Abstract
It was previously reported that Tn-4h and DpN1 cells have the endogenous capacity to efficiently sialylate secreted alkaline phosphatase (SEAP) when infected with a baculovirus expression vector. In contrast, it has been found that lepidopteran insect cell lines that are more widely used as hosts for baculovirus vectors typically fail to sialylate SEAP and other recombinant glycoproteins. Thus, the N-glycan processing capabilities of Tn-4h and DpN1 cells are of potential interest to investigators using the baculovirus expression system for recombinant glycoprotein production. In this study, we experimentally re-assessed the ability of Tn-4h and DpN1 cells to sialylate SEAP with Sf9 and glyco-engineered Sf9 cells (SfSWT-1) as negative and positive controls, respectively. Our results showed that the SEAP purified from SfSWT-1 cells was strongly sialylated and initially indicated that the SEAP purified from Tn-4h cells was weakly sialylated. However, further analyses suggested that the SEAP produced by Tn-4h cells only appeared to be sialylated because it was contaminated with an electrophoretically indistinguishable sialoglycoprotein derived from fetal bovine serum. We subsequently expressed, purified, and analyzed a second recombinant glycoprotein (GST-SfManI) from all four cell lines and found that only the SfSWT-1 cells were able to detectably sialylate this product. Together, these results showed that neither Tn-4h nor DpN1 cells efficiently sialylated SEAP or GST-SfManI when infected by baculovirus expression vectors. Furthermore, they suggested that previous reports of efficient SEAP sialylation by Tn-4h and DpN1 cells probably reflect contamination with a sialylated, co-migrating glycoprotein, perhaps bovine fetuin, derived from the serum used in the insect cell growth medium.
Keywords: baculovirus, insect, N-glycosylation, recombinant glycoproteins
Introduction
The production of therapeutic glycoproteins requires an expression system with the capacity for appropriate post-translational processing in order to ensure that the recombinant and native products will be structurally and functionally equivalent. The insect cell/baculovirus expression vector system (BEVS; Summers and Smith 1987; O'Reilly et al. 1992; Jarvis 2009) is commonly used to produce recombinant proteins requiring post-translational processing, including N-glycosylation. However, the BEVS falls short of the basic requirement given above because insect cell lines typically produce recombinant glycoproteins with N-glycans that are quite different from those found on the native products. More specifically, the processed N-glycans produced by insect cell lines usually have trimannosyl core (“paucimannosidic”) structures, whereas those on the native mammalian products have complex or hybrid structures, which have been elaborated beyond the trimannosyl core to include N-acetylglucosamine, galactose, and often, terminal sialic acid residues (reviewed by Marz et al. 1995; Altmann et al. 1999; Marchal et al. 2001; Tomiya et al. 2004; Harrison and Jarvis 2006; Shi and Jarvis 2007; Geisler and Jarvis 2009). This difference in N-glycan processing is a serious limitation of the BEVS because terminal sugars, particularly sialic acids, can dramatically influence the biological functions and clinical efficacies of therapeutic glycoprotein products.
In order to critically assess this important biotechnological limitation of the BEVS, considerable effort has been expended to study the protein N-glycosylation pathways of insect cell systems (reviewed by Marz et al. 1995; Altmann et al. 1999; Marchal et al. 2001; Tomiya et al. 2004; Harrison and Jarvis 2006; Shi and Jarvis 2007; Geisler and Jarvis 2009). The majority of these studies have generally supported the view that lepidopteran insect cells process N-glycans less extensively than mammalian cells, as detailed above. In stark contrast, a few reports have claimed that some lepidopteran insect cell lines can produce recombinant glycoproteins with high proportions of complex, even terminally sialylated N-glycans (Davidson et al. 1990; Davis and Wood 1995; Joshi et al. 2001; Joosten and Shuler 2003a, 2003b; Palomares et al. 2003).
While these reports have been greeted with skepticism, there is growing evidence that insects have at least a limited capacity for glycoprotein sialylation. For example, low levels of sialic acids have been detected in various tissues of various insects, including Lepidoptera (Roth et al. 1992; Karacali et al. 1997; Karacali et al. 1999; Malykh et al. 2000). In addition, extremely low levels of complex, sialylated N-glycans have been found in Drosophila melanogaster (Aoki et al. 2007; Koles et al. 2007). Finally, it is now well established that D. melanogaster encodes key enzymes involved in sialic acid and CMP-sialic acid biosynthesis and glycoprotein sialylation (Kim et al. 2002; Koles et al. 2004; Viswanathan et al. 2006). In light of these findings, it is inappropriate to simply dismiss the claim that native, non-glyco-engineered lepidopteran insect cell lines can produce sialylated recombinant glycoproteins. Instead, this claim should be assessed more objectively by further experimentation. Thus, the purpose of this study was to experimentally assess the claim that Tn-4h and DpN1 cells can efficiently sialylate recombinant secreted alkaline phosphatase (SEAP) when infected with a baculovirus expression vector (Joshi et al. 2001; Palomares et al. 2003).
Tn-4h and DpN1 are native, non-glyco-engineered lepidopteran insect cell lines derived from Trichoplusia ni (cabbage looper) and Danaus plexippus (monarch butterfly), respectively. We expressed and purified SEAP with these cell lines using the same conditions and methods described in previous reports (Joshi et al. 2001; Palomares et al. 2003). We also included Spodoptera frugiperda (Sf9; fall armyworm; Summers and Smith 1987) and glyco-engineered Sf9 cells (SfSWT-1; Hollister et al. 2002) as negative and positive controls, respectively. The N-glycan structures of the recombinant SEAP preparations produced by these four cell lines were then analyzed by lectin blotting with rigorous specificity controls. Finally, these analyses were extended to include another model glycoprotein, glutathione-S-transferase-tagged S. frugiperda mannosidase I (GST-SfManI), which was used previously to assess the N-glycan processing capabilities of native and glyco-engineered lepidopteran insect cell lines (Seo et al. 2001; Hollister et al. 2002; Aumiller et al. 2003). Ultimately, we found that neither SEAP nor GST-SfManI were detectably sialylated by Tn-4h or DpN1 cells. Furthermore, we discovered an explanation for the claims of SEAP sialylation in previous reports, which was that those preparations were probably contaminated with a co-purifying serum sialoglycoprotein.
Results
Purification and preliminary characterization of SEAP produced by Tn-4h, DpN1, SfSWT-1, and Sf9 cells
To re-examine SEAP sialylation by Tn-4h and DpN1 cells, we were obliged to precisely replicate the cell culture, baculovirus infection, and SEAP purification methods used in the relevant previous studies (Joshi et al. 2001; Palomares et al. 2003). Tn-4h cells were cultured in T. ni medium–formulation Hink (TNM-FH) medium supplemented with 10% fetal bovine serum and 5 mM N-acetylmannosamine. DpN1 cells were cultured in TNM-FH medium supplemented with 10% fetal bovine serum alone. Both cell lines were infected with AcSEAP, which is the same recombinant baculovirus, and cell-free media were harvested at the same times post-infection and used to purify SEAP by phosphate affinity chromatography, as described in the previous reports. In the present study, we also introduced Sf9 cells (Summers and Smith 1987) as a negative control and SfSWT-1 cells, a subclone of Sf9 cells glyco-engineered to produce sialylated recombinant glycoproteins (Hollister et al. 2002), as a positive control for recombinant glycoprotein sialylation.
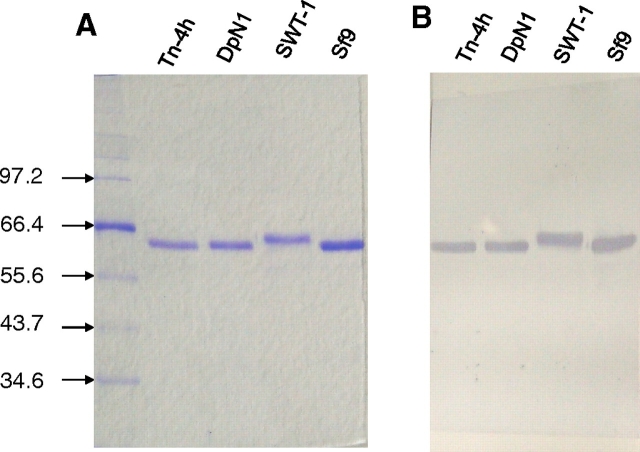
The relative purity of the SEAP preparations isolated from each of the four cell lines was examined by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with Coomassie Blue staining (Figure 1A). As expected, each SEAP preparation included a single protein band with a monomeric apparent molecular weight of about 64 kDa (Davis et al. 1992; Zhang et al. 2001). A parallel immunoblotting analysis with a SEAP-specific antibody indicated that the 64 kDa bands in each SEAP preparation consisted, at least in part, of bona-fide, immunoreactive SEAP protein (Figure 1B). Both analyses showed that the SEAP preparation from SfSWT-1 cells had a slightly slower electrophoretic mobility than the SEAP preparations isolated from the other cell lines. This was consistent with the ability of SfSWT-1 cells to produce SEAP containing complex, terminally sialylated N-glycans, which are larger than the paucimannosidic N-glycans typically produced by non-glyco-engineered insect cell lines. Conversely, SEAP from Tn-4h and DpN1 cells co-migrated with SEAP from Sf9 cells, which were expected to produce only paucimannosidic N-glycans. Thus, their relative electrophoretic mobilities provided a preliminary hint that the SEAP produced by Tn-4h and DpN1 cells had insect-type rather than mammalian-type N-glycosylation patterns.
Fig. 1.
Analysis of SEAP purified from various insect cell lines. Tn-4h, DpN1, SfSWT-1, and Sf9 cells were infected with AcSEAP, and recombinant SEAP was purified as described in Materials and methods. Equal aliquots of each SEAP preparation were then analyzed by (A) SDS-PAGE with Coomassie Brilliant Blue R250 staining or (B) SDS-PAGE and immunoblotting with a SEAP-specific antibody, as described in Materials and methods. The arrows and numbers on the left-hand side of the figure indicate the positions of protein standards and their sizes in kilodaltons.
Lectin blotting analysis of SEAP sialylation
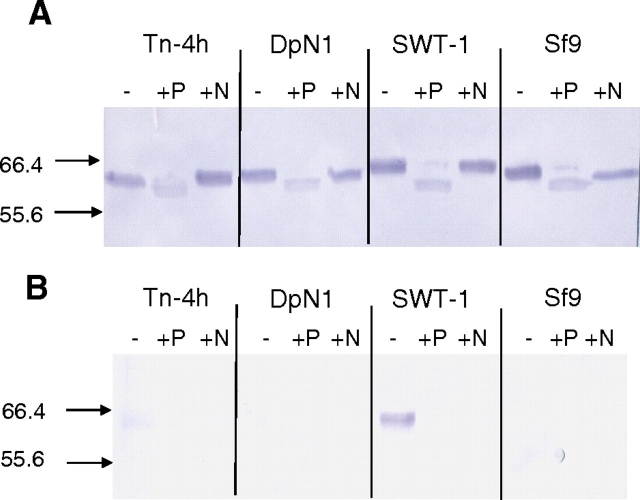
Subsequently, lectin blots with Sambucus nigra agglutinin (SNA) were performed to probe the SEAP preparations from all four cell lines for the presence of terminal α2,6-linked sialic acids (Figure 2B). The specificity of the lectin blotting method was monitored by pre-treating the SEAP preparations with either Flavobacterium meningosepticum peptide-N-glycosidase (PNGase-F; lanes marked P in Figure 2) or Arthrobacter urofaciens neuraminidase (lanes marked N in Figure 2). The integrity and electrophoretic mobility of each enzymatically digested, purified protein was monitored by immunoblotting with a SEAP-specific antibody (Figure 2A). The results showed that PNGase-F increased the electrophoretic mobilities of all four SEAP preparations relative to the untreated controls, indicating that each had been successfully deglycosylated by this enzyme. In contrast, neuraminidase had no detectable effect on the mobilities of the SEAP preparations, including the one from SfSWT-1 cells. This result was inconclusive because desialylation was not necessarily expected to detectably alter the electrophoretic mobility of these proteins.
Fig. 2.
N-Glycosylation of SEAP purified from various insect cell lines. Tn-4h, DpN1, SfSWT-1, and Sf9 cells were infected with AcSEAP, and recombinant SEAP was purified as described in Materials and methods. Equal aliquots of each SEAP preparation were then treated with buffer alone (−), PNGase-F (+P), or neuraminidase (+N) and analyzed by (A) SDS-PAGE and immunoblotting with a SEAP-specific antibody or (B) SDS-PAGE and SNA lectin blotting. The arrows and numbers on the left-hand side of the figure indicate the positions of protein standards and their sizes in kilodaltons.
The lectin blotting results showed that only the untreated SEAP preparation from SfSWT-1 cells reacted strongly with SNA (Figure 2B). Pre-treatment with PNGase-F or neuraminidase abolished the SNA reactivity, confirming that the lectin blotting assay was specific for terminally sialylated N-glycans. The lectin blotting results also revealed that the untreated SEAP preparation from Tn-4h cells reacted very weakly with SNA, although the band was very faint and does not show up very well in the image (Figure 2B). Again, pre-treatment with either PNGase-F or neuraminidase abolished the SNA reactivity, demonstrating the specificity of the lectin blotting analysis. From these results, we initially concluded that the SEAP preparation from SfSWT-1 cells was highly sialylated, the SEAP preparation from Tn-4h cells was marginally sialylated, and the SEAP preparations from DpN1 and Sf9 cells were not sialylated, at least not at levels that could be detected in these assays. However, the SNA reactivity of the SEAP preparation from Tn-4h cells was inconsistent with the fact that it had a faster electrophoretic mobility than the control, sialylated SEAP preparation from SfSWT-1 cells (Figure 1). Thus, we began to consider alternative explanations for the weak SNA reactivity of the SEAP preparation from Tn-4h cells.
A serum protein contaminant co-purifies with SEAP via phosphate affinity chromatography
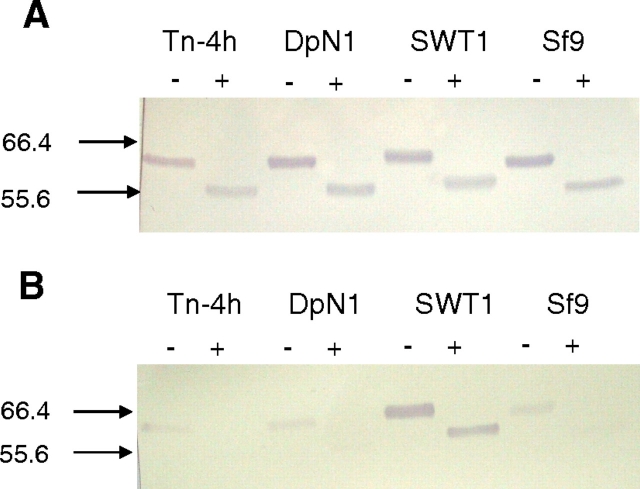
One potential alternative explanation was that the SEAP preparation from Tn-4h cells was contaminated with a sialoglycoprotein from the bovine serum in the growth medium, which had been co-purified with SEAP in the phosphoaffinity method and co-migrated with SEAP in SDS-PAGE. To examine this possibility, we performed sham phosphoaffinity purifications using the cell-free supernatant from mock-infected Tn-4h cells or fetal bovine serum, alone, as the starting material. The eluted fractions from the sham purifications were then examined by immunoblotting and SNA blotting, with the eluted SEAP fractions from AcSEAP-infected Tn-4h and SfSWT-1 cells as controls. Neuraminidase treatments were used to monitor the specificity of the SNA blotting analysis. The immunoblotting results confirmed that the control SEAP preparations from SfSWT-1 and Tn-4h cells contained immunoreactive bands with slightly different electrophoretic mobilities that were not altered by neuraminidase treatment (Figure 3A). These results also showed that there was no detectable immunoreactive SEAP in the elution fractions from the sham purifications performed with the cell-free supernatant from mock-infected Tn-4h cells or serum, alone, as expected. The lectin blotting results confirmed that the SEAP preparation from SfSWT-1 cells contained a strongly SNA-reactive protein and the SEAP preparation from Tn-4h cells contained a weakly SNA-reactive protein (Figure 3B), as observed previously (Figure 2B). However, the lectin blots also showed that both of the sham preparations also contained weakly SNA-reactive proteins that co-migrated with bona-fide SEAP (Figure 3B). In every case, the SNA reactivity was eliminated by pre-treatment with neuraminidase, indicating that the reactivity was sialic acid-specific (Figure 3B). These results strongly suggested that the weakly SNA-reactive protein observed in purified SEAP preparations from Tn-4h cells (Figures 2B and 3B) was not SEAP. Rather, the SNA-reactive protein appeared to be a contaminating sialoglycoprotein with an indistinguishable electrophoretic mobility that had been co-purified with SEAP by the phosphoaffinity method. Furthermore, the presence of this same SNA-reactive protein in the eluted fractions from the sham purification performed using serum alone indicated that it is derived from the bovine serum used in the insect cell growth medium. In the experiment shown in Figure 2B, this contaminating, SNA-reactive protein was only observed in the purified SEAP preparation from AcSEAP-infected Tn-4h cells. However, in other experiments, we also observed the contaminating, SNA-reactive protein in SEAP preparations purified from DpN1 and Sf9 cells (e.g., see Figure 4B).
Fig. 3.
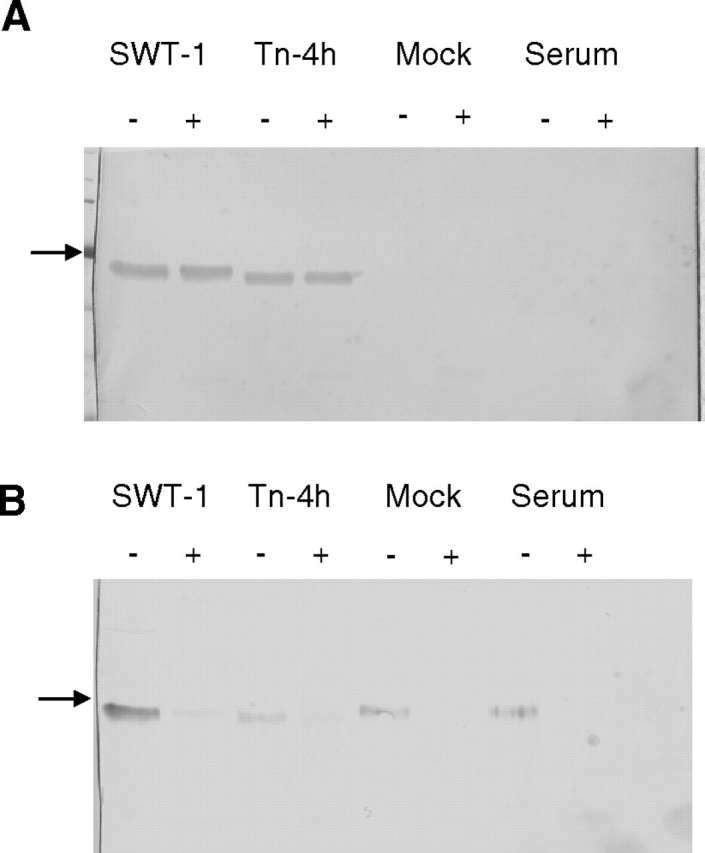
Co-purification of SEAP and a co-migrating sialoglycoprotein. Phosphoaffinity purifications were performed with the cell-free medium from AcSEAP-infected SfSWT-1 cells, AcSEAP-infected Tn-4h cells, or mock-infected Tn-4h cells or with fetal bovine serum alone, as the starting materials as described in Materials and methods. Equal aliquots of the purified column eluants were then treated with buffer alone (−) or neuraminidase (+) and analyzed by (A) SDS-PAGE and immunoblotting with a SEAP-specific antibody or (B) SDS-PAGE and SNA lectin blotting, as described in Materials and methods. The arrows on the left-hand side of the figure mark the position of the 66.4-kDa standard.
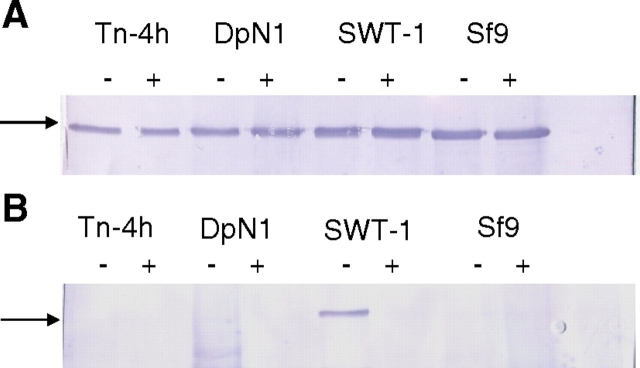
Fig. 4.
Effect of trypsin treatment on SNA reactivity of SEAP purified from various insect cell lines. Equal aliquots of purified SEAP preparations from Tn-4h, DpN1, SfSWT-1, and Sf9 cells were incubated with buffer alone (−) or trypsin (+), and the reaction products were analyzed by (A) SDS-PAGE and immunoblotting with a SEAP-specific antibody or (B) SDS-PAGE and SNA lectin blotting, as described in Materials and methods. The arrows and numbers on the left-hand side of the figure indicate the positions of protein standards and their sizes in kilodaltons.
The co-purification of a serum sialoglycoprotein with an electrophoretic mobility nearly identical to SEAP was an unexpected artifact that complicated our ability to analyze SEAP glycosylation by SDS-PAGE and lectin blotting. In essence, it was impossible to determine whether an SNA-reactive band in the appropriate molecular weight range reflected sialylation of SEAP or the presence of a contaminating, co-migrating sialoglycoprotein. To solve this problem and more clearly assess SEAP sialylation by various insect cell lines, we took advantage of a previous observation. Trypsin cleavage of native placental alkaline phosphatase yields a large, protease-resistant fragment, which is about 10 kDa smaller than the undigested protein and conveniently includes the N-linked glycan (Jemmerson and Stigbrand 1984; Millan 1986). We reasoned that trypsin treatment would degrade the contaminating sialoglycoprotein(s) in our purified SEAP preparations to small peptides that would run off the bottom of the gel, while leaving the ∼55 kDa protease-resistant SEAP fragment intact for analysis by our lectin blotting method.
Thus, SEAP was purified from AcSEAP-infected Tn-4h, DpN1, SfSWT-1, and Sf9 cells, incubated with buffer alone or buffer plus trypsin, resolved by SDS-PAGE, and analyzed by immunoblotting or SNA blotting, as described in Materials and methods. The immunoblotting results showed that trypsin produced immunoreactive SEAP fragments that were ∼10 kDa smaller than the buffer-treated controls, as expected (Figure 4A). The SNA blotting results showed that there was a strongly SNA-reactive protein in the untreated SEAP preparation from SfSWT-1 cells and very weakly SNA-reactive proteins in the untreated SEAP preparations from the other three cell lines (Figure 4B, lanes marked with negative symbol). However, the only SNA-reactive protein observed after trypsin treatment was the ∼55 kDa SEAP fragment from the control SfSWT-1 cells (Figure 4B, lanes marked with positive symbol). None of the SEAP fragments from any of the other insect cell lines, all of which were clearly visible in the immunoblot (Figure 2A), had any detectable SNA reactivity. These results strongly supported the idea that the SEAP produced by Tn-4h and DpN1 cells was not detectably sialylated but only appeared to be sialylated due to the presence of one or more contaminating, trypsin-sensitive sialoglycoproteins.
Lectin blotting analysis of GST-SfManI sialylation
Subsequently, we used a second model glycoprotein and protein purification method to circumvent the complication of a contaminating, co-migrating sialoglycoprotein(s) and extend our analysis of the endogenous capacity of Tn-4h and DpN1 cells for recombinant glycoprotein sialylation. We chose GST-SfManI as the second model for these studies because it is larger (∼96 kDa) than SEAP, it can be effectively purified by a different affinity method, and we had used it for several previous studies on the protein glycosylation patterns of native and engineered lepidopteran insect cell lines (Hollister and Jarvis 2001; Hollister et al. 2002; Aumiller et al. 2003; Hollister et al. 2003).
Tn-4h, DpN1, SfSWT-1, and Sf9 cells were infected with AcGST-SfManI under the same conditions used for SEAP production, as described above, and the GST-SfManI products were purified from the cell-free supernatants by a glutathione-affinity method, as described in Materials and methods. The purified GST-SfManI preparations were then analyzed by SDS-PAGE with immunoblotting or SNA blotting, as for SEAP. The results of the immunoblots showed that the GST-SfManI fusion proteins had similar electrophoretic mobilities, although the purified GST-SfManI from SfSWT-1 cells appeared to migrate as a poorly resolved doublet in which the upper band had a slightly retarded electrophoretic mobility (Figure 5A). The immunoblotting results also showed that neuraminidase had no detectable impact on the migration of any of the GST-SfManI preparations (Figure 5A). The SNA blotting results showed that the only clearly SNA-reactive GST-SfManI preparation was the one isolated from SfSWT-1 cells (Figure 5B). Neuraminidase digestion abolished the SNA reactivity, indicating it was specific for sialic acid. Thus, the results of these experiments indicated that GST-SfManI was not sialylated by Tn-4h, DpN1, or Sf9 cells, at least not at levels that could be detected by the lectin blotting assay used in this study.
Fig. 5.
N-Glycosylation of GST-SfManI purified from various insect cell lines. Tn-4h, DpN1, SfSWT-1, and Sf9 cells were infected with AcGST-SfManI, and the recombinant protein was purified as described in Materials and methods. Equal aliquots of each GST-SfManI preparation were then treated with buffer alone (−) or neuraminidase (+) and analyzed by (A) SDS-PAGE and immunoblotting with a GST-specific antibody or (B) SDS-PAGE and SNA lectin blotting, as described in Materials and methods. The arrows on the left-hand side of the figure mark the position of the 97.2-kDa standard.
Discussion
The ability of lepidopteran insect cells to produce glycoproteins with complex, terminally sialylated N-glycans has been a controversial subject. Initially, it was thought that insect cells generally lack sialic acid metabolism because they contained no detectable sialic acids, CMP-sialic acids, or sialyltransferase activities and produced non-sialylated native and recombinant glycoproteins (reviewed by Marz et al. 1995; Altmann et al. 1999; Marchal et al. 2001; Tomiya et al. 2004; Harrison and Jarvis 2006; Shi and Jarvis 2007; Geisler and Jarvis 2009). However, some studies were inconsistent with this view and indicated that some baculovirus-infected lepidopteran insect cell lines could produce at least some sialylated recombinant N-glycoproteins (Davidson et al. 1990; Davidson and Castellino 1991a, 1991b; Joshi et al. 2001; Palomares et al. 2003). Other studies showed that sialic acids could be found in various tissues of various insects, including Lepidoptera (Roth et al. 1992; Karacali et al. 1997, 1999; Malykh et al. 2000). Subsequently, genes encoding enzymes involved in sialic acid production (Kim et al. 2002; Viswanathan et al. 2006) and utilization (Koles et al. 2004) were identified in D. melanogaster, and it was shown that this insect contains low levels of sialylated N-glycans (Aoki et al. 2007; Koles et al. 2007). Thus, early views had to evolve to accommodate these observations and to include the idea that insects might have the endogenous capacity for sialic acid metabolism and glycoprotein sialylation. This newer view of insect glycobiology intensified our interest in previous reports of recombinant glycoprotein sialylation in baculovirus-infected insect cell lines, which had been greeted with some skepticism in the field.
We focused our attention on previous reports of SEAP sialylation by baculovirus-infected Tn-4h (Joshi et al. 2001) and DpN1 (Palomares et al. 2003) cells because these cell lines were relatively newly described, under-utilized hosts for baculovirus expression vectors. In fact, there were no data to refute the claim that these cells could produce recombinant glycoproteins with sialylated N-glycans. Moreover, the production of sialylated SEAP by baculovirus-infected adherent Tn-4h cell cultures required the addition of N-acetylmannosamine, which was consistent with the idea that these cells might utilize this sialic acid precursor in an endogenous pathway leading to recombinant glycoprotein sialylation. This pathway appeared to be quite efficient, as it was reported that baculovirus-infected Tn-4h cells cultured in the presence of N-acetylmannosamine produced SEAP with ∼20% terminally sialylated N-glycans (Joshi et al. 2001). In related studies, it was reported that a baculovirus-infected Tn-4h cell variant (Tn-4s) also produced recombinant SEAP with up to ∼20% sialylated N-glycans when cultured under various conditions (Joosten et al. 2003; Joosten and Shuler 2003a, 2003b). Finally, baculovirus-infected DpN1 cells, another relatively newly described host cell line, reportedly produced recombinant SEAP with ∼13% sialylated N-glycans when grown in a standard medium lacking N-acetylmannosamine (Palomares et al. 2003). Thus, these reports appeared to document examples of highly efficient recombinant glycoprotein sialylation by relatively new, potentially advantageous hosts for baculovirus expression vectors.
Our analysis of SEAP preparations isolated from Tn-4h, DpN1, SfSWT-1, and Sf9 cells revealed that they each consisted of single bands of immunoreactive protein with the expected electrophoretic mobilities. Notably, the SEAP preparation isolated from SfSWT-1 cells had a slightly slower electrophoretic mobility than the SEAP preparations from the other cell lines, reflecting the ability of SfSWT-1 cells to produce complex, terminally sialylated N-glycans (Hollister et al. 2002). This difference in their electrophoretic mobilities provided an early clue that the SEAP preparations from Tn-4h, DpN1, and Sf9 cells might not, after all, contain any complex, terminally sialylated N-glycans. However, SNA, which is a lectin specific for terminal α2,6-linked sialic acids, bound weakly, though sporadically, to SEAP preparations isolated from Tn-4h, DpN1, and Sf9 cells. While we expected the SEAP from Sf9 cells to serve as a negative control, we nevertheless considered that the SNA binding results might truly reflect the ability of all three of these cell lines to produce a sialylated form of SEAP. We also considered the possibility that the SEAP preparations isolated from the non-glyco-engineered insect cell lines were not sialylated but only appeared to be sialylated because they might be contaminated with a mammalian sialoglycoprotein that was electrophoretically indistinguishable from SEAP.
This latter possibility was strongly supported by the results of sham phosphoaffinity purifications performed with the growth medium from mock-infected cells or fetal bovine serum, alone, which obviously contained no recombinant SEAP, but nevertheless yielded an SNA-reactive protein with the electrophoretic mobility of SEAP. Further support was obtained by trypsin treatment of the SEAP preparations from all four insect cell lines, as this produced an immunoreactive ∼55 kDa SEAP fragment in each case but an SNA-reactive SEAP fragment only with the positive control preparation from SfSWT-1 cells.
Together, the results described above indicated that Tn-4h and DpN1 cells failed to efficiently sialylate recombinant SEAP when infected by a baculovirus expression vector. These cells also failed to detectably sialylate another recombinant N-glycoprotein, GST-SfManI, which was clearly sialylated by SfSWT-1 cells. Thus, the results obtained in this study do not support the idea that Tn-4h and DpN1 cells have the endogenous ability to produce recombinant glycoproteins with terminally sialylated N-glycans.
The results of this study also suggest a possible explanation for the previous reports of efficient SEAP sialylation by Tn-4h and DpN1 cells, which is that the SEAP preparations in those studies were likely contaminated with a sialylated, co-migrating glycoprotein derived from the serum used in the insect cell growth medium. We speculate that this contaminant might be bovine fetuin because fetuin has an apparent molecular weight of ∼63–64 kDa (Johnson and Heath 1986), biantennary and triantennary N-glycans with both terminal α2,3- and α2,6-linked sialic acids (Rohrer et al. 1993), and is a major component of bovine serum. Notably, the N-glycans previously identified in SEAP preparations from various insect cell lines included triantennary and tetraantennary structures, some with terminal α2,3-linked sialic acids. In contrast, the only sialylated N-glycans detected in total N-glycan preparations of D. melanogaster were monoantennary structures with terminal α2,6-linked sialic acids (Aoki et al. 2007). Even native placental alkaline phosphatase has only biantennary, complex N-glycans (Endo et al. 1988), so it seems unlikely that a recombinant form of this protein would acquire more highly branched structures. Thus, the detection of more highly branched and α2,3-sialylated N-glycans in recombinant SEAP preparations is consistent with the idea that these preparations were contaminated with bovine fetuin, which has these types of oligosaccharide side chains.
Another interesting observation pursuant to the previous claims of SEAP sialylation by Tn-4h and DpN1 cells is that we detected the co-migrating, SNA-reactive protein contaminant somewhat sporadically in our SEAP preparations and found that the presence of the contaminant was inversely related to the yields of SEAP obtained in various expression and purification experiments. Generally, Sf9 and SfSWT-1 cells produced ∼10-fold higher levels of SEAP than Tn-4h and DpN1 cells, and the latter SEAP preparations were more likely to contain the co-migrating, SNA-reactive protein contaminant. Based on these observations, we conclude that a lower expression level and, therefore, lower concentration of SEAP in the starting material for the phosphoaffinity purification step was a critical factor determining whether or not a given SEAP preparation would contain the co-migrating, SNA-reactive contaminant. We speculate that relatively non-specific phosphate-binding proteins can more effectively compete for sites on the affinity matrix in the presence of lower concentrations of SEAP. This speculation is indirectly supported by the fact that at least one baculovirus-derived protein was found to interact non-specifically with the phosphate affinity matrix when the SEAP concentration in the starting material was low (Zhang et al. 2001). Finally, this speculation is more directly supported by a survey of the literature reporting sialylation of SEAP by T. ni and DpN1 cells, which revealed an inverse correlation between the concentrations of SEAP in the starting material and the proportions of sialylation reported for SEAP (Table I).
Table I.
Comparison of SEAP expression levels and sialylation by insect cell lines
| Cell line | Reference | SEAP concentration in cell-free media (U/mL) | SEAP sialylation (%) |
|---|---|---|---|
| Tn-5B1-4 | Palomares et al. (2003) | 6.79 | ND |
| Tn-4s | Joosten et al. (2003) | 6.3 | 0.7 |
| Tn-4s | Joosten and Shuler (2003b) | 3.7 | 2.6 |
| Tn-4s | Joosten and Shuler (2003a, 2003b) | 1.4, 1.5 | 9.1, 20 |
| DpN1 | Palomares et al. (2003) | 0.51 | 13.3 |
| Tn-4h | Joshi et al. (2001) | NR | 20 |
ND, not detected; NR, not reported.
Materials and methods
Cells and viruses
DpN1 (Palomares et al. 2003), Tn-4h (Joshi et al. 2000), Sf9 (Summers and Smith 1987), and SfSWT-1 (Hollister et al. 2002) cells were all maintained at 28°C as adherent cultures in TNM-FH supplemented with 10% fetal bovine serum (Atlas Biologicals, Fort Collins, CO) and 0.1% Pluronic® F-68 (Sigma-Aldrich, St. Louis, MO).
AcSEAP and AcGST-SfManI, which are recombinant baculoviruses encoding SEAP or GST-SfManI under the control of the polyhedrin promoter, respectively, have been described previously (Davis et al. 1992; Kawar et al. 2000). All working virus stocks were produced by infecting 50–100 mL shake flask cultures of Sf9 cells in TNM-FH medium with low passage seed stocks at low multiplicities of infection (≤0.1 plaque-forming unit/cell). The cell-free culture media were harvested at 3–5 days after infection and stored at 4°C in the dark, and baculoviral titers were determined by plaque assays on Sf9 cells (Jarvis 2009).
Recombinant protein production
Recombinant proteins were produced by infecting adherent or semi-adherent cultures of Sf9, DpN1, Tn-4h, or SfSWT-1 cells in 75 cm2 flasks containing a total of 5–15 million cells/flask to conform to previous experiments (Joshi et al. 2001; Palomares et al. 2003). DpN1, Tn-4h, and Sf9/SfSWT-1 cells were infected with AcSEAP or AcGST-SfManI at multiplicities of infection of 20, 10, and 4 plaque-forming units/cell, respectively. In each case, the virus was allowed to adsorb for 2 h at 28°C, and then the cells were pelleted by centrifugation at 300 × g for 5 min in a swinging-bucket rotor and re-suspended in fresh TNM-FH medium supplemented with 10% (v/v) fetal bovine serum and 0.1% (w/v) Pluronic® F-68. The medium used to re-suspend the Tn-4h cells after infection was additionally supplemented with 5 mM N-acetylmannosamine to conform to conditions used in previous studies (Joshi et al. 2001). At 96 h post-infection, the culture media supernatants were harvested by centrifugation at 3200 × g for 10 min in a swinging-bucket rotor and were either used immediately for purification of SEAP or GST-SfManI or stored at −20°C for future purification.
Protein purifications
A phosphate affinity resin (4-aminobenzophosphonic acid-histidyl-agarose) was prepared and used to purify SEAP from the cell-free media of AcSEAP-infected insect cell cultures, as described previously (Kulakosky et al. 1998). Briefly, the cell-free media were centrifuged at 72,000 × g for 30 min in a Beckman 45Ti fixed-angle rotor to pellet debris and budded virus particles. SEAP and other proteins in the media were then concentrated and partially purified by adding solid ammonium sulfate to 55–60% saturation with stirring on ice. The precipitates were harvested by centrifugation at 17,000 × g in a Beckman JA20 fixed-angle rotor for 30 min at 4°C, re-dissolved in 5–10 mL of column buffer (20 mM Tris-HCl, 1 mM MgCl2, pH 8.0), and then dialyzed overnight against 2 L of the same buffer. The dialysates were then loaded onto phosphate affinity columns with a bed volume of ∼2 mL, which had been pre-equilibrated with column buffer. The columns were then extensively washed with at least 100 bed volumes of column buffer, once with 10 mL of column buffer containing 1 µM dibasic sodium phosphate, and finally eluted with 5–10 mL of 100 µM dibasic sodium phosphate in column buffer. The purity and enzymatic activity of SEAP were measured in each fraction by SDS-PAGE with Coomassie Brilliant Blue R-250 staining and a phosphatase activity assay, respectively, as described previously (Laemmli 1970; Davis et al. 1992).
Glutathione-affinity chromatography was used in analogous fashion to purify the GST-SfManI from cell-free supernatants of AcGST-SfManI-infected insect cell cultures after ammonium sulfate concentration, as described previously (Geisler et al. 2008), except 5 mM dithiothreitol was added to the protein solution during the GSH-agarose binding step.
SDS-PAGE, immunoblotting, and lectin blotting analyses
SDS-PAGE assays were performed under reducing conditions with 8–10% acrylamide resolving gels (Laemmli 1970), and proteins were either stained directly with Coomassie Brilliant Blue R-250 or electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes (Immobilon P, Millipore), as described previously (Towbin et al. 1979). SEAP and GST-SfManI were detected on PVDF membranes with primary rabbit polyclonal antibodies against human placental alkaline phosphatase (AbD-Serotec, Oxford, UK) or GST (Sigma-Aldrich), respectively. An anti-rabbit IgG-alkaline phosphatase conjugate (Sigma-Aldrich) was used as the secondary antibody. Glycans attached to SEAP and GST-SfManI proteins on membranes were detected by lectin blotting using biotinylated lectins (Vector Laboratories, Burlingame, CA) followed by incubation of lectin-treated membranes with alkaline phosphatase-conjugated streptavidin. Immune complexes and lectin–streptavidin complexes were visualized by the chromogenic nitroblue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate reaction (Blake et al. 1984).
Acknowledgments
This work was supported by Award Number R01GM49734 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health. The authors thank Dr. Laura Palomares of the Universidad Nacional Autonoma de Mexico, Dr. H. Alan Wood of Mississippi State University, and Dr. David Murhammer of the University of Iowa for providing DpN1 cells, Tn-4h cells, and AcSEAP, respectively. We also thank Drs. Michael Tiemeyer and Toshihiko Katoh of the Complex Carbohydrate Research Center at the University of Georgia for insightful discussions during the course of this study.
Glossary
Abbreviations
- BEVS
baculovirus expression vector system
- GST
glutathione-S-transferase
- GST-SfManI
Spodoptera frugiperda mannosidase I-glutathione-S-transferase fusion protein
- PVDF
polyvinylidene difluoride
- SDS-PAGE
Sodium dodecyl sulfate- polyacrylamide gel electrophoresis
- SEAP
human placental secreted alkaline phosphatase
- SNA
Sambucus nigra agglutinin
- TNM-FH
Trichoplusia ni medium–formulation Hink
References
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282:9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- Aumiller JJ, Hollister JR, Jarvis DL. A transgenic lepidopteran insect cell line engineered to produce CMP-sialic acid and sialoglycoproteins. Glycobiology. 2003;13:497–507. doi: 10.1093/glycob/cwg051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MS, Johnston KH, Russell-Jones GJ, Gotschlich EC. A rapid, sensitive method for detection of alkaline phosphatase conjugated anti-antibody on western blot. Analyt Biochem. 1984;36:175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Castellino FJ. Asparagine-linked oligosaccharide processing in lepidopteran insect cells. Temporal dependence of the nature of the oligosaccharides assembled on asparagine-289 of recombinant human plasminogen produced in baculovirus vector infected Spodoptera frugiperda (IPLB-SF-21AE) cells. Biochemistry. 1991a;30:6167–6174. doi: 10.1021/bi00239a013. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Castellino FJ. Structures of the asparagine-289-linked oligosaccharides assembled on recombinant human plasminogen expressed in a Mamestra brassicae cell line (IZD-MBO503) Biochemistry. 1991b;30:6689–6696. doi: 10.1021/bi00241a008. [DOI] [PubMed] [Google Scholar]
- Davidson DJ, Fraser MJ, Castellino FJ. Oligosaccharide processing in the expression of human plasminogen cDNA by lepidopteran insect (Spodoptera frugiperda) cells. Biochemistry. 1990;29:5584–5590. doi: 10.1021/bi00475a024. [DOI] [PubMed] [Google Scholar]
- Davis TR, Wood HA. Intrinsic glycosylation potentials of insect cell cultures and insect larvae. In Vitro Cell Dev Biol. 1995;31:659–663. doi: 10.1007/BF02634086. [DOI] [PubMed] [Google Scholar]
- Davis TR, Trotter KM, Granados RR, Wood HA. Baculovirus expression of alkaline phosphatase as a reporter gene for evaluation of production, glycosylation and secretion. Nat Biotechnol. 1992;10:1148–1150. doi: 10.1038/nbt1092-1148. [DOI] [PubMed] [Google Scholar]
- Endo T, Ohbayashi H, Hayashi Y, Ikehara Y, Kochibe N, Kobata A. Structural study on the carbohydrate moiety of human placental alkaline phosphatase. J Biochem. 1988;103:182–187. doi: 10.1093/oxfordjournals.jbchem.a122228. [DOI] [PubMed] [Google Scholar]
- Geisler C, Jarvis DL. Insect cell glycosylation patterns in the context of biopharmaceuticals. In: Walsh B, editor. Post-translational Modification of Protein Biopharmaceuticals. Weinheim: Wiley-Blackwell; 2009. pp. 165–191. [Google Scholar]
- Geisler C, Aumiller JJ, Jarvis DL. A fused lobes gene encodes the processing beta-N-acetylglucosaminidase in Sf9 cells. J Biol Chem. 2008;283:11330–11339. doi: 10.1074/jbc.M710279200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison RL, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell expression system and engineering of insect cells to produce “mammalianized” recombinant glycoproteins. Adv Vir Res. 2006;68:159–191. doi: 10.1016/S0065-3527(06)68005-6. [DOI] [PubMed] [Google Scholar]
- Hollister J, Jarvis DL. Engineering lepidopteran insect cells for sialoglycoprotein production by genetic transformation with mammalian ß1, 4-galactosyltransferase and α2, 6-sialyltransferase genes. Glycobiology. 2001;11:1–9. doi: 10.1093/glycob/11.1.1. [DOI] [PubMed] [Google Scholar]
- Hollister JR, Conradt HO, Jarvis DL. Evidence for a sialic acid salvaging pathway in lepidopteran insect cell lines. Glycobiology. 2003;13:487–495. doi: 10.1093/glycob/cwg053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister JR, Grabenhorst E, Nimtz M, Conradt HO, Jarvis DL. Engineering the protein N-glycosylation pathway in insect cells for production of biantennary, complex N-glycans. Biochemistry. 2002;41:15093–15104. doi: 10.1021/bi026455d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis DL. Baculovirus-insect cell expression systems. Meth Enzymol. 2009;463:191–222. doi: 10.1016/S0076-6879(09)63014-7. [DOI] [PubMed] [Google Scholar]
- Jemmerson R, Stigbrand T. Monoclonal antibodies block the trypsin cleavage site on human placental alkaline phosphatase. FEBS Lett. 1984;173:357–359. doi: 10.1016/0014-5793(84)80805-4. [DOI] [PubMed] [Google Scholar]
- Johnson WV, Heath EC. Structural features of bovine fetuin revealed from analysis of the primary translation product: anomalous behavior on sodium dodecyl sulfate-polyacrylamide gel electrophoresis is due largely to peptide and not solely to carbohydrate. Arch Biochem Biophys. 1986;251:732–737. doi: 10.1016/0003-9861(86)90383-8. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Effect of culture conditions on the degree of sialylation of a recombinant glycoprotein expressed in insect cells. Biotechnol Progr. 2003a;19:739–749. doi: 10.1021/bp0201049. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Shuler ML. Production of a sialylated N-linked glycoprotein in insect cells: role of glycosidases and effect of harvest time on glycosylation. Biotechnol Progr. 2003b;19:193–201. doi: 10.1021/bp025695h. [DOI] [PubMed] [Google Scholar]
- Joosten CE, Park TH, Shuler ML. Effect of silkworm hemolymph on N-linked glycosylation in two Trichoplusia ni insect cell lines. Biotechnol Bioengr. 2003;83:695–705. doi: 10.1002/bit.10696. [DOI] [PubMed] [Google Scholar]
- Joshi L, Shuler ML, Wood HA. Production of a sialylated N-linked glycoprotein in insect cells. Biotechnol Progr. 2001;17:822–827. doi: 10.1021/bp010071h. [DOI] [PubMed] [Google Scholar]
- Joshi L, Davis TR, Mattu TS, Rudd PM, Dwek RA, Shuler ML, Wood HA. Influence of baculovirus-host cell interactions on complex N-linked glycosylation of a recombinant human protein. Biotechnol Progr. 2000;16:650–656. doi: 10.1021/bp000057p. [DOI] [PubMed] [Google Scholar]
- Karacali S, Kirmizigul S, Deveci R. Sialic acids in developing testis of Galleria mellonella (Lepidoptera) Invert Repr Dev. 1999;35:225–229. [Google Scholar]
- Karacali S, Kirmizigul S, Deveci R, Deveci O, Onat T, Gurcu B. Presence of sialic acid in prothoracic glands of Galleria mellonella (Lepidoptera) Tiss Cell. 1997;29:315–321. doi: 10.1016/s0040-8166(97)80007-9. [DOI] [PubMed] [Google Scholar]
- Kawar Z, Romero PA, Herscovics A, Jarvis DL. N-Glycan processing by a lepidopteran insect alpha1, 2-mannosidase. Glycobiology. 2000;10:347–355. doi: 10.1093/glycob/10.4.347. [DOI] [PubMed] [Google Scholar]
- Kim K, Lawrence SM, Park J, Pitts L, Vann WF, Betenbaugh MJ, Palter KB. Expression of a functional Drosophila melanogaster N-acetylneuraminic acid (Neu5Ac) phosphate synthase gene: evidence for endogenous sialic acid biosynthetic ability in insects. Glycobiology. 2002;12:73–83. doi: 10.1093/glycob/12.2.73. [DOI] [PubMed] [Google Scholar]
- Koles K, Irvine KD, Panin VM. Functional characterization of a Drosophila sialyltransferase. J Biol Chem. 2004;279:4346–4357. doi: 10.1074/jbc.M309912200. [DOI] [PubMed] [Google Scholar]
- Koles K, Lim JM, Aoki K, Porterfield M, Tiemeyer M, Wells L, Panin V. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology. 2007;17:1388–1403. doi: 10.1093/glycob/cwm097. [DOI] [PubMed] [Google Scholar]
- Kulakosky PC, Shuler ML, Wood HA. N-glycosylation of a baculovirus-expressed recombinant glycoprotein in three insect cell lines. In Vitro Cell Dev Biol Anim. 1998;34:101–108. doi: 10.1007/s11626-998-0091-0. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malykh YN, Krisch B, Gerardy-Schahn R, Lapina EB, Shaw L, Schauer R. The presence of N-acetylneuraminic acid in Malpighian tubules of larvae of the cicada Philaenus spumarius. Glycoconj J. 2000;16:731–739. doi: 10.1023/a:1007115627708. [DOI] [PubMed] [Google Scholar]
- Marchal I, Jarvis DL, Cacan R, Verbert A. Glycoproteins from insect cells: sialylated or not? Biol Chem. 2001;382:151–159. doi: 10.1515/BC.2001.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marz L, Altmann F, Staudacher E, Kubelka V. Protein glycosylation in insects. In: Montreuil J, Vliegenthart JFG, et al., editors. Glycoproteins. Amsterdam: Elsevier; 1995. pp. 543–563. [Google Scholar]
- Millan JL. Molecular cloning and sequence analysis of human placental alkaline phosphatase. J Biol Chem. 1986;261:3112–3115. [PubMed] [Google Scholar]
- O'Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors. New York: W.H. Freeman and Company; 1992. [Google Scholar]
- Palomares L, Joosten CE, Hughes PR, Granados RR, Shuler ML. Novel insect cell line capable of complex N-glycosylation and sialylation of recombinant proteins. Biotechnol Progr. 2003;19:185–192. doi: 10.1021/bp025598o. [DOI] [PubMed] [Google Scholar]
- Rohrer JS, Cooper GA, Townsend RR. Identification, quantification, and characterization of glycopeptides in reversed-phase HPLC separations of glycoprotein proteolytic digests. Anal Biochem. 1993;212:7–16. doi: 10.1006/abio.1993.1283. [DOI] [PubMed] [Google Scholar]
- Roth J, Kempf A, Reuter G, Schauer R, Gehring W. Occurrence of sialic acids in Drosophila melanogaster. Science. 1992;256:673–675. doi: 10.1126/science.1585182. [DOI] [PubMed] [Google Scholar]
- Seo NS, Hollister JR, Jarvis DL. Mammalian glycosyltransferase expression allows sialoglycoprotein production by baculovirus-infected insect cells. Prot Expr Purif. 2001;22:234–241. doi: 10.1006/prep.2001.1432. [DOI] [PubMed] [Google Scholar]
- Shi X, Jarvis DL. Protein N-glycosylation in the baculovirus-insect cell system. Curr Drug Targ. 2007;8:1116–1125. doi: 10.2174/138945007782151360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers MD, Smith GE. A manual of methods for baculovirus vectors and insect cell culture procedures. Tx Ag Expt Stn Bull No 1555. 1987 [Google Scholar]
- Tomiya N, Narang S, Lee YC, Betenbaugh MJ. Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj J. 2004;21:343–360. doi: 10.1023/B:GLYC.0000046275.28315.87. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan K, Tomiya N, Singh S, Park J, Lee YC, Palter K, Betenbaugh MJ. Expression of a functional Drosophila melanogaster CMP-sialic acid synthetase: differential localization of the Drosophila and human enzymes. J Biol Chem. 2006;281:15929–15940. doi: 10.1074/jbc.M512186200. [DOI] [PubMed] [Google Scholar]
- Zhang F, Wolff MW, Williams D, Busch K, Lang SC, Murhammer DW, Linhardt RJ. Affinity purification of secreted alkaline phosphatase produced by baculovirus expression vector system. Appl Biochem Biotechnol. 2001;90:125–136. doi: 10.1385/abab:90:2:125. [DOI] [PubMed] [Google Scholar]