Abstract
Endoglycan is a mucin-like glycoprotein expressed by endothelial cells and some leukocytes and is recognized by L-selectin, a C-type lectin important in leukocyte trafficking and extravasation during inflammation. Here, we show that recombinant L-selectin and human T lymphocytes expressing L-selectin bind to synthetic glycosulfopeptides (GSPs). These synthetic glycosulfopeptides contain 37 amino acid residues modeled after the N-terminus of human endoglycan and contain one or two tyrosine sulfates (TyrSO3) along with a nearby core-2-based Thr-linked O-glycan with sialyl Lewis x (C2-SLex). TyrSO3 at position Y118 was more critical for binding than at Y97. C2-SLex at T124 was required for L-selectin recognition. Interestingly, under similar conditions, neither L-selectin nor T lymphocytes showed appreciable binding to the sulfated carbohydrate epitope 6-sulfo-SLex. P-selectin also bound to endoglycan-based GSPs but with lower affinity than toward GSPs modeled after PSGL-1, the physiological ligand for P- and L-selectin that is expressed on leukocytes. These results demonstrate that TyrSO3 residues in association with a C2-SLex moiety within endoglycan and PSGL-1 are preferentially recognized by L-selectin.
Keywords: endoglycan, glycosulfopeptide, L-selectin, O-glycan, tyrosine sulfate
Introduction
The selectin family of cell adhesion molecules together with their glycoconjugate ligands participate in leukocyte trafficking to sites of inflammation and to lymphoid organs (McEver 2002). P- and E-selectins are expressed on activated vascular endothelial cells where they mediate initial tethering and rolling of leukocytes on endothelial cells by binding to P-selectin glycoprotein ligand-1 (PSGL-1) present on the surface of leukocytes (McEver and Cummings 1997; Carlow et al. 2009). P-selectin is also expressed on activated platelets. L-selectin is expressed on the surface of leukocytes and mediates leukocyte–leukocyte interactions by binding to PSGL-1 present on the surface of other leukocytes promoting leukocyte accumulation to the inflammatory sites (Guyer et al. 1996; Spertini et al. 1996; Tu et al. 1996; Walcheck et al. 1996). All selectins recognize the sialyl Lewis x epitope (SLex, NeuAcα2-3Galβ1-4(Fucα1-3)GlcNAcβ1-) on glycoconjugate ligands. However, selectin binding to SLex determinant alone is very low affinity and is necessary but not sufficient for physiological interactions. Thus, selectins require additional post-translational modifications or peptide components for high-affinity binding to their ligands.
P- and L-selectin both bind to the extreme N-terminus of PSGL-1 and interact with three clustered tyrosine sulfate residues and a nearby core-2-based O-glycan with sialyl Lewis x epitope (C2-SLex). The N-terminus of human PSGL-1 contains three potential tyrosine sulfation sites (Y46, Y48 and Y51) and two potential O-glycan attachment sites (T44 and T57). Site-directed mutagenesis experiments using recombinant PSGL-1 constructs with substitutions in targeted N-terminal tyrosine or threonine residues suggested that at least one sulfated tyrosine (TyrSO3) and an O-glycan at T57 were required for L-selectin-dependent rolling (Ramachandran et al. 1999) and that Y51 was the most important sulfation site on PSGL-1 for L-selectin binding (Bernimoulin et al. 2003). Studies utilizing synthetic glycosulfopeptides (GSPs) modeled after N-terminus of PSGL-1 indicated that L-selectin bound with relatively high affinity (Kd ∼5 μM) to a glycosulfopeptide (2-GSP-6) containing three TyrSO3 residues and a C2-SLex O-glycan at T57 (Leppänen et al. 2003). SLex on extended core-1 O-glycan on a PSGL-1 model glycosulfopeptide or on CHO cell transfectants with PSGL-1 supported weaker binding to L-selectin than 2-GSP-6 or CHO cell transfectants containing C2-SLex O-glycan (Leppänen et al. 2003; Mitoma et al. 2003).
During lymphocyte homing to secondary lymphoid organs, lymphocytes bind to high endothelial cells present on high endothelial venules (HEV) of lymphoid organs, which enables lymphocyte exit from the blood circulation. Initial tethering and rolling of lymphocytes on endothelial cell layer is mediated by L-selectin on lymphocytes and L-selectin ligands present on the surface of high endothelial cells (Rosen 2004). Several candidate L-selectin ligands have been identified in the HEV of secondary lymphoid organs, including glycosylation-dependent cell adhesion molecule (GlyCAM-1) (Imai et al. 1991; Lasky et al. 1992), CD34 (Imai et al. 1991; Baumhueter et al. 1993), podocalyxin-like protein (Sassetti et al. 1998), MadCAM-1 (Berg et al. 1993), Sgp200 (Hemmerich et al. 1994), endomucin (Morgan et al. 1999; Samulowitz et al. 2002), endoglycan (Sassetti et al. 2000), nepmucin (Umemoto et al. 2006) and PSGL-1 (Rivera-Nieves et al. 2006). They are sialomucins containing a highly sialylated and O-glycosylated peptide backbone. Many of these endothelial mucins express sulfated sialyl Lewis x structures on O-glycans (Hemmerich et al. 1995; Satomaa et al. 2002). 6-Sulfo-SLex (NeuAcα2-3Galβ1-4(Fucα1-3)(SO3-6)GlcNAcβ1-) has been shown to contribute to L-selectin recognition (Scudder et al. 1994; Mitsuoka et al. 1998; Bistrup et al. 1999; Kimura et al. 1999; Hemmerich et al. 2001). Recently, we showed that human tonsil-derived CD34 comprised two glycoforms, a major glycoform lacking 6-sulfo-SLex on O-glycans and a minor form containing relatively high amounts of O-glycans with 6-sulfo-SLex (Hernandez Mir et al. 2009).
The evidence that sulfated carbohydrate residues are important for L-selectin recognition is partly based on the observation that a monoclonal antibody MECA-79, which binds to a group of endothelial mucins called peripheral lymph node vascular addressin (PNAd) on lymph node HEV, inhibits lymphocyte homing to lymph nodes in mice (Streeter et al. 1988; Berg et al. 1991). MECA-79 recognizes a 6-sulfated N-acetyllactosamine epitope on extended core-1 O-glycan (Galβ1-4(SO3-6)GlcNAcβ1-3Galβ1-3GalNAc) expressed on endothelial ligands (Yeh et al. 2001). Although MECA-79 binds 6-sulfo-SLex epitope only when present on extended core-1 O-glycans, 6-sulfo-SLex expressed on core-2 O-glycans can function as L-selectin ligands in lymphocyte homing in mice (Yeh et al. 2001; Hiraoka et al. 2004). Studies with mice deficient in two of the 6-sulfotransferases responsible for synthesis of 6-sulfo-SLex epitope on endothelial cells of lymph nodes indicated that sulfotransferase double-deficient mice had impaired lymphocyte homing to lymph nodes, although MECA-79 epitopes were completely absent in endothelial cells of sulfotransferase double-deficient mice (Kawashima et al. 2005; Uchimura et al. 2005). This suggests that MECA-79-independent ligands for L-selectin are expressed in lymph nodes in mice and that they contribute significantly to lymphocyte homing to secondary lymphoid organs. A study with mice deficient in core-1 extension and core-2 branching enzymes has indicated that 6-sulfo-SLex on N-glycans of murine CD34 can contribute to L-selectin-dependent lymphocyte homing (Mitoma et al. 2007). Potential 6-sulfo-SLex epitope has also been identified on N-glycans of human endothelial CD34 (Hernandez Mir et al. 2009). Other MECA-79-independent ligand candidates for L-selectin have been reported to be present in human, porcine and rat lymphoid tissues, but the identity of these ligands is unknown (Clark et al. 1998; Derry et al. 1999; Tu et al. 1999; Khan et al. 2002).
The initial studies indicating that 6-sulfo-SLex contributes to L-selectin ligand function were carried out primarily in the murine system, and the interaction of human L-selectin with endothelial ligands on human lymphoid tissues are still poorly understood. MECA-79 and 6-sulfo-SLex epitopes are expressed on HEV of human lymphoid tissues, and MECA-79 has been shown to partially inhibit lymphocyte binding to HEV or to purified PNAd (Berg et al. 1991; Michie et al. 1993; Clark et al. 1998; Mitsuoka et al. 1998). Two MECA-79 reactive sialomucins, CD34 (Puri et al. 1995) and podocalyxin-like protein (Sassetti et al. 1998) isolated from human tonsils, have been shown to support L-selectin-dependent lymphocyte rolling in vitro. Moreover, endomucin (Samulowitz et al. 2002) and several MECA-79-independent candidate L-selectin ligands, including endoglycan (Clark et al. 1998; Tu et al. 1999; Sassetti et al. 2000), have also been identified from human lymphoid tissues and endothelial cells. Recent structural analysis of O- and N-glycans of human CD34 revealed that L-selectin binding glycoforms of human endothelial CD34 carry relatively high density of 6-sulfo-SLex epitopes on extended core-1 and core-2-based O-glycans (Hernandez Mir et al. 2009). Potential 6-sulfo-SLex epitopes were also identified on N-glycans of L-selectin-bound form of CD34. Partial inhibition of lymphocyte binding to HEV by MECA-79 monoclonal antibody (mAb) could be explained by the inability of MECA-79 to inhibit L-selectin binding to 6-sulfo-SLex on core-2 O-glycans or on N-glycans. However, N-glycans on human CD34 have not been shown to have a major role for L-selectin binding.
Endoglycan is a member of the CD34 family of sialomucins (Sassetti et al. 2000). It is a dimeric mucin expressed on vascular endothelium, leukocyte subpopulations and hematopoietic precursor cells. Endoglycan has an acidic N-terminal domain that is not present in the other CD34 family members, CD34 and podocalyxin. Endoglycan is extensively glycosylated and contains a large number of potential O-glycosylation sites and several potential N-glycan and glycosaminoglycan attachment sites and also contains chondroitin sulfate (Sassetti et al. 2000). Moreover, the acidic N-terminus of endoglycan contains two potential tyrosine sulfation sites at residues Y97 and Y118. Arylsulfatase, which cleaves sulfate from TyrSO3 residues, has been shown to remove sulfate from recombinant endoglycan (Fieger et al. 2003). Recombinant and native human endoglycan can function as a ligand in vitro for L-selectin (Fieger et al. 2003; Sarangapani et al. 2004) and for vascular selectins (Kerr et al. 2008). Sialylation and α1,3-fucosylation of recombinant endoglycan were required for L-selectin ligand activity in vitro, whereas GlcNAc 6-sulfation of endoglycan appeared to be less important for L-selectin binding (Fieger et al. 2003). Site-directed mutagenesis using recombinant endoglycan constructs with substitutions in targeted N-terminal tyrosine and threonine residues suggested that at least one tyrosine (Y97 or Y118) and threonine 124 contribute for binding to L-selectin (Fieger et al. 2003). The role of individual tyrosine residues for binding to L-selectin has not been studied. Because functional endoglycan was expressed in transfected COS-7 cells expressing recombinant fucosyltransferase VII, endogenous core-2 N-acetylglucosaminyl-transferase and sialyltransferase, the fucosylated and sialylated O-glycans on recombinant endoglycan were likely of core-2 type. The presence of SLex (or sulfo-SLex) on recombinant endoglycan was verified by HECA-452 reactivity. However, the role of SLex on core-2-based O-glycan at T124 on endoglycan for binding to L-selectin is unclear. The potential L-/P-selectin binding site on human endoglycan resembles L-/P-selectin binding site on human PSGL-1. The most striking similarity can be found around amino acids Y118 and T124 of endoglycan and Y51 and T57 of PSGL-1. Interestingly, T57 and Y51 have been shown to be critical modification sites on PSGL-1 for L-selectin binding (Ramachandran et al. 1999; Bernimoulin et al. 2003; Leppänen et al. 2003).
Such studies prompted our investigation into the importance of the potential glycosulfopeptide region of endoglycan for L- and P-selectin recognition. In the present study, glycosulfopeptides modeled after acidic N-terminal region of human endoglycan were synthesized to explore the site-specific contribution of each of the two potential TyrSO3 residues and the role of core-2-based O-glycan with SLex at T124 of endoglycan for binding to L- and P-selectin. Binding of selectins to endoglycan glycosulfopeptides (EG-GSPs) was compared with glycosulfopeptides modeled after N-terminus of human PSGL-1 (P-GSPs). The role of the 6-sulfo-SLex epitope for binding to selectins was also evaluated. The results indicate that recombinant L-selectin and T lymphocytes expressing natural L-selectin recognize EG- and P-GSPs containing TyrSO3 residues and a nearby core-2-based O-glycan with sLex (C2-SLex). The contribution of sulfate at Y118 on EG-GSPs was more important than sulfate at Y97 for L-selectin binding. EDTA and antibodies to L-selectin and endoglycan were able to inhibit the interaction of L-selectin/T lymphocytes with EG-GSPs. We also show that L-selectin binds poorly to the sulfated carbohydrate epitope 6-sulfo-SLex. Our results also indicate that P-selectin recognizes EG-GSPs, and binding is dependent on TyrSO3 residues and a core-2-based O-glycan with sLex at Thr57. However, P-selectin binds to EG-GSPs with considerably lower affinity than to P-GSPs, further refining the specificity of P-selectin toward PSGL-1.
Results
Synthesis of glycosulfopeptides modeled after human endoglycan
Human endoglycan contains two potential tyrosine sulfation sites (Y97, Y118) and a nearby potential O-glycosylation site (T124) at the N-terminus that have been suggested to contribute to L-selectin binding (Fieger et al. 2003). Model glyco(sulfo)peptides (EG-GSPs) corresponding to amino acid residues 92–127 of human endoglycan were synthesized chemoenzymatically to study the role of site-specific tyrosine sulfation and O-glycosylation of endoglycan for binding to L-selectin (Figure 1). The sulfated peptide backbone with a TyrSO3 residue at position Y97 or Y118 or at both positions and the first monosaccharide residue (α-linked GalNAc) attached to T124 were synthesized using N-(9-fluorenyl)methoxycarbonyl (Fmoc) chemistry on an automated peptide synthesizer. Fmoc derivatives of TyrSO3 and tri-O-acetyl GalNAc-Thr were used during the solid-phase peptide synthesis. An extra cysteine residue was incorporated at the C-terminus of each peptide for future coupling purposes. The peptides were cleaved from the solid support, tri-O-acetyl GalNAc was de-O-acetylated, and the peptides were purified by reversed-phase high-performance liquid chromatography (rpHPLC) and characterized by mass spectrometry (EG-GSP-1 series, Figure 1, Table I). Nonsulfated EG-GP-1 was prepared from monosulfated EG-GSP(97)-1 using acid treatment. A core-2-based O-glycan with a SLex epitope (C2-SLex) at T124 was synthesized enzymatically on each peptide using recombinant or highly purified glycosyltransferases to get EG-GSP-6 series of peptides (Figure 1) (Leppänen et al. 2000). EG-GSP-4 series of peptides were intermediate products containing a galactosylated core-2 O-glycan at T124 (Figure 1). The completeness of each glycosyltransferase reaction was monitored by HPLC and mass spectrometry. The masses of the final products as well as the intermediate products from glycosyltransferase reactions were verified by matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) (see Table I for mass data of the final products). The mass spectra of disulfated peptides showed signals that represented partially desulfated peptides (−80 Da), in addition to fully sulfated peptides. Sulfate on tyrosine is labile, and desulfation of peptides occurs during normal mass spectrometric conditions (Önnerfjord et al. 2004). The retention time of each member of the EG-GSP-6 series (and EG-GSP-1 series) of peptides were different according to the number and position of tyrosine sulfate residues in an analytical C-18 reversed-phase HPLC column (not shown). This verifies the homogeneity of the glycosulfopeptide products and that desulfation of peptides occurs during MALDI-TOF mass spectrometric analysis. Glyco(sulfo)peptides [P-GSP-6, P-GSP(46)-6, P-GSP(48)-6, P-GSP(51)-6, P-GP-6 and P-GP-1 in Figure 1] corresponding to amino acid residues 45–61 of human PSGL-1 have been synthesized earlier (Leppänen et al. 2000) and used as reference peptides in binding assays of the present study.
Fig. 1.

The structures of glyco(sulfo)peptides and sugars used in the present study. Tyrosine sulfate and O-glycan attachment sites are numbered according to the sequence of human endoglycan (EG) or PSGL-1 (P). The number in the parenthesis indicates the position of tyrosine sulfate residues, and the last number indicates the number of sugar residues. All peptides contain an extra C-terminal cysteine residue
Table I.
MALDI-TOF mass spectrometric analysis of HPLC-purified endoglycan glyco(sulfo)peptide samples (see Figure 1 for structures)
| Glyco(sulfo)peptide | m/z [M–H]− | |
|---|---|---|
| Calculated | Observed | |
| EG-GP-1 | 4538.9 | 4539.8 |
| EG-GSP(97)-1 | 4619.0 | 4619.9 |
| EG-GSP(118)-1 | 4619.0 | 4619.4 |
| EG-GSP-1* | 4699.1 | 4699.6; 4618.2 |
| EG-GP-6 | 5503.8 | 5505.0 |
| EG-GSP(97)-6 | 5583.9 | 5583.0 |
| EG-GSP(118)-6 | 5583.9 | 5584.7 |
| EG-GSP-6* | 5664.0 | 5667.1; 5586.9 |
The calculated and observed values for the major [M–H]− molecular ions are indicated, except for disulfated EG-GSP-1 and EG-GSP-6 marked with an asterisk (*) where the observed values represented molecular ions [M–H]− and partially desulfated molecular ions ([M–H]−-80). Partial desulfation of disulfated GSPs occurs during MALDI-TOF analysis (see text for details).
The C-terminal cysteine of each of EG-GSP-6, EG-GSP-1 and P-GSP-6 series of peptides was biotinylated, and the biotinylated peptides were used in fluorescence-based solid-phase binding assays. Radiolabeled EG-GSP-6 and EG-GSP-4 series of peptides were synthesized using nonlabeled precursor peptide series (nonfucosylated EG-GSP-5 and nongalactosylated EG-GSP-3 series) as acceptors and GDP-[3H]Fuc or UDP-[3H]Gal as a donor in reactions with α1,3-fucosyltransferase or β1,4-galactosyltransferase, respectively. Radiolabeled GSPs were utilized in L-selectin affinity chromatography.
L-selectin binds to immobilized EG-GSP-6, and binding is dependent on tyrosine sulfation and a C2-SLex O-glycan of EG-GSP-6: sulfate at Y118 contributes more than sulfate at Y97 for binding
EG-GSPs were synthesized to study the role of site-specific tyrosine sulfation and O-glycosylation of endoglycan for binding to L-selectin. We used highly sensitive fluorescence-based solid-phase assay to study the binding of L-selectin to immobilized EG-GSPs. The method has been used earlier to study the binding of P- and L-selectin to model GSPs of human PSGL-1 (Leppänen et al. 2002, 2003). Reduced salt concentration below physiologic level increases the binding affinity of selectins to their ligands (Koenig et al. 1997; Leppänen et al. 2000, 2003). In this study, we utilized reduced salt concentration in addition to physiologic salt concentration to increase the sensitivity of the binding assay. Equimolar amounts of biotinylated EG-GSPs and P-GSPs were immobilized on streptavidin-coated plates (Figure 1). L-selectin IgG chimera (L-sel-Ig) was incubated with immobilized GSPs in the presence of either Ca2 and Mg2 or EDTA at physiologic salt (150 mM NaCl) or at low salt (50 mM NaCl) conditions, and bound L-sel-Ig was detected with fluorescently labeled anti-human IgG. L-sel-Ig showed relatively weak but distinct binding to EG-GSP-6 series of peptides containing a C2-SLex O-glycan at T124, at physiologic and low salt conditions (Figure 2A and B, respectively). L-sel-Ig bound to disulfated EG-GSP-6 with higher affinity than to monosulfated or nonsulfated EG-GSPs. Binding to monosulfated EG-GSP(118)-6 was better than to isomeric EG-GSP(97)-6, indicating that a sulfate at Y118 contributes more for binding to L-selectin than a sulfate at Y97. However, L-sel-Ig bound to nonsulfated EG-GP-6 with ∼2-fold weaker affinity than to EG-GSP(97)-6, showing that a sulfate at Y97 also contributes for binding. L-sel-Ig showed almost undetectable binding to incompletely glycosylated EG-GSP-1 series of peptides under physiologic and low salt conditions, even in the presence of two tyrosine sulfates (EG-GSP-1), indicating that C2-SLex O-glycan at T124 contributes more than tyrosine sulfates to L-selectin binding. L-sel-Ig bound to EG-GSP-6 with similar affinity compared with the nonsulfated glycopeptide (GP) P-GP-6. However, L-sel-Ig recognized P-GSP-6 containing three sulfated tyrosines with ∼10-fold higher affinity than EG-GSP-6 (Figure 2A and B, the insets). These results show that L-selectin recognizes EG-GSP-6 series of peptides and that binding is specific but relatively weak. L-selectin requires at least one tyrosine sulfate residue, preferably at position Y118, and an O-glycan with C2-SLex at T124 for binding to EG-GSPs. C2-SLex O-glycan contributes more for binding to L-selectin than two tyrosine sulfates within EG-GSP-6.
Fig. 2.
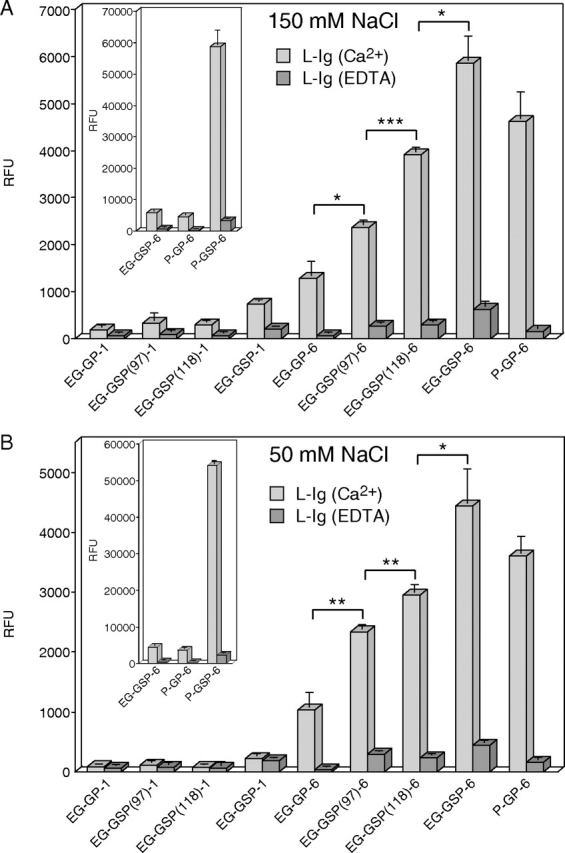
Binding of L-sel-Ig to immobilized glyco(sulfo)peptides in a fluorescence-based solid-phase assay at physiologic and low salt buffer. Biotinylated glyco(sulfo)peptides were immobilized on streptavidin-coated microtiter wells (A) 20 pmol/well; (B) 10 pmol/well). (A) L-sel-Ig (40 μg/ml) was incubated with the immobilized GSPs in physiologic salt buffer (20 mM MOPS, pH 7.5, 150 mM NaCl, 1% BSA, 0.05% Tween 20, 0.02% NaN3) containing 2 mM CaCl2 and 2 mM MgCl2 (light gray bars) or 5 mM EDTA (dark gray bars). (B) L-sel-Ig (20 μg/ml) was incubated with the immobilized GSPs in low salt buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 1% BSA, 0.05% Tween 20, 0.02% NaN3) containing 2 mM CaCl2 and 2 mM MgCl2 (light gray bars) or 5 mM EDTA (dark gray bars). Fluorescently labeled anti-human IgG was used to detect the bound L-sel-Ig. The inset shows binding of L-sel-Ig to immobilized P-GSP-6. All assays were performed in triplicate, and the results represent the mean ± SD of three determinations. *P < 0.05; **P < 0.01; ***P < 0.001
One tyrosine sulfate residue enhances the relative binding affinity of L-selectin to EG and PSGL-1 glycosulfopeptides similarly, but the nonsulfated peptide backbone together with C2-SLex O-glycan contributes more for L-selectin binding to PSGL-1 glycopeptides than to EG glycopeptides
Next, we studied the contribution of one tyrosine sulfate residue within monosulfated EG-GSPs versus monosulfated P-GSPs for binding to L-selectin. Consistent with our earlier results, L-sel-Ig binding to monosulfated P-GSPs was not clearly dependent on the position of the sulfate residue (Leppänen et al. 2003) (Figure 3A). L-sel-Ig recognized monosulfated P-GSPs with ∼2–3-fold higher affinity in comparison with nonsulfated P-GP-6. Similarly L-sel-Ig bound monosulfated EG-GSPs with ∼2–5-fold higher affinity than nonsulfated EG-GP-6 (Figures 2A and B and 3A). In conclusion, one tyrosine sulfate residue within EG-GSPs and P-GSPs enhances the binding affinity for L-selectin similarly. However, L-selectin recognized P-GP-6 with significantly higher affinity than EG-GP-6, indicating that the nonsulfated peptide backbone together with C2-SLex O-glycan contributes more for L-selectin binding to PSGL-1 glycopeptides than to EG glycopeptides. The results also show that L-selectin does not detectably bind to the nonsulfated peptide backbones without C2-SLex O-glycan (EG-GP-1 and P-GP-1 in Figure 3A).
Fig. 3.
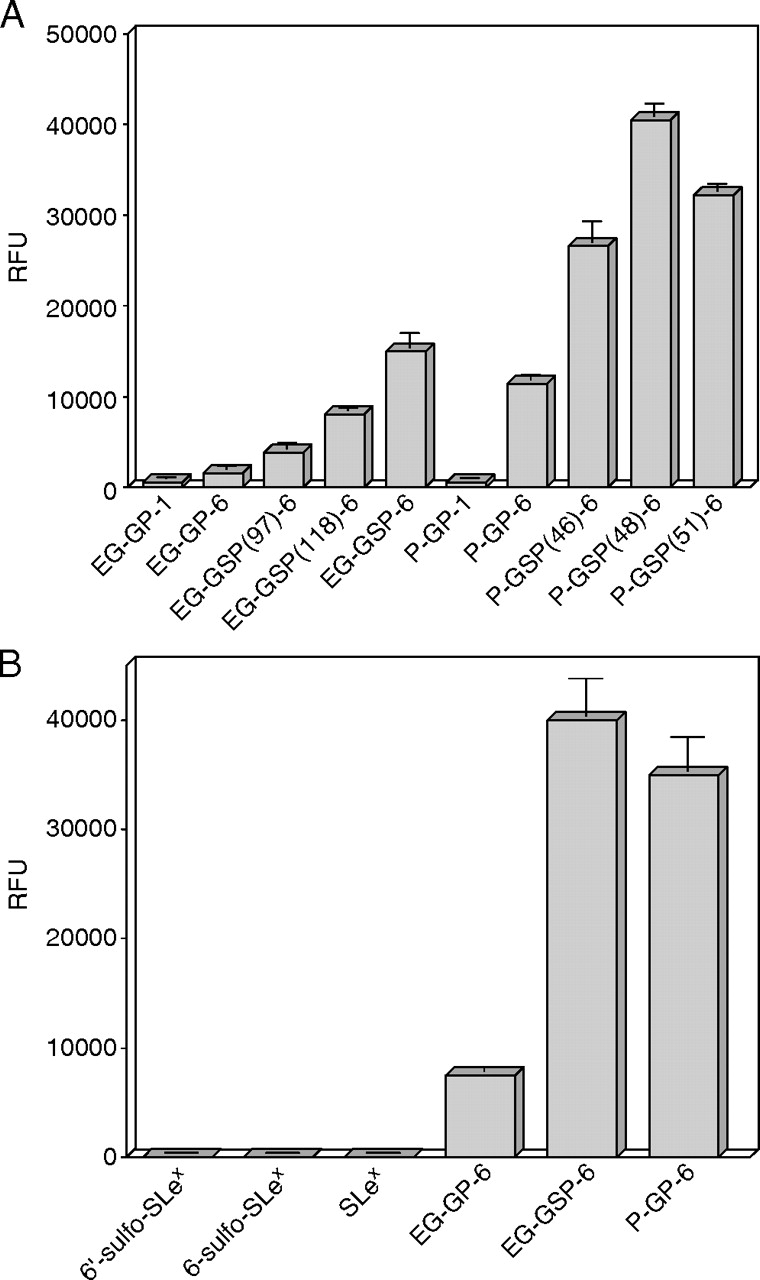
Binding of L-sel-Ig to immobilized glyco(sulfo)peptides and sugars in a fluorescence-based solid-phase assay at low salt buffer. Biotinylated glyco(sulfo)peptides and sugars were immobilized on streptavidin-coated microtiter wells (10 pmol/well). L-sel-Ig (A) 20 μg/ml or (B) 40 μg/ml was incubated with the immobilized ligands in low salt buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% BSA, 0.05% Tween 20, 0.02% NaN3). Fluorescently labeled anti-human IgG was used to detect the bound L-sel-Ig. All assays were performed in triplicate, and the results represent the mean ± SD of three determinations
L-selectin does not detectably bind to immobilized monomeric 6-sulfo sialyl Lewisx
Because L-selectin binds to 6-sulfo-SLex carbohydrate epitope present on various HEV sialomucins, we used fluorescence-based solid-phase assay to compare L-selectin binding to sulfated carbohydrates versus sulfated peptide epitopes. Biotinylated monomeric 6-sulfo-SLex, 6′-sulfo-SLex and SLex carbohydrates and equimolar amounts of biotinylated glyco(sulfo)peptides, EG-GSP-6, EG-GP-6 and P-GP-6 were immobilized on streptavidin plates (10 pmol/well). L-sel-Ig (40 μg/ml) was incubated in the wells under low salt conditions. L-sel-Ig did not show detectable binding to immobilized 6-sulfo-SLex, 6′-sulfo-SLex or SLex (Figure 3B), whereas L-selectin bound to glyco(sulfo)peptides as expected from Figures 2 and 3A. The experiment was repeated using 5-fold higher density of immobilized ligands (50 pmol/well) under low salt conditions. L-selectin showed less than 0.2% binding to 6-sulfo-SLex in comparison with control P-GP-6 and no detectable binding to 6′-sulfo-SLex or SLex (data not shown). To show that the immobilized ligands were available for L-selectin binding, we used HECA-452 mAb, which recognizes 6-sulfo-SLex, 6′-sulfo-SLex and SLex (Mitsuoka et al. 1998), to detect immobilized carbohydrates on the plate. The antibody was able to recognize all immobilized structures (not shown). Thus, our results demonstrate that L-selectin binds to monomeric 6-sulfo-SLex epitope with very low affinity relative to the appropriate glycosulfopeptides.
Binding of L-selectin to EG-GSPs is inhibited by anti-QPP, an antibody to a peptide epitope on EG-GSPs
Monospecific antibodies against two peptide epitopes on endoglycan were generated. Anti-QPP epitope corresponds to amino acid residues 93–110 and anti-SGF epitope corresponds to amino acid residues 79–92 of human endoglycan. The epitope of anti-QPP is part of the peptide sequence of EG-GSPs, whereas the epitope of anti-SGF is not. The ability of anti-QPP to inhibit L-selectin binding to EG-GSPs was tested using solid-phase assay. Biotinylated glyco(sulfo)peptides were captured on streptavidin plates at a density of 5 pmol/well. Antibodies were incubated with the immobilized peptides before L-sel-Ig (complexed with fluorescently labeled anti-human IgG) was added to the wells. Incubation with L-sel-Ig was performed in low salt buffer (50 mM NaCl). Binding of L-sel-Ig to all EG-GSP-6 series of peptides was inhibited by anti-QPP but not by anti-SGF (Figure 4). Anti-QPP inhibited L-selectin binding to monosulfated EG-GSP(118)-6 and EG-GSP(97)-6 by 70–80%, to nonsulfated EG-GP-6 by ∼70% and to disulfated EG-GSP-6 by ∼60%. By contrast, anti-QPP did not inhibit L-selectin binding to P-GP-6. L-selectin binding to any of the glyco(sulfo)peptides was not inhibited by anti-SGF (Figure 4). The results indicate that anti-QPP antibody that recognizes the N-terminal region of EG-GSPs partially blocks L-selectin binding to EG-GSPs.
Fig. 4.
Inhibition of L-sel-Ig binding to immobilized glyco(sulfo)peptides by anti-EG peptide antibodies. Biotinylated glyco(sulfo)peptides were immobilized on streptavidin-coated microtiter wells (5 pmol/well). Anti-QPP and anti-SGF antibodies (100 μg/ml) were first incubated with the immobilized GSPs in PBS containing 1% BSA and 0.05% Tween 20, and unbound antibodies were removed by washing. L-sel-Ig (10 μg/ml) was preincubated with fluorescently labeled anti-human IgG in low salt buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% BSA, 0.05% Tween 20, 0.02% NaN3) before adding to the wells. Assays with anti-QPP and without an antibody were performed in triplicate, assays with anti-SGF were performed in duplicate, and the results represent the mean ± SD of three or two determinations, respectively
EG-GSP-6 binds to immobilized L-selectin, and binding is dependent on tyrosine sulfation and a C2-SLex O-glycan of EG-GSP-6
Experiments utilizing fluorescence-based solid-phase assay showed that L-selectin bound to immobilized EG-GSP-6, and binding was dependent on TyrSO3 residues and a C2-SLex O-glycan of EG-GSP-6. To confirm the findings using a different assay method, we prepared radiolabeled EG-GSP-6 series of peptides to test their binding to immobilized L-selectin using affinity chromatography. L-sel-Ig was immobilized on Protein A-Sepharose at density 5 mg/ml. To increase the sensitivity of binding, chromatography on immobilized L-sel-Ig was performed at low salt (50 mM NaCl) concentration. Radiolabeled PSGL-1 glycopeptides P-GSP-6 and P-GP-6 were used as controls as in fluorescence-based solid-phase assay. Fully sulfated P-GSP-6 bound to immobilized L-sel-Ig with high affinity and eluted out of the column by replacing divalent cations with EDTA in the elution buffer, whereas nonsulfated P-GP-6 showed only weak affinity to immobilized L-selectin eluting as a broad, retarded peak with buffer containing divalent cations (Figure 5A). Replacement of divalent cations with 1 mM EDTA in the elution buffer completely inhibited the binding of P-GP-6 to L-selectin, showing that binding was strictly Ca2-dependent (Figure 5A). However, binding of P-GSP-6 to L-selectin was not completely inhibited by EDTA, showing weak residual Ca2-independent binding to L-selectin (Figure 5A). The elution of each EG-GSP-6 series of peptides was clearly retarded on immobilized L-selectin, indicating that all peptides have some affinity for L-selectin (Figure 5B). However, the elution profiles of individual peptides were not identical on immobilized L-selectin, suggesting that they have different affinity for L-selectin. The elution of disulfated EG-GSP-6 was most retarded, suggesting that it has the highest affinity for immobilized L-selectin. The elution of EG-GSP(118)-6 was more retarded than the elution of EG-GSP(97)-6 and EG-GP-6, suggesting that sulfate at tyrosine 118 is more important for binding to L-selectin than sulfate at tyrosine 97, which is in good agreement with results obtained in solid-phase assay. The elution order of each EG-GSP was consistent in several independent experiments. The interaction of EG-GSPs with L-selectin was strictly Ca2-dependent, since it was inhibited by 1 mM EDTA in the elution buffer (Figure 5C). A set of radiolabeled EG-GSP-4 series of peptides containing one, two or no tyrosine sulfate(s) and a galactosylated core-2 O-glycan (without sialic acid and fucose residues) at T124 (Figure 1) were used to test the role of C2-SLex at T124 for binding to immobilized L-selectin. The EG-GSP-4 series of peptides did not show any detectable binding to immobilized L-sel-Ig in the presence of Ca2 (Figure 5D). The result indicates that the C2-SLex O-glycan of EG-GSP-6 is more important for binding to L-selectin than TyrSO3 residues, which is consistent with results obtained in the solid-phase assays.
Fig. 5.
Affinity chromatography of glyco(sulfo)peptides on immobilized L-sel-Ig at low salt buffer. The indicated radiolabeled glyco(sulfo)peptides were loaded into the L-sel-Ig column in low salt buffer (20 mM MOPS, 50 mM NaCl, 0.02% NaN3, pH 7.5) containing 2 mM CaCl2 and 2 mM MgCl2 (A), (B) and (D) or 1 mM EDTA (C). In (A), dashed lines represent control experiments with the indicated glycosulfopeptides where chromatography was performed in buffer containing 1 mM EDTA. The arrow indicates the fraction number (21) where 10 mM EDTA was used to replace divalent cations in buffer. Experiments shown are representative of three independent experiments
L-selectin binds EG-GSP-6 with 12-fold lower affinity than P-GSP-6
The binding affinity of L-sel-Ig for P-GSP-6 is relatively high (Kd ∼5 μM) at physiologic salt concentration as determined earlier by equilibrium gel filtration (Leppänen et al. 2003). The fluorescence-based solid-phase assay is a semi-quantitative method and can provide relative binding affinities between a glycan binding protein and an array of different ligands. Here, we utilized fluorescence-based solid-phase assay to determine an apparent Kd for L-sel-Ig binding to immobilized P-GSP-6, P-GP-6 and EG-GSP-6 under reduced salt concentration (50 mM NaCl). Glyco(sulfo)peptides were captured quantitatively on streptavidin plates at the same density (10 pmol/well). L-sel-Ig was incubated in the wells at different concentrations, and bound L-sel-Ig was detected with fluorescently labeled anti-human IgG. Binding isotherms were obtained, and apparent dissociation constants were derived from the binding curves using nonlinear curve fitting with one site saturation equation. L-sel-Ig bound to P-GSP-6, P-GP-6 and EG-GSP-6 with apparent Kd ∼20.4, ∼233 and ∼241 nM, respectively (Figure 6). The results show that L-sel-Ig binds to P-GP-6 and EG-GSP-6 with similar affinity, but the binding affinity to P-GSP-6 is 11–12-fold higher than to P-GP-6 and EG-GSP-6. The results are consistent with the results obtained by fluorescence-based solid-phase assay using a single concentration of L-sel-Ig under physiologic and low salt conditions (Figure 2).
Fig. 6.

Equilibrium-binding affinity of L-sel-Ig for P-GSP-6, P-GP-6 and EG-GSP-6 in a fluorescence-based solid-phase assay at low salt buffer. Biotinylated glyco(sulfo)peptides were immobilized on streptavidin-coated microtiter wells (10 pmol/well). Various concentrations of L-sel-Ig were incubated with the immobilized (A) P-GSP-6, (B) P-GP-6 and (C) EG-GSP-6 in low salt buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 1% BSA, 0.05% Tween 20, 0.02% NaN3). Fluorescently labeled anti-human IgG (40 μg/ml) was used to detect the bound L-sel-Ig. Assays were performed in triplicate (A) and (B) or in duplicate (C), and the results represent the mean ± standard error of the mean. Experiments shown are representative of three independent experiments
Human T lymphocytes bind to immobilized EG-GSP-6 and P-GSP-6 but not to 6-sulfo-SLex
Our results show that recombinant human L-sel-Ig recognizes a sulfated peptide backbone together with a nearby C2-SLex O-glycan present on glycosulfopeptides modeled after human EG and PSGL-1. Moreover, the data also indicate that L-selectin binds very poorly to a sulfated carbohydrate epitope, 6-sulfo-SLex. To investigate whether natural L-selectin present on the surface of human T lymphocytes is able to recognize glycosulfopeptides, we used purified human T lymphocytes in the solid-phase assay. Fluorescently labeled T lymphocytes were incubated with immobilized sugars and glyco(sulfo)peptides (10 or 5 pmol/well) in physiologic buffer containing Ca2 and Mg2. In control experiments, 5 mM EDTA or alternatively a function-blocking L-selectin mAb, DREG-56, was preincubated with the cells before adding to the wells. T lymphocytes showed high affinity binding to P-GSP-6 and moderate binding to nonsulfated P-GP-6 (Figure 7A, the inset). Binding to nonsulfated P-GP-6 was better than expected from experiments with recombinant L-sel-Ig (Figure 2A and B, the insets). T lymphocytes also recognized disulfated EG-GSP-6 and monosulfated EG-GSPs but with lower affinity than P-GP-6 (Figure 7A). Cells bound better to monosulfated EG-GSP(118)-6 than to isomeric EG-GSP(97)-6 consistent with binding assays using L-sel-Ig. T lymphocytes also recognized weakly nonsulfated EG-GP-6, but binding to incompletely glycosylated EG-GP-1 was undetectable, demonstrating the critical importance of C2-SLex O-glycan for binding. T-lymphocyte binding to EG-GSPs and P-GSPs was completely inhibited by EDTA and DREG-56, showing that interaction was strictly dependent on Ca2 and L-selectin (Figure 7B). T lymphocytes lacked binding to immobilized 6-sulfo-SLex (Figure 7A). These results are in good agreement with the results obtained using recombinant L-sel-Ig with an exception that T lymphocytes showed better binding to P-GP-6 than L-sel-Ig.
Fig. 7.

Binding of human T lymphocytes to immobilized glyco(sulfo)peptides and sugars in a fluorescence-based solid-phase assay at physiologic buffer. Biotinylated glyco(sulfo)peptides and sugars were immobilized on streptavidin-coated microtiter wells (10 pmol/well). Purified, fluorescently labeled human T lymphocytes (A) 190,000 cells/well or (B) 65,000 cells/well were incubated with the immobilized ligands in Hank's balanced salt solution (with Ca2 and Mg2) containing 1% BSA (light gray bars). (B) In control experiments, a function-blocking mAb to L-selectin, DREG-56 (20 μg/ml) (medium gray bars), or 5 mM EDTA (dark gray bars) were preincubated with the cells in HBSS before adding to the wells. The inset in (A) shows binding of T lymphocytes to immobilized P-GSP-6. All assays were performed in triplicate, and the results represent the mean ± SD of three determinations. Experiment shown in (A) is a representative of eight independent experiments using T cells isolated from five different donors. *P < 0.05; **P < 0.01
P-selectin binds weakly to EG-GSP-6, and binding is dependent on tyrosine sulfation and C2-SLex O-glycan of EG-GSP-6
Our earlier data indicated that P-selectin requires a dual recognition of sulfated tyrosine residues and a nearby O-glycan with C2-SLex for high-affinity binding to a glycosulfopeptide (P-GSP-6) modeled after N-terminus of human PSGL-1 (Leppänen et al. 1999, 2000, 2003). Because endoglycan contains two potential tyrosine sulfation sites and endoglycan and PSGL-1 share sequence similarity in that region, we were prompted to test whether P-selectin recognizes EG-GSPs. This is of physiological interest because endoglycan is also expressed on subsets of leukocytes, and it has recently been suggested to serve as a ligand for endothelial P-selectin (Kerr et al. 2008). P-selectin IgG chimera (P-sel-Ig) was incubated with immobilized GSPs and sugars (10 pmol/well) in the presence of either Ca2 or EDTA at physiologic salt conditions. P-sel-Ig bound to EG-GSP-6 with similar affinity compared with nonsulfated P-GP-6 (Figure 8). P-sel-Ig binding to monosulfated EG-GSPs was weaker than to disulfated EG-GSP-6 with preferential recognition of tyrosine sulfate at Y118 rather than at Y97, thus resembling the binding preference of L-selectin towards EG-GSPs. P-sel-Ig bound weakly to nonsulfated EG-GP-6 but did not bind to EG-GSP-1 series of peptides lacking C2-SLex O-glycan, showing the importance of C2-SLex O-glycan at T124 for binding. P-sel-Ig did not bind to any of the immobilized carbohydrate epitopes, SLex, 6-sulfo-SLex or 6′-sulfo-SLex even under reduced salt concentration (not shown). Based on our earlier data indicating that soluble P-selectin binds to P-GP-6 with relatively low affinity (Kd ∼20–30 μM) (Leppänen et al. 2000), the present results suggest that P-sel-Ig binds to EG-GSP-6 with similar affinity. The binding affinity is approximately 40-fold lower than P-sel-Ig binding to fully sulfated P-GSP-6 (Leppänen et al. 2000).
Fig. 8.
Binding of P-sel-Ig to immobilized sugars and glyco(sulfo)peptides in a fluorescence-based solid-phase assay at physiologic buffer. Biotinylated sugars and glyco(sulfo)peptides were immobilized on streptavidin-coated microtiter wells (10 pmol/well). P-sel-Ig (20 μg/ml) was incubated with the immobilized GSPs in physiologic salt buffer (20 mM MOPS, pH 7.5, 150 mM NaCl, 1% BSA, 0.05% Tween 20, 0.02% NaN3) containing 2 mM CaCl2 and 2 mM MgCl2 (light gray bars) or 5 mM EDTA (dark gray bars). Fluorescently labeled anti-human IgG was used to detect the bound P-sel-Ig. All assays were performed in triplicate, and the results represent the mean ± SD of three determinations. Experiment shown is a representative of three independent experiments. *P < 0.05; **P < 0.01
Discussion
A set of glycosulfopeptides (EG-GSPs) of 37 amino acid residues modeled after N-terminal domain of human endoglycan were synthesized chemoenzymatically to evaluate the contribution of individual TyrSO3 residues and a core-2-based O-glycan with SLex (C2-SLex) at T124 on endoglycan for binding to L-selectin (Figure 1). L-sel-Ig and T lymphocytes showed highest affinity binding to EG-GSP-6 containing two TyrSO3 residues (Y97 and Y118) and a C2-SLex O-glycan at T124. Sulfate at Y118 was more important for L-selectin recognition than at Y97. L-selectin showed weak binding to nonsulfated glycopeptide EG-GP-6 containing C2-SLex O-glycan, but it bound poorly to sulfated glycopeptides lacking the SLex epitope, indicating that C2-SLex O-glycan contributes more to L-selectin binding than the two TyrSO3 residues. Our results also indicate that binding of T lymphocytes to EG-GSPs was strictly L-selectin dependent, since binding was completely inhibited by EDTA and DREG-56, a function-blocking mAb to L-selectin. Moreover, binding of L-sel-Ig to EG-GSPs was partially inhibited by an antibody to N-terminal peptide epitope of EG-GSPs. We also found that L-sel-Ig and T lymphocytes do not bind appreciably to the sulfated carbohydrate epitope 6-sulfo-sLex under the same conditions where L-selectin bound to glycosulfopeptides.
Our present results show that both TyrSO3 residues on EG-GSP-6 contribute for binding to L-selectin. This is in agreement with our earlier results indicating that the contribution of each tyrosine sulfate residue on model glycosulfopeptides from PSGL-1 for binding to L-selectin was additive (Leppänen et al. 2003). However, the interaction of L-selectin with P-GSPs was not clearly dependent on the specific sulfation site, in contrast to P-selectin that preferred sulfate at position Y48. By comparing the L-selectin binding epitopes between PSGL-1 and endoglycan, the most striking similarity can be found around amino acids Y118 and T124 of endoglycan and Y51 and T57 of PSGL-1. Tyrosine 118 on endoglycan corresponds in position to tyrosine 51 on PSGL-1. The present data indicating that Y118 of endoglycan has a major role for L-selectin binding are in agreement with earlier results obtained by utilizing recombinant PSGL-1 constructs with substitutions at specific tyrosine residues suggesting that tyrosine 51 plays a major role for L-selectin binding to PSGL-1 (Bernimoulin et al. 2003). Although spacing between TyrSO3 residues and an O-glycan attachment site in endoglycan and PSGL-1 is very similar, individual tyrosine sulfation sites are very differently organized in PSGL-1 and endoglycan. Three TyrSO3 residues are clustered together in PSGL-1 (Y46, Y48, Y51), whereas the two potential tyrosine sulfation sites are 20 amino acid residues apart in endoglycan (Y97, Y118). Therefore, our results showing that sulfate at Y97 on EG-GSPs has a minor contribution for binding to L-selectin is not surprising. Moreover, based on our results with P-selectin showing that spacing between a cluster of tyrosine sulfates and an O-glycan at T57 of PSGL-1 is very important for P-selectin binding (Leppänen et al. in preparation), we anticipate that spacing between these amino acid residues is likely important for L-selectin recognition as well.
Our present data show that the contribution of both tyrosine sulfate residues of EG-GSP-6 for L-selectin binding is additive. Our results also indicate that one tyrosine sulfate residue within EG-GSPs and P-GSPs enhances the binding for L-selectin ∼2–5-fold in comparison with nonsulfated EG-GP-6 and P-GP-6 (Figures 2A and B and 3A). However, L-selectin recognized P-GP-6 with significantly higher affinity than EG-GP-6, indicating that the nonsulfated peptide backbone together with C2-SLex O-glycan contributes more for L-selectin binding to PSGL-1 glycopeptides than to EG glycopeptides. L-selectin did not bind to peptide backbones of P-GP-1 or EG-GP-1, indicating the important role of C2-SLex O-glycan for L-selectin binding to P and EG peptide sequences (Figure 3A). Although sialic acid and fucose residues of C2-SLex O-glycan are important for binding to L-selectin, the contribution of core-2 type O-glycan is also critical because L-selectin recognized poorly isomeric C1-P-GSP-6 containing SLex on extended core-1 O-glycan at T57 (Leppänen et al. 2003). In the present experiment, L-selectin did not detectably bind to immobilized monomeric SLex under any conditions, indicating that SLex epitope is a very poor ligand for L-selectin.
We used affinity chromatography on immobilized L-selectin as an alternative method to test the ability of EG-GSPs to bind to L-selectin and to verify the results from solid-phase assay. EG-GSP-6 showed relatively weak but strictly Ca2-dependent binding to immobilized L-selectin, and binding was dependent on the presence and position of TyrSO3 residues and a C2-SLex O-glycan (Figure 5). The results also indicated that the C2-SLex O-glycan of EG-GSP-6 was more important for binding to L-selectin than TyrSO3 residues. Nonsulfated EG-GP-6 and P-GP-6 showed weak binding to immobilized L-selectin, suggesting that a C2-SLex O-glycan together with nonsulfated peptide backbones contribute to recognition by L-selectin. The results obtained by two different methods are in good agreement with each other. We were even able to detect differences in the elution order between disulfated and monosulfated EG-GSP-6 series of peptides using affinity chromatography that are in agreement with results from solid-phase assay. The elution order was consistent from experiment to experiment. We have shown earlier using immobilized P-selectin and GSPs modeled after PSGL-1 that the elution positions of GSPs on affinity chromatography is a direct reflection of the equilibrium binding in solution (Leppänen et al. 2000). Taken together, our present and earlier data indicate that affinity chromatography and solid-phase binding assay can both be used as semi-quantitative methods to estimate relative binding affinities of different ligands for a selectin.
Our results clearly demonstrate preferential binding of L-selectin to glycosulfopeptides containing sulfated tyrosines compared with sulfated carbohydrate epitopes. L-selectin did not show detectable interaction with immobilized 6-sulfo-SLex, 6′-sulfo-SLex or SLex even under low salt conditions that were used to increase the sensitivity of the assay (Figure 3B). Increasing the ligand density on microtiter wells by 5–10-fold did not improve the binding of L-selectin to sulfated carbohydrates (not shown). The sensitivity of our assay is in the range of Kd ∼1 mM under conditions used in the experiment here. Thus, we speculate that the dissociation constant for L-selectin binding to 6-sulfo-SLex must be in the millimolar range, which is consistent with earlier results (Scudder et al. 1994; Poppe et al. 1997). Moreover, human T lymphocytes expressing natural L-selectin at the cell surface also did not have detectable affinity for sulfated carbohydrates (Figure 7). The 6-sulfo-SLex epitope is commonly expressed on sialomucins present on HEV of lymphoid tissues. The peptide backbone of these sulfated mucins has not been shown to be involved in binding to L-selectin. Although the binding affinity of L-selectin to monovalent 6-sulfo-SLex is weak, it is commonly thought that multivalent epitope expression on individual mucins lead to high-affinity binding due to enhanced avidity. In fact, direct biochemical binding experiments have been carried out only with L-selectin and GlyCAM-1, and the results show that there is some enhancement in avidity either due to the peptide determinants or large number of sulfated glycans, since L-selectin bound to purified murine GlyCAM-1 with a dissociation constant (Kd) of 108 μM (Nicholson et al. 1998). O-glycan analysis of murine GlyCAM-1 has revealed that 25% of total O-glycans on GlyCAM-1 contains a 6-sulfo-SLex epitope (Kawashima et al. 2005). Moreover, recent glycan analysis of human CD34 indicated that approximately 20% of total O-glycans of L-selectin binding glycoforms of human endothelial CD34 carry 6-sulfo-SLex (Hernandez Mir et al. 2009). Recent data have also indicated that murine and human CD34 contain N-glycans with potential 6-sulfo-SLex epitopes (Mitoma et al. 2007; Hernandez Mir et al. 2009) and that N-glycans with 6-sulfo-SLex can contribute for L-selectin-mediated lymphocyte homing at least in mice (Mitoma et al. 2007). In GlyCAM-1 and CD34, the enhancement in binding affinity to L-selectin is likely due to clustering of sulfated glycans. However, the binding mechanism of L-selectin to PSGL-1 glycosulfopeptide and 6-sulfo-SLex is different (Klopocki et al. 2008). L-selectin binding to monomeric P-GSP-6 is relatively high affinity, and enhancement in binding may not be required. Although L-selectin binds to monomeric EG-GSP-6 with an order of magnitude lower affinity than to P-GSP-6, L-selectin likely binds EG-GSP-6 with similar affinity than clustered 6-sulfo-SLex epitopes, and enhancement of binding may not be required. Experiments under flow conditions are required to reveal the differences in recognition by L-selectin between sulfated peptides and clustered sulfated carbohydrates.
L-selectin is expressed on subsets of peripheral blood T lymphocytes (Ley and Kansas 2004). It is constitutively expressed on all naïve T lymphocytes, but L-selectin is shed upon activation of T lymphocytes. Therefore, only a subset of memory T cells express L-selectin. We explored the ability of fluorescently labeled, highly purified human CD3 T lymphocytes to bind to glycosulfopeptides modeled after PSGL-1 and endoglycan in solid-phase assay. T lymphocytes showed highest affinity for P-GSP-6, and binding to EG-GSP-6 was one order of magnitude lower affinity (Figure 7A, the inset), consistent with results obtained using recombinant L-sel-Ig. T lymphocytes showed 2–4-fold weaker binding to monosulfated EG-GSP(118)-6 and EG-GSP(97)-6 than to fully sulfated EG-GSP-6 preferring binding to EG-GSP(118)-6 containing sulfate at Y118, which is consistent with results obtained using L-sel-Ig (Figure 7A and B). There was weak binding to nonsulfated EG-GP-6 but no binding to incompletely glycosylated EG-GP-1, indicating that C2-SLex O-glycan at T124 together with the EG peptide backbone is critical for L-selectin recognition. Although the binding preference of L-sel-Ig and T lymphocytes towards immobilized GSPs was very similar, there was one noticeable difference. T lymphocytes recognized nonsulfated P-GP-6 with higher affinity relative to P-GSP-6 and EG-GSPs than recombinant L-sel-Ig (the insets in Figures 7A and 2A and B). Binding to P-GP-6 was dependent on L-selectin because a function-blocking mAb to L-selectin (DREG-56) and EDTA were able to inhibit the interaction (Figure 7B).
In addition to endothelial expression, endoglycan is also expressed on human B cells, T cells and monocytes (Kerr et al. 2008). Recently, leukocyte endoglycan has been shown to function as a ligand for vascular selectins in vitro (Kerr et al. 2008). Because endoglycan and PSGL-1 share structural similarity near tyrosine sulfation sites, it prompted us to explore the ability of P-selectin to bind to EG-GSPs. The present experiments indicate that P-sel-Ig recognizes disulfated EG-GSP-6 but with relatively low affinity (Figure 8). P-sel-Ig bound to EG-GSP-6 with similar affinity to nonsulfated P-GP-6. Our earlier data indicated that soluble P-selectin bound to P-GP-6 with Kd ∼25 μM (Leppänen et al. 2000). P-sel-Ig also recognized monosulfated EG-GSPs but with lower affinity than disulfated EG-GSP-6 and preferred to bind to EG-GSP(118)-6 containing a tyrosine sulfate at position Y118. P-sel-Ig also bound to nonsulfated EG-GP-6 but with lower affinity than sulfated EG-GSPs. The binding pattern of P-sel-Ig to EG-GSPs was very similar to that of L-sel-Ig, suggesting that P- and L-selectin bind endoglycan by a related mechanism. It is not surprising that P-selectin recognizes EG-GSP-6 with relatively low affinity, since spacing between individual tyrosine sulfates is very different in PSGL-1 compared with endoglycan. Moreover, P-selectin prefers tyrosine sulfate at position Y48 for binding to PSGL-1 (Leppänen et al. 2000; Somers et al. 2000). Sulfation site at Y118 on endoglycan corresponds to sulfation site at Y51 on PSGL-1, suggesting that P-selectin binding to endoglycan may not be optimal. However, tyrosine sulfate at position Y97 also contributes to P-selectin binding, suggesting that when bound to P-selectin, EG-GSP-6 may obtain a conformation that brings two TyrSO3 residues close together. A crystal structure of P-selectin (C-type lectin domain EGF domain) complexed with an N-terminal glycosulfopeptide of PSGL-1 indicated that P-selectin interacts with only two TyrSO3 residues at positions Y48 and Y51 of PSGL-1 (Somers et al. 2000). However, our results have indicated that a third tyrosine sulfate at position Y46 in the PSGL-1-modeled glycosulfopeptides also contributes to binding (Leppänen et al. 2000). Thus, conformations of glycopeptides that may be adopted in complex with a selectin might promote the interactions of determinants with the receptor binding sites. Thus, it will be interesting in future structural studies to define the conformation of glycosulfopeptides modeled from endoglycan in complex with selectins.
Materials and methods
Synthesis of glycosulfopeptides
Glycosulfopeptide backbones corresponding to amino acid residues 92–127 of human endoglycan with an extra C-terminal cysteine were synthesized on an Applied Biosystems 433A peptide synthesizer using a preloaded H-Cys(Trt)-2-chlorotrityl resin (Novabiochem, Darmstadt, Germany) with Fmoc chemistry essentially as described (Leppänen et al. 2000). Briefly, one or two tyrosine sulfate residues (Y97 and/or Y118) and a GalNAcα residue (T124) were incorporated into the peptide backbone using Fmoc-Tyr(-SO3H)-H sodium salt (Bachem, Switzerland) and tri-O-acetyl-GalNAcα-Fmoc-Thr (GlycoTech, Rockville, MD), respectively, during the solid-phase peptide synthesis. Sulfated peptides were cleaved from the resin by mixing in 90% aqueous trifluoroacetic acid (TFA) for 8 h on ice to avoid desulfation. A nonsulfated peptide was prepared from a monosulfated peptide (EG-GSP(97)-1) still attached to the resin by incubating in 90% TFA for 2.5 h at room temperature. The cleaved peptides were precipitated with tert-butyl methyl ether. Tri-O-acetyl GalNAc was deacetylated, and C-terminal cysteine was protected as described (Leppänen et al. 1999, 2000). Peptides were purified by reversed phase HPLC and analyzed by MALDI-TOF MS) (Table I).
C2-SLex O-glycan was synthesized at T124 in each peptide using EG-GP-1, EG-GSP(97)-1, EG-GSP(118)-1 and EG-GSP-1 as acceptors for glycosyltransferase reactions. Glycosyltransferase reactions were accomplished step by step by adding one purified or recombinant glycosyltransferase and one donor at a time to the reaction mixture as described (Leppänen et al. 1999, 2000). The completeness of each reaction was followed by rpHPLC from a small aliquot of the reaction mixture, and each intermediate product was analyzed by MALDI-TOF MS. At the end, each reaction mixture was purified on rpHPLC and analyzed by MALDI-TOF MS (Table I).
Radiolabeled [3H]EG-GP-6, [3H]EG-GSP(97)-6, [3H]EG-GSP(118)-6 and [3H]EG-GSP-6 were synthesized using purified nonfucosylated EG-GP-5, EG-GSP(97)-5, EG-GSP(118)-5 and EG-GSP-5 as acceptors (150 pmol) and GDP-[3H]Fuc (Perkin Elmer, Boston, MA) (3900 cpm/pmol) as a donor in a α1,3-fucosyltransferase VI (Calbiochem) reaction. Radiolabeled [3H]EG-GP-4, [3H]EG-GSP(97)-4, [3H]EG-GSP(118)-4 and [3H]EG-GSP-4 were synthesized using purified EG-GP-3, EG-GSP(97)-3, EG-GSP(118)-3 and EG-GSP-3 as acceptors (130 pmol) and UDP-[3H]Gal (American Radiolabeled Chemicals, St. Louis, MO) (4900 cpm/pmol) as a donor in a β1,4-galactosyltransferase (Sigma, St. Louis, MO) reaction. Radiolabeled products were purified by HPLC.
Selectin-Ig chimeras, antibodies and biotinylated glycans
Expression and purification of recombinant L- and P-selectin-Ig chimeric proteins have been described earlier (Leppänen et al. 2003). A monoclonal anti-L-selectin antibody (DREG-56) and purified rat anti-human CLA (HECA-452) were from BD Pharmingen, San Diego, CA. Alexa FluorTM488 goat anti-human IgG (H+//0) and Alexa FluorTM488 goat anti-rat IgM (μ chain) were from Molecular Probes, Eugene, OR. 6-Sulfo-SLex-sp-Biotin, 6′-sulfo-SLex-sp-Biotin and SLex-sp-Biotin [sp = –O(CH2)3NH–CO(CH2)5NH–] were from Lectinity Holdings, Inc., Moscow, Russia.
Peptide sequences containing amino acid residues 79–92 [(C)SGFPSEENEESRIL] and 93–110 [QPPQYFWEEEEELNDSSL(C)] of human endoglycan with additional cysteine residues were synthesized, coupled to a carrier protein and used to immunize rabbits (Innovagen AB, Lund, Sweden). Polyclonal antibodies (anti-QPP and anti-SGF) were purified by affinity chromatography on a column where the antigen peptide was coupled to UltraLink® Iodoacetyl gel (Pierce) (2 mg/ml, total 1 ml) according to manufacturer's instructions.
L-selectin affinity chromatography
L-selectin-Ig was immobilized on Protein A-SepharoseTM 4 Fast Flow (Amersham Biosciences AB, Uppsala, Sweden) at a density 5 mg/ml. L-selectin column (∼1 ml, 0.7 × 2.8 cm) was equilibrated with buffer containing subphysiologic salt concentration (20 mM 4-[N-morpholine]propanesulfonic acid (MOPS), pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.02% NaN3). Radiolabeled glyco(sulfo)peptides (1000–2000 cpm, 0.3–2 pmol) were dissolved in 200 μl of equilibration buffer and chromatographed in the L-selectin column by collecting 0.5-ml fractions at a flow rate of ∼ 0.6 ml/min. Tightly bound peptides were eluted by replacing divalent cations by 10 mM EDTA in buffer. In some control experiments, 1 mM EDTA was used instead of divalent cations throughout the analysis. Radioactivity in all fractions was determined by liquid scintillation counting.
Reversed-phase high-performance liquid chromatography
Glyco(sulfo)peptides were filtered using Costar Spin-x centrifuge tube nylon filters (Corning Incorporated, Corning, NY) and analyzed by rpHPLC on a C18 column (Vydac 218TP54, 5 μm, 300 Å, 4.6 × 250 mm, Grace Vydac, Hesperia, CA) on a Waters 626 HPLC system. For elution, a linear gradient of acetonitrile (20–45% in 60 min) in 0.1% trifluoroacetic acid was used at a flow rate of 1.0 ml/min. The UV absorbance at 215 nm was followed, and/or the radioactivity of the collected fractions was measured. Glycosulfopeptides collected from HPLC were neutralized by adding 25 mM NH4HCO3 and dried under vacuum.
Biotinylation of glyco(sulfo)peptides and fluorescence-based solid-phase assay
The C-terminal cysteine of each EG-GSPs was biotinylated using biotin-HPDP (N-(6-[biotinamido]-3′-(2-pyridyldithio)propion-amide) (Pierce, Rockford, IL) as described (Leppänen et al. 2002). Biotinylated GSPs were dissolved in 20 mM MOPS, pH 7.5, containing 150 mM NaCl, and the concentration of each peptide solution was determined by UV absorbance at 215 nm of a sample injected to HPLC.
Fluorescence-based solid-phase assay was performed as described (Leppänen et al. 2003). Biotinylated GSPs were captured on Reacti-BindTM streptavidin-coated high-binding capacity black 96-well plates (Pierce) in 20 mM MOPS, pH 7.5, 150 mM NaCl, 0.05% NaN3 or in phosphate-buffered saline (PBS). The wells were then incubated for 1 h with 50 μl of L-sel-Ig or P-sel-Ig chimera at buffer containing physiologic salt (20 mM MOPS, pH 7.5, 150 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.02% NaN3 containing 1% bovine serum albumin (BSA) and 0.05% Tween-20) or low salt (20 mM MOPS, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.02% NaN3 containing 1% BSA and 0.05% Tween-20). The wells were subsequently incubated for 1 h with 50 μl of Alexa FluorTM 488 goat anti-human IgG (H+//0) (Molecular Probes) at physiologic or low salt buffer. Control experiments with EDTA were performed at physiologic or low salt buffer containing 5 mM EDTA instead of divalent cations. Fluorescence was measured using a Victor2 (Wallac, Turku, Finland) microtiter plate reader with excitation wavelength at 490 nm and emission wavelength at 535 nm. All incubations were performed at room temperature, and the wells were washed with physiologic or low salt buffer (without BSA) between incubations. Background fluorescence reading without immobilized peptide was subtracted from each sample.
Antibody inhibition assay
Biotinylated GSPs were captured on streptavidin plates at a density of 5 pmol/well. The wells were then incubated for 30 min with 50 μl of anti-QPP or anti-SGF antibody (100 μg/ml in PBS containing 0.05% Tween-20 and 1% BSA). The wells were washed with PBS containing 0.05% Tween-20 and incubated for 1 h with 50 μl of L-sel-Ig (10 μg/ml) preincubated for 15 min with Alexa FluorTM 488 goat anti-human IgG (10 μg/ml) at low salt buffer (20 mM MOPS, pH 7.5, 50 mM NaCl, 2 mM CaCl2, 2 mM MgCl2, 0.02% NaN3 containing 1% BSA and 0.05% Tween-20). The wells were washed with low salt buffer (without BSA), and fluorescence was measured. Control incubations with human IgG instead of L-sel-Ig were performed to determine the background fluorescence reading, which was subtracted from each sample.
T-lymphocyte isolation and cell adhesion assay
T lymphocytes were purified from buffy coats obtained from the Finnish Red Cross Transfusion Service (Helsinki, Finland). Mononuclear leukocytes were isolated by Ficoll-Paque Plus (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation according to manufacturer's instructions. T lymphocytes were further purified using Scrubbed Nylon Fiber (ZeptoMetrix Corp., Buffalo, NY) column and magnetic cell separation technology (Miltenyi Biotech GmbH, Bergisch Gladbach, Germany). T lymphocytes purified by this method were >99% CD3 as determined by flow cytometric analysis with PE-conjugated anti-human CD3 mAb (Immunotools GmbH, Friesoythe, Germany).
Purified T lymphocytes (∼8 × 106 cells) were labeled with 1 μM Calcein-AM (acetoxymethyl) (Molecular Probes, Invitrogen) in PBS at 37°C for 30 min. Labeled cells were washed three times with PBS and suspended in Hank's balanced salt solution (HBSS, with 1.3 mM Ca2 and 0.9 mM Mg2) containing 1% BSA. Biotinylated sugars and glyco(sulfo)peptides were immobilized into streptavidin-coated microtiter plates at a density of 5 or 10 pmol/well. Labeled cells (100,000–200,000 cells/well) were incubated with immobilized sugars and glycopeptides in HBSS containing 1% BSA (50 μl/well) at room temperature for 30 min. Microtiter wells were washed four times with HBSS containing 1% BSA, and bound fluorescence was determined using a microtiter plate reader. Parallel control experiments with EDTA and DREG-56 mAb (Pharmingen) were performed by preincubating cells (in HBSS containing 1% BSA) for 15 min with 5 mM EDTA or DREG-56 (20 μg/ml), respectively, before incubating the cells with the immobilized ligands. Control wells with EDTA were washed four times with HBSS containing 1% BSA and 5 mM EDTA, and control wells with DREG-56 were washed four times with HBSS (including 1.3 mM Ca2 and 0.9 mM Mg2) containing 1% BSA.
Mass spectrometric analysis of glycosulfopeptides
MALDI-TOF MS was performed in the linear negative mode with an Ultraflex TOF/TOF instrument (Bruker Daltonik GmbH, Bremen, Germany) equipped with a nitrogen laser operating at 337 nm. HPLC-purified glycopeptide samples were dissolved in 30% aqueous acetonitrile, and a 0.5-µl aliquot (about 2.5 pmol) was mixed with 0.5 µl of 2,4,6trihydroxyacetophenone matrix (3 mg/ml in acetonitrile, 20 mM aqueous diammonium citrate, 1:1, by volume) and immediately dried under reduced pressure. The spectra were externally calibrated with the ubiquitin [M–H]– and [M–2H]2− signals.
Statistical analysis
Data are presented as the mean ± SD unless indicated otherwise. The two-tailed, unpaired Student's t-test was used for statistical comparison of two samples. P values less than 0.05 were considered to be statistically significant.
Acknowledgments
We thank Carmela Kantor-Aaltonen and Jussi Hepojoki for help in the peptide synthesis. We also thank Dr. Matti Autero and Liisa Uotila for valuable advice and discussions.
Glossary
Abbreviations
- BSA
bovine serum albumin
- C2-SLex
core 2-based O-glycan with SLex
- EG
endoglycan
- Fmoc
N-(9-fluorenyl)methoxycarbonyl
- GlyCAM-1
glycosylation-dependent cell adhesion molecule
- GP
glycopeptide
- GSP
glycosulfopeptide
- HBSS
Hank's balanced salt solution
- HEV
high endothelial venule
- L-sel-Ig
L-selectin IgG chimera
- mAb
monoclonal antibody
- MALDI-TOF MS
matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- MOPS
4-[N-morpholine]propanesulfonic acid
- PBS
phosphate-buffered saline
- PNAd
peripheral lymph node vascular addressin
- P-sel-Ig
P-selectin IgG chimera
- PSGL-1
P-selectin glycoprotein ligand-1
- rpHPLC
reversed-phase high-performance liquid chromatography
- SLex
sialyl Lewis x
- TFA
trifluoroacetic acid
Funding
This work was supported by the Academy of Finland [106908 and 118469 to A.L.], Magnus Ehrnrooth Foundation [to A.L.] and the National Institutes of Health [HL085607 to R.D.C.].
Conflict of interest statement
None declared.
References
- Baumhueter S, Singer MS, Henzel W, Hemmerich S, Renz M, Rosen SD, Lasky LA. Binding of L-selectin to the vascular sialomucin CD34. Science. 1993;262:436–438. doi: 10.1126/science.7692600. [DOI] [PubMed] [Google Scholar]
- Berg EL, McEvoy LM, Berlin C, Bargatze RF, Butcher EC. L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature. 1993;366:695–698. doi: 10.1038/366695a0. [DOI] [PubMed] [Google Scholar]
- Berg EL, Robinson MK, Warnock RA, Butcher EC. The human peripheral lymph node vascular addressin is a ligand for LECAM-1, the peripheral lymph node homing receptor. J Cell Biol. 1991;114:343–349. doi: 10.1083/jcb.114.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernimoulin MP, Zeng XL, Abbal C, Giraud S, Martinez M, Michielin O, Schapira M, Spertini O. Molecular basis of leukocyte rolling on PSGL-1. Predominant role of core-2 O-glycans and of tyrosine sulfate residue 51. J Biol Chem. 2003;278:37–47. doi: 10.1074/jbc.M204360200. [DOI] [PubMed] [Google Scholar]
- Bistrup A, Bhakta S, Lee JK, Belov YY, Gunn MD, Zuo FR, Huang CC, Kannagi R, Rosen SD, Hemmerich S. Sulfotransferases of two specificities function in the reconstitution of high endothelial cell ligands for L-selectin. J Cell Biol. 1999;145:899–910. doi: 10.1083/jcb.145.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. PSGL-1 function in immunity and steady state homeostasis. Immunol Rev. 2009;230:75–96. doi: 10.1111/j.1600-065X.2009.00797.x. [DOI] [PubMed] [Google Scholar]
- Clark RA, Fuhlbrigge RC, Springer TA. L-Selectin ligands that are O-glycoprotease resistant and distinct from MECA-79 antigen are sufficient for tethering and rolling of lymphocytes on human high endothelial venules. J Cell Biol. 1998;140:721–731. doi: 10.1083/jcb.140.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry CJ, Faveeuw C, Mordsley KR, Ager A. Novel chondroitin sulfate-modified ligands for L-selectin on lymph node high endothelial venules. Eur J Immunol. 1999;29:419–430. doi: 10.1002/(SICI)1521-4141(199902)29:02<419::AID-IMMU419>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Fieger CB, Sassetti CM, Rosen SD. Endoglycan, a member of the CD34 family, functions as an L-selectin ligand through modification with tyrosine sulfation and sialyl Lewis x. J Biol Chem. 2003;278:27390–27398. doi: 10.1074/jbc.M304204200. [DOI] [PubMed] [Google Scholar]
- Guyer DA, Moore KL, Lynam EB, Schammel CMG, Rogelj S, McEver RP, Sklar LA. P-selectin glycoprotein ligand-1 (PSGL-1) is a ligand for L-selectin in neutrophil aggregation. Blood. 1996;88:2415–2421. [PubMed] [Google Scholar]
- Hemmerich S, Bistrup A, Singer MS, van Zante A, Lee JK, Tsay D, Peters M, Carminati JL, Brennan TJ, Carver-Moore K, et al. Sulfation of L-selectin ligands by an HEV-restricted sulfotransferase regulates lymphocyte homing to lymph nodes. Immunity. 2001;15:237–247. doi: 10.1016/s1074-7613(01)00188-1. [DOI] [PubMed] [Google Scholar]
- Hemmerich S, Butcher EC, Rosen SD. Sulfation-dependent recognition of high endothelial venules (HEV)-ligands by L-selectin and MECA 79, and adhesion-blocking monoclonal antibody. J Exp Med. 1994;180:2219–2226. doi: 10.1084/jem.180.6.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmerich S, Leffler H, Rosen SD. Structure of the O-glycans in GlyCAM-1, an endothelial-derived ligand for L-selectin. J Biol Chem. 1995;270:12035–12047. doi: 10.1074/jbc.270.20.12035. [DOI] [PubMed] [Google Scholar]
- Hernandez Mir G, Helin J, Skarp KP, Cummings RD, Mäkitie A, Renkonen R, Leppänen A. Glycoforms of human endothelial CD34 that bind L-selectin carry sulfated sialyl Lewis x capped O- and N-glycans. Blood. 2009;114:733–741. doi: 10.1182/blood-2009-03-210237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Kawashima H, Petryniak B, Nakayama J, Mitoma J, Marth JD, Lowe JB, Fukuda M. Core 2 branching β1, 6-N-acetylglucosaminyltransferase and high endothelial venule-restricted sulfotransferase collaboratively control lymphocyte homing. J Biol Chem. 2004;279:3058–3067. doi: 10.1074/jbc.M311150200. [DOI] [PubMed] [Google Scholar]
- Imai Y, Singer MS, Fennie C, Lasky LA, Rosen SD. Identification of a carbohydrate-based endothelial ligand for a lymphocyte homing receptor. J Cell Biol. 1991;113:1213–1221. doi: 10.1083/jcb.113.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima H, Petryniak B, Hiraoka N, Mitoma J, Huckaby V, Nakayama J, Uchimura K, Kadomatsu K, Muramatsu T, Lowe JB, et al. N-acetylglucosamine-6-O-sulfotransferases 1 and 2 cooperatively control lymphocyte homing through L-selectin ligand biosynthesis in high endothelial venules. Nat Immunol. 2005;6:1096–1104. doi: 10.1038/ni1259. [DOI] [PubMed] [Google Scholar]
- Kerr SC, Fieger CB, Snapp KR, Rosen SD. Endoglycan, a member of the CD34 family of sialomucins, is a ligand for the vascular selectins. J Immunol. 2008;181:1480–1490. doi: 10.4049/jimmunol.181.2.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AI, Haskard DO, Malhotra R, Landis RC. Identification and characterization of L-selectin ligands in porcine lymphoid tissue. Immunology. 2002;105:441–449. doi: 10.1046/j.1365-2567.2002.01393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N, Mitsuoka C, Kanamori A, Hiraiwa N, Uchimura K, Muramatsu T, Tamatani T, Kansas GS, Kannagi R. Reconstitution of functional L-selectin ligands on a cultured human endothelial cell line by cotransfection of α1–>3 fucosyltransferase VII and newly cloned GlcNAcβ:6-sulfotransferase cDNA. Proc Natl Acad Sci USA. 1999;96:4530–4535. doi: 10.1073/pnas.96.8.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopocki AG, Yago T, Mehta P, Yang J, Wu T, Leppänen A, Bovin NV, Cummings RD, Zhu C, McEver RP. Replacing a lectin domain residue in L-selectin enhances binding to P-selectin glycoprotein ligand-1 but not to 6-sulfo sialyl Lewis x. J Biol Chem. 2008;283:11493–11500. doi: 10.1074/jbc.M709785200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig A, Jain R, Vig R, Norgard-Sumnicht KE, Matta KL, Varki A. Selectin inhibition: synthesis and evaluation of novel sialylated, sulfated and fucosylated oligosaccharides, including the major capping group of GlyCAM-1. Glycobiology. 1997;7:79–93. doi: 10.1093/glycob/7.1.79. [DOI] [PubMed] [Google Scholar]
- Lasky LA, Singer MS, Dowbenko D, Imai Y, Henzel WJ, Grimley C, Fennie C, Gillett N, Watson SR, Rosen SD. An endothelial ligand for L-selectin is a novel mucin-like molecule. Cell. 1992;69:927–938. doi: 10.1016/0092-8674(92)90612-g. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Mehta P, Ouyang YB, Ju T, Helin J, Moore KL, van Die I, Canfield WM, McEver RP, Cummings RD. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J Biol Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Penttilä L, Renkonen O, McEver RP, Cummings RD. Glycosulfopeptides with O-glycans containing sialylated and polyfucosylated polylactosamine bind with low affinity to P-selectin. J Biol Chem. 2002;277:39749–39759. doi: 10.1074/jbc.M206281200. [DOI] [PubMed] [Google Scholar]
- Leppänen A, White SP, Helin J, McEver RP, Cummings RD. Binding of glycosulfopeptides to P-selectin requires stereospecific contributions of individual tyrosine sulfate and sugar residues. J Biol Chem. 2000;275:39569–39578. doi: 10.1074/jbc.M005005200. [DOI] [PubMed] [Google Scholar]
- Leppänen A, Yago T, Otto VI, McEver RP, Cummings RD. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem. 2003;278:26391–26400. doi: 10.1074/jbc.M303551200. [DOI] [PubMed] [Google Scholar]
- Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nature Rev. 2004;4:1–11. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. 2002;14:581–586. doi: 10.1016/s0955-0674(02)00367-8. [DOI] [PubMed] [Google Scholar]
- McEver RP, Cummings RD. Perspectives series: cell adhesion in vascular biology. Role of PSGL-1 binding to selectins in leukocyte recruitment. J Clin Invest. 1997;100:485–491. doi: 10.1172/JCI119556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- Mitoma J, Bao X, Petryanik B, Schaerli P, Gauguet JM, Yu SY, Kawashima H, Saito H, Ohtsubo K, Marth JD, et al. Critical functions of N-glycans in L-selectin-mediated lymphocyte homing and recruitment. Nat Immunol. 2007;8:409–418. doi: 10.1038/ni1442. [DOI] [PubMed] [Google Scholar]
- Mitoma J, Petryniak B, Hiraoka N, Yeh JC, Lowe JB, Fukuda M. Extended core 1 and core 2 branched O-glycans differentially modulate sialyl Lewis x-type L-selectin ligand activity. J Biol Chem. 2003;278:9953–9961. doi: 10.1074/jbc.M212756200. [DOI] [PubMed] [Google Scholar]
- Mitsuoka C, Sawada-Kasugai M, Ando-Furui K, Izawa M, Nakanishi H, Nakamura S, Ishida H, Kiso M, Kannagi R. Identification of a major carbohydrate capping group of the L-selectin ligand on high endothelial venules in human lymph nodes as 6-sulfo sialyl Lewis x. J Biol Chem. 1998;273:11225–11233. doi: 10.1074/jbc.273.18.11225. [DOI] [PubMed] [Google Scholar]
- Morgan SM, Samulowitz U, Darley L, Simmons DL, Vestweber D. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood. 1999;93:165–175. [PubMed] [Google Scholar]
- Nicholson MW, Barclay AN, Singer MS, Rosen SD, van der Merwe PA. Affinity and kinetic analysis of L-selectin (CD62L) binding to glycosylation-dependent cell-adhesion molecule-1. J Biol Chem. 1998;273:763–770. doi: 10.1074/jbc.273.2.763. [DOI] [PubMed] [Google Scholar]
- Poppe L, Brown GS, Philo JS, Nikrad PV, Shah BH. Conformation of sLex tetrasaccharide, free in solution and bound to E-, P-, and L-selectin. J Am Chem Soc. 1997;119:1727–1736. [Google Scholar]
- Puri KD, Finger EB, Gaudernack G, Springer TA. Sialomucin CD34 is the major L-selectin ligand in human tonsil high endothelial venules. J Cell Biol. 1995;131:261–270. doi: 10.1083/jcb.131.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Nollert MU, Qiu H, Liu WJ, Cummings RD, Zhu C, McEver RP. Tyrosine replacement in P-selectin glycoprotein ligand-1 affects distinct kinetic and mechanical properties of bonds with P- and L-selectin. Proc Natl Acad Sci USA. 1999;96:13771–13776. doi: 10.1073/pnas.96.24.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Nieves J, Burcin TL, Olson TS, Morris MA, McDuffie M, Cominelli F, Ley K. Critical role of endothelial P-selectin glycoprotein ligand 1 in chronic murine ileitis. J Exp Med. 2006;203:907–917. doi: 10.1084/jem.20052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Samulowitz U, Kuhn A, Brachtendorf G, Nawroth R, Braun A, Bankfalvi A, Böcker W, Vestweber D. Human endomucin: distribution pattern, expression on high endothelial venules, and decoration with the MECA-79 epitope. Am J Pathol. 2002;160:1669–1681. doi: 10.1016/S0002-9440(10)61114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangapani KK, Yago T, Klopocki AG, Lawrence MB, Fieger CB, Rosen SD, McEver RP, Zhu C. Low force decelerates L-selectin dissociation from P-selectin glycoprotein ligand-1 and endoglycan. J Biol Chem. 2004;279:2291–2298. doi: 10.1074/jbc.M310396200. [DOI] [PubMed] [Google Scholar]
- Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med. 1998;187:1965–1975. doi: 10.1084/jem.187.12.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassetti C, Van Zante A, Rosen SD. Identification of endoglycan, a member of the CD34, podocalyxin family of sialomucins. J Biol Chem. 2000;275:9001–9010. doi: 10.1074/jbc.275.12.9001. [DOI] [PubMed] [Google Scholar]
- Satomaa T, Renkonen O, Helin J, Kirveskari J, Mäkitie A, Renkonen R. O-Glycans on human high endothelial CD34 putatively participating in L-selectin recognition. Blood. 2002;99:2609–2611. doi: 10.1182/blood.v99.7.2609. [DOI] [PubMed] [Google Scholar]
- Scudder PR, Shailubhai K, Duffin KL, Streeter PR, Jacob GS. Enzymatic synthesis of a 6′-sulphated sialyl-Lewis x which is an inhibitor of L-selectin binding to peripheral addressin. Glycobiology. 1994;4:929–932. doi: 10.1093/glycob/4.6.929. [DOI] [PubMed] [Google Scholar]
- Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLex and PSGL-1. Cell. 2000;103:467–479. doi: 10.1016/s0092-8674(00)00138-0. [DOI] [PubMed] [Google Scholar]
- Spertini O, Cordey AS, Monai N, Giuffre L, Schapira M. P-selectin glycoprotein ligand 1 is a ligand for L-selectin on neutrophils, monocytes, and CD34 hematopoietic progenitor cells. J Cell Biol. 1996;135:523–531. doi: 10.1083/jcb.135.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter PR, Rouse BTN, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu L, Chen A, Delahunty MD, Moore KL, Watson SR, McEver RP, Tedder TF. L-selectin binds to P-selectin glycoprotein ligand-1 on leukocytes. Interactions between the lectin, epidermal growth factor, and consensus repeat domains of the selectins determine ligand binding specificity. J Immunol. 1996;157:3995–4004. [PubMed] [Google Scholar]
- Tu L, Delahunty MD, Ding H, Luscinskas FW, Tedder TF. The cutaneous lymphocyte antigen is an essential component of the L-selectin ligand induced on human vascular endothelial cells. J Exp Med. 1999;189:241–252. doi: 10.1084/jem.189.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchimura K, Gauguet JM, Singer MS, Tsay D, Kannagi R, Muramatsu T, von Andrian UH, Rosen SD. A major class of L-selectin ligands is eliminated in mice deficient in two sulfotransferases expressed in high endothelial venules. Nat Immunol. 2005;6:1105–1113. doi: 10.1038/ni1258. [DOI] [PubMed] [Google Scholar]
- Umemoto E, Tanaka T, Kanda H, Jin S, Tohya K, Otani K, Matsutani T, Matsumoto M, Ebisuno Y, Jang MH, et al. Nepmucin, a novel HEV sialomucin, mediates L-selectin-dependent lymphocyte rolling and promotes lymphocyte adhesion under flow. J Exp Med. 2006;203:1603–1614. doi: 10.1084/jem.20052543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J Clin Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JC, Hiraoka N, Petryniak B, Nakayama J, Ellies LG, Rabuka D, Hindsgaul O, Marth JD, Lowe JB, Fukuda M. Novel sulfated lymphocyte homing receptors and their control by a core1 extension β1, 3-N-acetylglucosaminyltransferase. Cell. 2001;105:957–969. doi: 10.1016/s0092-8674(01)00394-4. [DOI] [PubMed] [Google Scholar]
- Önnerfjord P, Heathfield TF, Heinegård D. Identification of tyrosine sulfation in extracellular leucine-rich repeat proteins using mass spectrometry. J Biol Chem. 2004;279:26–33. doi: 10.1074/jbc.M308689200. [DOI] [PubMed] [Google Scholar]





