Abstract
Background. Serum alkaline phosphatase (ALP) increases in patients with chronic kidney disease (CKD) and high-turnover bone disease. ALP may represent an adjunct marker of high bone turnover devoid of drawbacks of serum parathyroid hormone (PTH), and it may also be associated with cardiovascular calcification in CKD. Higher ALP has been recently associated with increased mortality and coronary calcification in dialysis patients. In pre-dialysis CKD patients, this association is not clear.
Methods. We examined the association of baseline, time-varying and time-averaged ALP with all-cause mortality and the composite of pre-dialysis mortality or end-stage renal disease in a historical prospective cohort of 1158 male veterans with pre-dialysis CKD from a single institution by using multivariable-adjusted Cox models.
Results. Higher ALP was associated with increased mortality irrespective of the statistical model. Time-averaged ALP displayed a consistent linear association with mortality: a 50-U/L higher serum ALP was associated with a multivariable-adjusted death hazard ratio (95% confidence interval) of 1.17 (1.08–1.28), P < 0.001. Baseline and time-varying ALP showed non-linear associations with mortality, with serum levels above 70 U/L in all models and with lower levels in time-varying models. Associations between ALP levels and the composite outcomes were similar. However, compared to serum PTH, mortality predictability of ALP appeared more incremental.
Conclusions. Elevated ALP is associated with increased mortality in patients with pre-dialysis CKD. Low ALP appears to be associated with short-term mortality.
Keywords: alkaline phosphatase, chronic kidney disease, mortality
Introduction
In patients with chronic kidney disease (CKD), including those undergoing maintenance hemodialysis (CKD 5D) therapy and those with pre-dialysis CKD stages (CKD 1–5), various abnormalities related to mineral and bone disorders (MBD) have been implicated as novel risk factors of mortality [1–6]. Many of the abnormalities that characterize CKD-MBD such as hyperphosphataemia, abnormal serum calcium and parathyroid hormone (PTH) represent clinical treatment targets, hence epidemiologic studies examining outcomes associated with these variables may be confounded by the use of medical interventions. Confounding by medical indication may distort the natural association between a certain risk factor and various outcomes.
Alkaline phosphatase (ALP) is an enzyme measurable in most body fluids and usually originates from the liver or bone. In CKD patients without liver disease, ALP can be elevated in high-turnover bone disease [7–9]. However, measuring this readily available and inexpensive biomarker has not been singled out as an individual therapeutic target of CKD-MBD [10]. Since moderately high or low ALP does not usually trigger a change in routine CKD patient care, ALP likely represents a less biassed tool to assess the risk(s) imparted by CKD-MBD in epidemiologic studies. Furthermore, elevated ALP may be causally involved in the cardiovascular calcification of CKD [11–14], making it a potentially important independent risk factor. Higher ALP has been shown to be associated with mortality and coronary artery calcification in CKD 5D [9] and in patients without CKD [15]. Similar findings were reported for bone-specific ALP in a small study of patients with CKD 1–5 [16] and, more recently, baseline ALP was associated with mortality in the African-American Study of Kidney Disease (AASK) [17]. It is unclear if similar associations exist in CKD 1–5 populations of mixed racial composition, and it is also unknown if the associations between ALP and mortality are different when accounting for temporal changes in ALP. We examined the association of baseline, time-varying and time-averaged ALP with all-cause mortality and the composite of pre-dialysis mortality or end-stage renal disease (ESRD) in a large number of males with CKD 1–5 at a single institution.
Materials and methods
Study population and data collection
We studied all 1259 patients evaluated for CKD 1–5 at Salem Veterans Affairs Medical Center (VAMC) between 1 January 1990 and 30 June 2007 and followed them up until 1 April 2009. Seventy-two patients with no ALP measurement, 10 female patients, 6 patients whose race was other than white or black and 13 patients whose ALP was first measured after starting dialysis were excluded. The final cohort consisted of 1158 male patients.
Baseline characteristics recorded at the time of the initial evaluation in the nephrology clinic were extracted retrospectively, including demographic and anthropometric characteristics, comorbidities and laboratory results, as detailed elsewhere [18,19]. Follow-up clinical and laboratory data recorded during follow-up in relation to outpatient visits was also extracted and utilized in time-varying analyses. Medication use including that of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (ACEI/ARB), statins, phosphate binders and calcitriol was also assessed over the entire follow-up. Glomerular filtration rate (GFR) was estimated using the abbreviated equation developed for the Modification of Diet in Renal Disease Study [20] and categorized according to the staging system introduced by the Kidney/Dialysis Outcome Quality Initiative Clinical Practice Guidelines for CKD [21]. All the biochemical measurements were performed in a single laboratory at the Salem VAMC.
Statistical analyses
Missing data points for comorbidity index (1% missing), body mass index (BMI; 15%), serum aspartate transaminase (AST; 1%), alanine transaminase (ALT; 13%), albumin (1%), phosphorus (2%), blood cholesterol (2%), haemoglobin (1%), white blood cell count (WBC; 1%), percent lymphocytes in WBC (1%) and 24-h urine protein (3%) were imputed using multiple imputations. Sixty-five percent of patients had a PTH measurement performed; only unimputed PTH was used in multivariable models that were restricted to this subgroup. Smoking (5% missing) was analysed as a categorical variable with the creation of a dummy category for missing status. We used different statistical models to analyse ALP: baseline values were assessed due to their practical relevance as prognostic indicators; time-varying values were examined in order to assess outcomes occurring after shorter exposure to abnormal ALP, reflecting potential acute effects of such levels (especially hypophosphatasaemia [22]); and finally, time-averaged values were assessed to alleviate potential misclassification stemming from single measurements and to assess the impact of chronic long-term exposure to higher ALP. Multivariable models of time-varying and time-averaged ALP were adjusted for time-varying and time-averaged blood pressure, BMI, medication use and all laboratory parameters.
Outcomes analysis. The starting time for survival analysis was the date of the first encounter in the nephrology clinic. Patients were considered lost to follow-up if no contact was documented for more than 6 months and were censored at the date of the last documented contact. The primary outcome measure was overall (pre-dialysis and post-dialysis) all-cause mortality (ascertained from Virginia electronic records), and the secondary outcome measure was the composite of pre-dialysis mortality or ESRD (defined as initiation of maintenance dialysis therapy and ascertained from local hospital records including Medicare Forms 2728).
The associations of baseline, time-varying and time-averaged ALP with outcomes were evaluated in adjusted Cox models. Selection of variables to be included in the multivariable models was done a priori by determining probable confounders [23] based on differences in baseline characteristics between patients with different ALP levels and based on theoretical considerations. To account for the different time periods of study enrolment, multivariable models were also adjusted for a dummy variable corresponding to the enrolment period (1990–95, 1996–2000 or post-2000). Models were constructed to assess unadjusted, case mix-adjusted (age, race, comorbidities, blood pressure, BMI, smoking, medication use and enrolment period) and case mix plus biochemical characteristics-adjusted (estimated GFR, albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes and 24-h urine protein) models. Additional adjustment for PTH was performed in the subgroup of 755 patients with available PTH measurements. Nonlinearity of associations was tested by categorizing ALP into quartiles, by including polynomial terms and by using restricted cubic splines; analyses were restricted to values above the first and below the 99th percentiles of the predictor variable in order to increase the stability of the spline models. The joint significance of the polynomial terms was assessed with the Wald test.
Interactions were assessed by including interaction terms and by performing subgroup analyses. Sensitivity analyses were performed by performing time-varying analyses with 30- and 90-day lag periods, by using only unimputed values of independent variables and by restricting analyses to a more contemporary cohort of patients enrolled after 1 January 2001. Proportionality of non-time-varying models was tested by using Schoenfeld residuals. P-values < 0.05 were considered significant. Statistical analyses were performed using the STATA statistical software version 10 (STATA Corporation, College Station, TX). The study protocol was approved by the Research and Development Committee at Salem VAMC.
Results
The mean (±SD) age of the cohort at baseline was 68 ± 11 years, 24% of the patients were black and the mean estimated GFR was 37 ± 17 mL/min/1.73 m2. Most patients had CKD Stages 3 (55%) and 4 (31%), with few patients categorized as CKD Stages 1 (2%), 2 (8%) and 5 (4%). The mean (±SD) baseline and time-averaged ALP were 91 ± 51 and 92 ± 44 U/L, respectively. Patients had a median number of five ALP measurements performed during follow-up (interquartile range [IQR], 2–9); the mean (95% confidence interval [95% CI]) intra-individual change in ALP during follow-up was an increase of 1.9 U/L/year (0.9–2.8). Seven hundred and twelve patients (62%) were enrolled after 1 January 2001. Six hundred and fifty-one patients died (mortality rate, 128/1000 patient-years; 95% CI, 118–138), and 742 patients reached the composite outcome of pre-dialysis death or ESRD (172/1000 patient-years, 95% CI, 160–185) during a median follow-up of 3.5 years (IQR, 1.3–5.5 years); the median time from the last time-updated visit to the end of follow-up was 140 days (IQR, 37–317 days). Forty-one patients (3%) were lost to follow-up and their characteristics were not significantly different (data not shown).
Baseline characteristics in patients divided by quartiles of ALP are shown in Table 1. Patients with higher ALP were younger, more likely to be black, active smokers and to use phosphate binders, were less likely to be diabetic and to use ACEI/ARB or statins, had lower BMI, estimated GFR, albumin, calcium, bicarbonate, haemoglobin and percentage of lymphocyte in WBC and had higher comorbidity index, blood pressure, AST, ALT, phosphorus, WBC and 24-h proteinuria levels.
Table 1.
Baseline characteristics of individuals stratified by quartiles of baseline serum ALP level
| Serum ALP (U/L) |
P for trend | ||||
|---|---|---|---|---|---|
| <66 (N = 282) | 66–82 (N = 296) | 83–105 (N = 302) | >105 (N = 278) | ||
| Age (years) | 69.9 ± 9.9 | 69.0 ± 10.6 | 67.1 ± 11.1 | 67.4 ± 11.6 | 0.001 |
| Race (black) | 60 (21) | 69 (23) | 76 (25) | 77 (28) | 0.06 |
| DM | 172 (61) | 164 (55) | 165 (55) | 142 (51) | 0.022 |
| ASCVD | 161 (57) | 168 (57) | 175 (58) | 159 (57) | 0.8 |
| Smoking | 49 (18) | 67 (23) | 81 (28) | 85 (34) | <0.001 |
| Comorbidity index | 2.3 ± 1.5 | 2.4 ± 1.7 | 2.5 ± 1.8 | 2.6 ± 1.7 | 0.027 |
| Calcitriol use | 99 (34) | 91 (31) | 102 (34) | 78 (28) | 0.16 |
| Calcium-containing binder use | 73 (25) | 98 (33) | 106 (35) | 95 (34) | 0.029 |
| Sevelamer HCl use | 20 (8) | 27 (7) | 26 (9) | 61 (24) | <0.001 |
| Aspirin use | 169 (59) | 182 (61) | 196 (65) | 159 (56) | 0.8 |
| ACEI/ARB use | 229 (80) | 229 (77) | 225 (74) | 197 (70) | 0.004 |
| Statin use | 222 (78) | 208 (70) | 188 (62) | 145 (51) | <0.001 |
| BMI (kg/m2) | 29.9 ± 5.8 | 29.4 ± 5.8 | 29.2 ± 5.9 | 28.4 ± 6.4 | 0.007 |
| SBP (mmHg) | 142 ± 26 | 149 ± 25 | 152 ± 24 | 150 ± 28 | <0.001 |
| DBP (mmHg) | 70 ± 14 | 74 ± 16 | 76 ± 15 | 75 ± 16 | <0.001 |
| eGFR (mL/min/1.73 m2) | 38.8 ± 16.5 | 38.6 ± 17.5 | 36.7 ± 17.0 | 35.9 ± 18.8 | 0.023 |
| Serum albumin (g/dL) | 3.6 ± 0.5 | 3.7 ± 0.5 | 3.6 ± 0.4 | 3.5 ±0.6 | 0.005 |
| Serum AST (U/L) | 25 ± 13 | 24 ± 11 | 26 ± 18 | 29 ± 24 | 0.002 |
| Serum ALT (U/L) | 23 ± 15 | 21 ± 13 | 24 ± 19 | 26 ± 27 | 0.045 |
| Total cholesterol (mg/dL) | 184 ± 57 | 193 ± 50 | 194 ± 55 | 188 ± 64 | 0.4 |
| Serum calcium (mg/dL) | 9.5 ± 0.5 | 9.4 ± 0.5 | 9.4 ± 0.5 | 9.3 ± 0.5 | <0.001 |
| Serum phosphorus (mg/dL) | 3.8 ± 0.7 | 3.8 ± 0.8 | 3.8 ± 0.8 | 4.0 ± 0.8 | 0.019 |
| Serum bicarbonate (mEq/L) | 26.4 ± 3.0 | 25.5 ± 3.5 | 25.6 ± 3.4 | 25.1 ± 3.7 | <0.001 |
| Blood Hgb (g/dL) | 12.7 ± 1.9 | 12.8 ± 2.1 | 12.7 ± 1.8 | 12.3 ± 1.8 | 0.02 |
| Blood WBC (1000/mm3) | 7.2 ± 2.2 | 7.7 ± 2.1 | 7.7 ± 2.4 | 7.9 ± 2.5 | 0.003 |
| Blood lymphocytes (% WBC) | 24.0 ± 9.0 | 23.4 ± 8.6 | 23.3 ± 8.0 | 22.4 ± 8.9 | 0.047 |
| Proteinuria (mg/24 h) | 577 (485–687) | 638 (533–764) | 810 (676–971) | 998 (812–1154) | <0.0001 |
Data is presented as means ± SD, number (% of total) or geometric means (95% CI). DM, diabetes mellitus; ASCVD, atherosclerotic cardiovascular disease; ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; AST, aspartate transaminase; ALT, alanine transaminase; Hgb, haemoglobin; WBC, white blood cell count. Comparisons were made by chi-square test for linear trend.
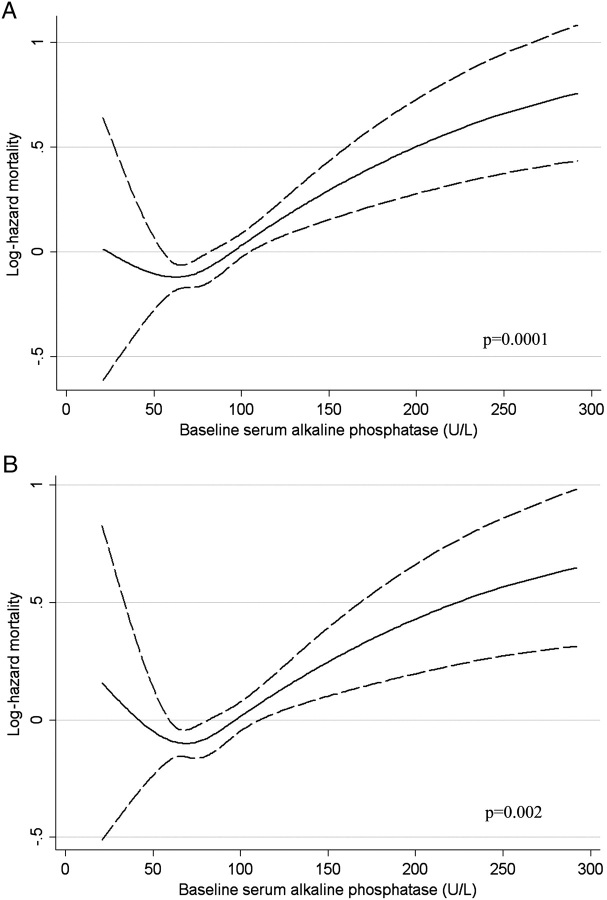
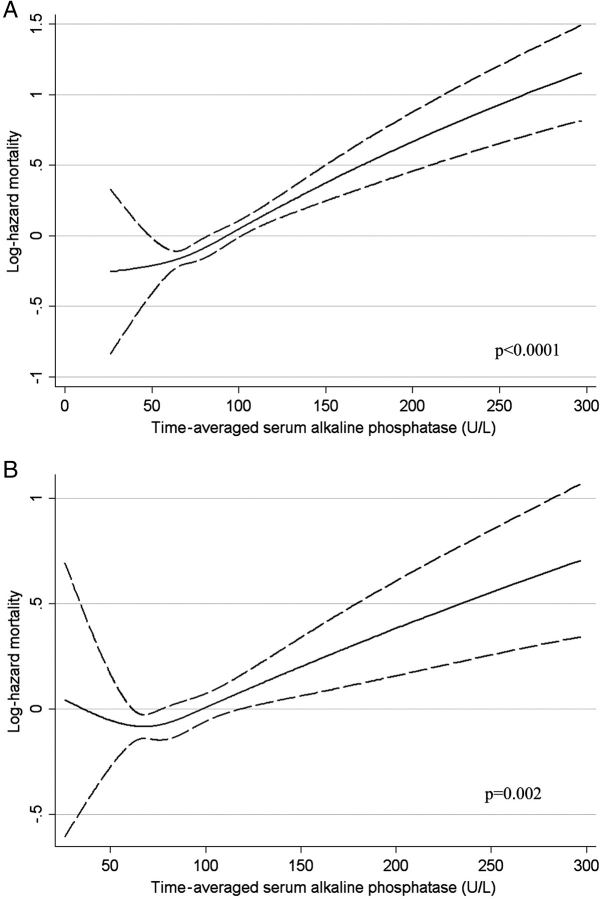
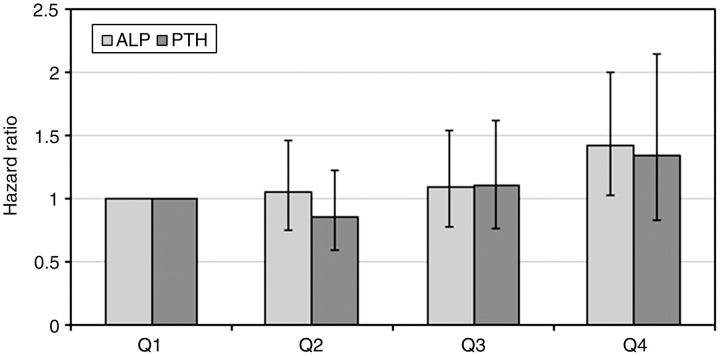
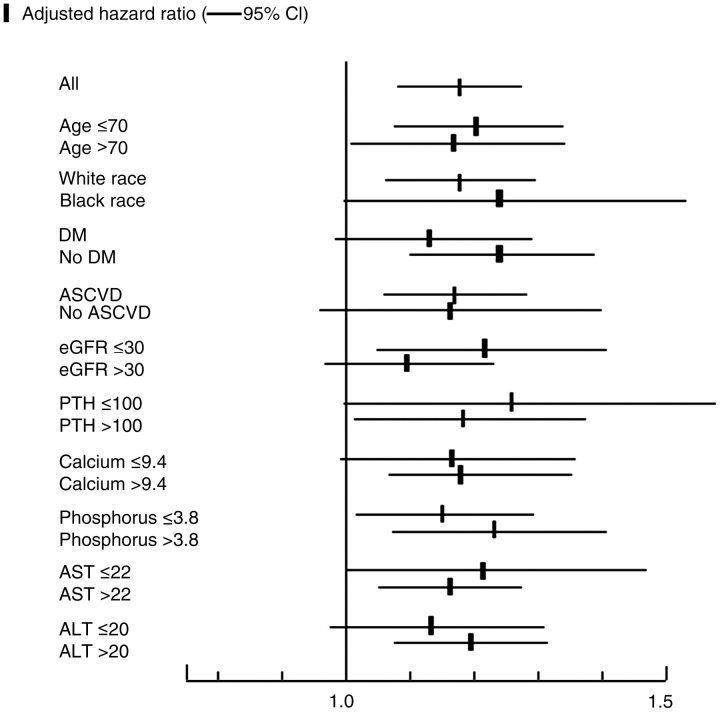
Higher ALP was associated with increased mortality in baseline (Figure 1), time-varying (Figure 2) and time-averaged (Figure 3) models, even after adjustments. The association between time-averaged ALP and mortality appeared robust and linear (Figure 3); a 50-U/L higher time-averaged ALP was associated with a multivariable-adjusted hazard ratio for death of 1.17 (95% CI, 1.08–1.28, P < 0.001). A U-shaped association was however evident in the case of time-varying (Figure 2) and somewhat for baseline ALP (Figure 1), with an inflection point at an ALP of approximately 70 U/L. A comparison between time-averaged ALP and PTH revealed consistently increasing risks of death for higher quartiles of ALP, but a U-shaped association for PTH (Figure 4). Adjustment for PTH did not substantially alter the associations between ALP and mortality in any of the models: the joint significance of the polynomial terms were P = 0.0008 in baseline, P = 0.0009 in time-averaged and P = 0.0002 in time-varying models after including PTH. The associations between ALP and the composite outcome were similar to the associations with all-cause mortality (data not shown). No significant interactions were found. Higher ALP was associated with increased mortality in various subgroup analyses, including patients with higher and lower serum PTH, AST, ALT and estimated GFR levels (Figure 5). The results did not change significantly in sensitivity analyses using 30- or 90-day lag periods, when adjusting for unimputed variables or when examining a more contemporary cohort enrolled after 1 January 2001 (data not shown).
Fig. 1.
Unadjusted (A) and multivariable-adjusted (B) log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality associated with baseline levels of serum ALP in Cox models. Multivariable models were adjusted for age, race, comorbidities, blood pressure, BMI, smoking status, medication use, enrolment period, estimated GFR, serum albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes in WBC and 24-h urine protein. P-values represent the joint significance of the polynomial terms for ALP by the Wald test.
Fig. 2.
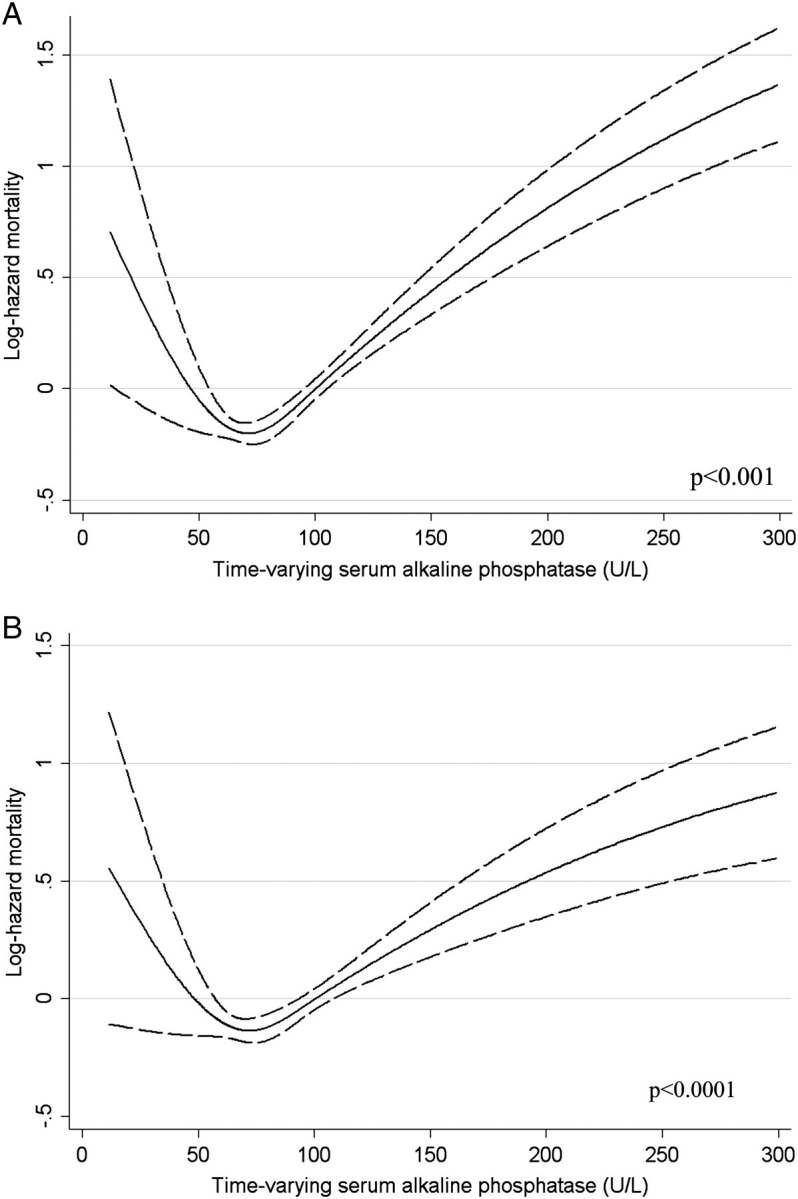
Unadjusted (A) and multivariable-adjusted (B) log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality associated with time-varying levels of serum ALP in Cox models. Multivariable models were adjusted for age, race, comorbidities, blood pressure, BMI, smoking status, medication use, enrolment period, estimated GFR, serum albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes in WBC and 24-h urine protein. P-values represent the joint significance of the polynomial terms for ALP by the Wald test.
Fig. 3.
Unadjusted (A) and multivariable-adjusted (B) log hazards (solid lines) and 95% CI (dashed lines) of all-cause mortality associated with time-averaged levels of serum ALP in Cox models. Multivariable models were adjusted for age, race, comorbidities, blood pressure, BMI, smoking status, medication use, enrolment period, estimated GFR, serum albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes in WBC and 24-h urine protein. P-values represent the joint significance of the polynomial terms for ALP by the Wald test.
Fig. 4.
Multivariable-adjusted associations between quartiles of time-averaged serum ALP and serum PTH and all-cause mortality. Patients in the first quartiles served as the reference group. Multivariable models were adjusted for age, race, comorbidities, blood pressure, BMI, smoking status, medication use, enrolment period, estimated GFR, serum albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes in WBC and 24-h urine protein.
Fig. 5.
Multivariable-adjusted hazard ratios (95% CI) of all-cause mortality associated with a 50-U/L higher time-averaged serum ALP level in select subgroups. Multivariable models were adjusted for age, race, comorbidities, blood pressure, BMI, smoking status, medication use, enrolment period, estimated GFR, serum albumin, bicarbonate, AST, ALT, calcium, phosphorus, blood haemoglobin, WBC, percentage of lymphocytes in WBC and 24-h urine protein.
Discussion
We describe significant associations between higher total (non-tissue-specific) ALP and increased all-cause mortality in patients with CKD 1–5. These associations were most robust for time-averaged ALP, which may reflect the effects of cumulative exposure to higher levels over time, but were also present in baseline and time-varying models for patients with ALP levels >70 U/L, most likely because intra-individual variability of ALP over time was modest and single baseline measurements appeared to be representative of ALP measured subsequently. Low ALP was also associated with mortality in time-varying models. We found that, while both ALP and PTH were independently associated with higher mortality, the death risk imparted by higher time-averaged ALP was linearly increasing, as opposed to a U-shaped association between time-averaged PTH and mortality.
ALP is a hydrolyze enzyme that dephosphorylates various molecules, most effectively operating in an alkaline environment. ALP is fairly ubiquitous in the human body, but it is especially concentrated in the bone, liver, placenta, leukocytes and kidneys. Pathologic conditions most commonly associated with elevations in ALP include diseases of the bones (such as high-turnover bone disease in CKD) and the liver. The origin of circulating ALP can be determined by measuring tissue-specific ALP such as bone-specific ALP. Elevations in total ALP are a known feature of CKD-MBD [10], yet no specific therapeutic interventions are used to target ALP levels, and there are currently no defined ‘desirable’ serum levels for this enzyme. The lack of specific interventions triggered by elevations in ALP makes it possible to assess it as a risk factor without being confounded by therapeutic measures.
Associations similar to the ones described in our study were reported by a small study in patients with pre-dialysis CKD [16] and by a post hoc analysis from the AASK study [17]. The latter study examined baseline ALP in an African-American cohort with relatively low mortality rates and found a significant association between higher ALP and all-cause mortality. PTH levels were not available in the AASK study; thus, residual confounding from this covariate was possible. To the best of our knowledge, our study is the first to examine associations of time-varying and time-averaged ALP with mortality in a large mixed-ethnicity cohort and to also compare head-to-head ALP and PTH as risk factors for mortality in CKD 1–5.
A possible explanation for the described association between ALP and mortality is that ALP is a marker of high-turnover bone disease and, as such, it is associated with serum PTH, which itself has been linked to increased mortality [2,6]. Lower ALP could also be indicative of low-turnover bone disease and, hence, explain the association between lower ALP and mortality in some of our models, although the fact that such associations were mostly seen in time-varying models indicates that the mechanism of action may be an acute effect (vide infra) rather than a chronic one such as vascular calcification. A potential advantage of ALP over PTH as a diagnostic marker of CKD-MBD is that it is devoid of some of the sample collection and assay variability problems that have raised significant concerns about the validity of PTH; this has resulted in recommendations to use ALP as an adjunct measure when evaluating CKD-MBD [24]. However, ALP may be more than a mere marker of bone turnover. Higher ALP has been shown to result in increased hydrolysis of pyrophosphate [11], which is a potent inhibitor of vascular calcification [25,26]. The effect of ALP on pyrophosphate could be the link that explains why lower levels of the former are associated with a linear decrease in mortality, especially since inhibitors of ALP have been shown to lower smooth muscle calcification in animal models [27]. Supporting a possible direct role for ALP in adverse outcomes is the fact that, in our study, ALP levels were associated with increased mortality independent of PTH and ALP remained a predictor of mortality even in patients with low serum PTH and was equally predictive of outcomes in white and black patients even though these groups display significant differences in their CKD-MBD [28]. Another plausible explanation for the observed association is a link between higher ALP and lower 25(OH) vitamin D levels [29–31] which themselves were associated with mortality in CKD 5D [32].
The association between chronic elevations in ALP and long-term mortality is in contrast with the potential acute effects of ALP, as indicated by the association of low ALP with higher short-term mortality in our time-varying models. It has been suggested that ALP has potentially beneficial acute anti-inflammatory effects [33,34] possibly through detoxification of endotoxin via dephosphorylation of lipopolysaccharides [35]. In a small clinical trial, the administration of exogenous bovine ALP to patients with sepsis-associated acute kidney injury had beneficial effects on kidney function [22], but it remains unclear if mortality could be affected by such a strategy. Our study excluded ALP levels linked with acute hospitalizations and our lag-time analyses also did not yield different results; hence, we probably did not capture such an acute effect related to states of acute inflammation which might have weakened associations reflecting the chronic deleterious effects of ALP. Nevertheless, discrepancies between the short-term and long-term effects of ALP may explain why the association of time-varying ALP with mortality in our study was non-linear, with higher mortality also seen in patients with the lowest ALP levels. Any future study examining interventions directly targeting ALP should consider the complex actions of this molecule and the possible discrepancy between its long-term and short-term effects.
Our study should be qualified for potential limitations. Its historical and observational nature allows us to establish associations, but not causal relationships. Our study was limited to male patients from a single institution; hence, our results may not apply to the larger population with CKD. Our enrolment of patients over an extended period of time makes it possible that secular trends in medical practices could have affected patient outcomes. To address this issue, we adjusted for the time of enrolment and we examined more contemporary patients separately and found no differences in outcomes. Patients with elevated ALP had more severe cardiovascular risk factors that could have confounded the association between ALP and mortality; we adjusted our models for several such confounders, but residual confounding from unmeasured variables (such as vascular calcification, C-reactive protein or lipid levels) could have affected our results. We did not have data on causes of death; hence, we could not specifically test our hypothesis that higher ALP was associated with increased death due to higher cardiovascular mortality. We only had data on PTH in a subgroup of patients; hence, we could not account for the confounding effects of it in the entire population. The results in this subgroup were, however, not different from the ones in the overall study population even after adjusting for PTH. We did not have bone-specific ALP available; hence, it is possible that elevated ALP levels were markers of not only CKD-MBD, but also liver disease. The association between ALP and mortality persisted in spite of adjustments for levels of liver enzymes and was similar in subgroups of patients with higher and lower AST and ALT levels, indicating an effect that was independent from hepatic pathology. Furthermore, the putative mechanism of action whereby ALP could be instrumental in causing vascular calcification [11,12] is the same irrespective of the source of ALP, thus potentially broadening the scope of our findings beyond CKD-MBD. We examined time-updated ALP in order to capture the effects of temporal changes on outcomes associated with it and to attenuate potential misclassification resulting from single baseline measurements, but differences from baseline models could also have been the result of unmeasured confounders driving more frequent visits for patients with higher numbers of ALP measurements and thus biassing the results of our time-averaged and time-varying models.
Higher ALP is associated with higher death risk in individuals with CKD 1–5. Low ALP is also associated with increased short-term mortality. Further research is needed to clarify the clinical significance of ALP and determine the efficacy and safety of interventions meant to manipulate its serum concentration.
Acknowledgments
Parts of this material were presented at the American Society of Nephrology Renal Week 2009, October 27–November 1, 2009, San Diego, California. This study was supported by an investigator-initiated grant (without salary support) from Shire Development, Inc. to CPK and by grant 1R01DK078106-01 to CPK and KKZ. The study sponsor(s) provided no input on data analysis and interpretation and exerted no influence on manuscript preparation which was solely the work of the listed authors.
Conflict of interest statement. CPK and KKZ have received grant support and/or honoraria from Genzyme, Shire and Fresenius.
References
- 1.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 2.Kalantar-Zadeh K, Kuwae N, Regidor DL, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 3.Kovesdy CP, Kalantar-Zadeh K. Bone and mineral disorders in pre-dialysis CKD. Int Urol Nephrol. 2008;40:427–440. doi: 10.1007/s11255-008-9346-7. [DOI] [PubMed] [Google Scholar]
- 4.Kestenbaum B, Sampson JN, Rudser KD, et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol. 2005;16:520–528. doi: 10.1681/ASN.2004070602. [DOI] [PubMed] [Google Scholar]
- 5.Voormolen N, Noordzij M, Grootendorst DC, et al. High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant. 2007;22:2909–2916. doi: 10.1093/ndt/gfm286. [DOI] [PubMed] [Google Scholar]
- 6.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Secondary hyperparathyroidism is associated with higher mortality in men with moderate to severe chronic kidney disease. Kidney Int. 2008;73:1296–1302. doi: 10.1038/ki.2008.64. [DOI] [PubMed] [Google Scholar]
- 7.Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MW, Larsson L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 8.Torres PU. Bone alkaline phosphatase isoforms in chronic renal failure. Kidney Int. 2002;61:1178–1179. doi: 10.1046/j.1523-1755.2002.00241-1.x. [DOI] [PubMed] [Google Scholar]
- 9.Regidor DL, Kovesdy CP, Mehrotra R, et al. Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol. 2008;19:2193–2203. doi: 10.1681/ASN.2008010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eknoyan G, Levin A, Levin N. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:1–201. [Google Scholar]
- 11.Lomashvili KA, Garg P, Narisawa S, Millan JL, O'Neill WC. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Neill WC. Pyrophosphate, alkaline phosphatase, and vascular calcification. Circ Res. 2006;99:e2. doi: 10.1161/01.RES.0000234909.24367.a9. [DOI] [PubMed] [Google Scholar]
- 13.Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? . Kidney Int. 2008;73:989–991. doi: 10.1038/ki.2008.104. [DOI] [PubMed] [Google Scholar]
- 14.Shantouf R, Kovesdy CP, Kim Y, et al. Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol. 2009;4:1106–1114. doi: 10.2215/CJN.06091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tonelli M, Curhan G, Pfeffer M, et al. Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation. 2009;120:1784–1792. doi: 10.1161/CIRCULATIONAHA.109.851873. [DOI] [PubMed] [Google Scholar]
- 16.Fahrleitner-Pammer A, Herberth J, Browning SR, et al. Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res. 2008;23:1850–1858. doi: 10.1359/jbmr.080610. [DOI] [PubMed] [Google Scholar]
- 17.Beddhu S, Ma X, Baird B, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1805–1810. doi: 10.2215/CJN.01560309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kovesdy CP, Trivedi BK, Anderson JE. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv Chronic Kidney Dis. 2006;13:183–188. doi: 10.1053/j.ackd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Kovesdy CP, Trivedi BK, Kalantar-Zadeh K, Anderson JE. Association of low blood pressure with increased mortality in patients with moderate to severe chronic kidney disease. Nephrol Dial Transplant. 2006;21:1257–1262. doi: 10.1093/ndt/gfk057. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 21.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 22.Heemskerk S, Masereeuw R, Moesker O, et al. Alkaline phosphatase treatment improves renal function in severe sepsis or septic shock patients. Crit Care Med. 2009;37:417–423. doi: 10.1097/CCM.0b013e31819598af. e1. [DOI] [PubMed] [Google Scholar]
- 23.Thadhani R, Tonelli M. Cohort studies: marching forward. Clin J Am Soc Nephrol. 2006;1:1117–1123. doi: 10.2215/CJN.00080106. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009;76:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 25.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 26.Schibler D, Russell RG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 27.Narisawa S, Harmey D, Yadav MC, O'Neill WC, Hoylaerts MF, Millan JL. Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res. 2007;22:1700–1710. doi: 10.1359/jbmr.070714. [DOI] [PubMed] [Google Scholar]
- 28.Stehman-Breen CO, Sherrard D, Walker A, Sadler R, Alem A, Lindberg J. Racial differences in bone mineral density and bone loss among end-stage renal disease patients. Am J Kidney Dis. 1999;33:941–946. doi: 10.1016/s0272-6386(99)70430-0. [DOI] [PubMed] [Google Scholar]
- 29.Lips P, Duong T, Oleksik A, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 30.Sahota O, Masud T, San P, Hosking DJ. Vitamin D insufficiency increases bone turnover markers and enhances bone loss at the hip in patients with established vertebral osteoporosis. Clin Endocrinol (Oxf) 1999;51:217–221. doi: 10.1046/j.1365-2265.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 32.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72:1004–1013. doi: 10.1038/sj.ki.5002451. [DOI] [PubMed] [Google Scholar]
- 33.van Veen SQ, van Vliet AK, Wulferink M, Brands R, Boermeester MA, van Gulik TM. Bovine intestinal alkaline phosphatase attenuates the inflammatory response in secondary peritonitis in mice. Infect Immun. 2005;73:4309–4314. doi: 10.1128/IAI.73.7.4309-4314.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij WR, Bentala H, Huizinga-vanderVlag A, et al. Protection against an Escherichia coli-induced sepsis by alkaline phosphatase in mice. Shock. 2004;22:174–179. doi: 10.1097/01.shk.0000132485.05049.8a. [DOI] [PubMed] [Google Scholar]
- 35.Koyama I, Matsunaga T, Harada T, Hokari S, Komoda T. Alkaline phosphatases reduce toxicity of lipopolysaccharides in vivo and in vitro through dephosphorylation. Clin Biochem. 2002;35:455–461. doi: 10.1016/s0009-9120(02)00330-2. [DOI] [PubMed] [Google Scholar]