. San Antonio, TX: The Psychological Corp.; 1987. [Google Scholar]
- Zemel RS, Sejnowski T. A model for encoding multiple object motions and self-motion in area MST of primate visual cortex. J Neurosci. 1998;18:531–47. doi: 10.1523/JNEUROSCI.18-01-00531.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]



