Abstract
Background. Non-diabetic forms of nephropathy commonly lead to end-stage renal disease (non-DM ESRD). Previous studies have demonstrated that African Americans are more susceptible to non-DM ESRD compared to other ethnic groups, and this risk has a strong genetic component. A genome-wide scan for ESRD in African American families enriched for non-DM ESRD showed evidence for linkage in chromosome 13q33.3, and a candidate gene in this region, klotho, was selected for a detailed analysis in a follow-up case-control association study.
Methods. Thirty-four single-nucleotide polymorphisms (SNPs) in the klotho gene were genotyped in 317 unrelated African American non-DM ESRD cases and 354 non-nephropathy controls, including 12 SNPs identified by re-sequencing a region around exon 4.
Results. Two SNPs demonstrated modest admixture-adjusted evidence of association with non-DM ESRD, rs650439 (P = 0.013, recessive model) and rs643780 (P = 0.017, recessive model), while rs17643698 approached significance (P = 0.0953, two degrees of freedom test). Eight of the most significant SNPs were tested for replication in a second case-control collection (557 African American non-DM ESRD cases and 187 controls), and there was no evidence of association in replicate cases and controls; nor when the samples were combined for a total of 874 non-DM cases and 541 controls. Cox proportional hazards models were computed to test for association between polymorphisms in klotho and age at onset of ESRD. A three-SNP haplotype, rs526906, rs525014 and rs571118 (T/T/A), was associated with age of onset of ESRD [P = 0.007, recessive model; hazard ratio (HR) = 0.70]. Subjects homozygous for this haplotype had a mean 4 years later onset of ESRD, suggesting a slower disease progression. HapMap subjects homozygous for this haplotype had increased expression of klotho, further supporting a protective role of this variant in ESRD.
Conclusion. We conclude that three SNPs in intron 1 of the klotho gene are associated with delayed age at onset of non-DM ESRD in African Americans.
Keywords: genetics, klotho, non-diabetic ESRD
Introduction
Non-diabetic forms of end-stage renal disease (non-DM ESRD), typically coded as hypertension-associated and secondary to chronic glomerular diseases, contribute to >40% of ESRD in African Americans. African Americans are particularly vulnerable to non-DM ESRD, having a 4-fold increased risk compared to European Americans. We [1] reported previously that African Americans are nine times more likely to develop ESRD if they have a family member with renal disease, even when access to healthcare, socioeconomic status, and increased prevalence and severity of hypertension in African Americans are considered.
In 2004, we [2] reported the first genome linkage scan for non-DM ESRD in African American families. This study identified evidence for linkage in four regions, 1q25.2, 9p21.3, 13q13.1 (with early onset ESRD) and 13q33.3 [with increased body mass index (BMI)]. Within the linkage interval, there were several genes which may play a functional role in an increased risk for ESRD. For example, podocin (NPHS2), (1q25.2), has been investigated in our non-DM ESRD African American cases [3]. Another gene, klotho, (13q33.3) appears to be an ideal functional candidate gene for association with ESRD.
Klotho is a β-glucoronidase which is associated with longevity [4], cardiovascular disease [5], and calcium and phosphorous homeostasis in the kidney (reviewed in [6]). Klotho is highly expressed by the kidney, and this expression is dramatically decreased in patients with chronic kidney disease [7]. Animal experiments have shown that increased expression of klotho in a rat model of renal disease resulted in improved renal function [8].
Several studies have identified polymorphisms in klotho and association with a variety of phenotypes. Two studies have reported an association between a functional variant of klotho, known as KL-VS, and longevity in Caucasians and African Americans [5,9]. KL-VS is also associated with cardiovascular disease in Caucasians and African Americans [10]. Two other variants, G-395A and C1818T, are associated with reduced cardiovascular disease rates in Korean women [11].
Given the high incidence of non-DM ESRD in African Americans, the biological characteristics of klotho and the location of this gene near a linkage peak, we tested klotho for association with non-DM ESRD in African Americans.
Materials and methods
Sample collection
A total of 874 African American cases with non-DM forms of ESRD were recruited from dialysis centres in North Carolina, South Carolina, Georgia, Virginia and Tennessee. Patients were classified as having non-DM ESRD if they had hypertension or chronic glomerular diseases listed as their primary cause of renal disease. Cases confirmed the onset of high blood pressure prior to development of ESRD, in the absence of other risk factors for kidney disease such as diabetes mellitus. Hypertension-associated ESRD was typically diagnosed in the presence of proteinuria ≤1.5 g/day, urinalysis ≤100 mg/dL albumin or spot urine protein:creatinine ratio ≤1.5 g/g when available, with either EKG evidence of left ventricular hypertrophy or presence of hypertensive retinopathy. Chronic glomerulonephritis was diagnosed in those with kidney biopsy evidence or higher levels of proteinuria. Patients with polycystic kidney disease, Alport’s syndrome, urologic disease or surgical nephrectomy were excluded. We also recruited 541 unrelated African American controls from hospital waiting rooms, churches and shopping malls in North Carolina. They were self-identified healthy African Americans born in North Carolina, age ≥18 years, and denying a personal or family history of kidney disease or diabetes in first degree relatives. Each participant provided 40 mL blood for DNA isolation. DNA was isolated from whole blood using an AutoPure LS automated DNA extraction robot (Gentra Systems, Minneapolis, MN, USA). Recruitment and sample collection procedures were approved by the Institutional Review Board at Wake Forest University.
SNP genotyping
A total of 22 SNPs from the klotho gene were initially genotyped in the first set of 317 non-diabetic ESRD cases and 354 controls. Sixteen of the SNPs were chosen as tagging SNPs based on their ability to capture genetic information in the Yoruba samples from Ibadan, Nigeria from HapMap (www.hapmap.org) using the program Tagger (Haploview [12]). Tagged SNPs had a minor allele frequency >0.1 and an inter-SNP r2 value <0.8. We also identified nine coding SNPs, five of which were synonymous and 4 which were non-synonymous. Of these 25 SNPs, two SNPs, G-395T and rs564481 (C1818T), were included because they have previously been associated with longevity [4] and cardiovascular disease [5]. The SNP rs9536314 was also included because it defines the functional KL-VS variant. Genotyping primers were designed using the MassARRAY Assay Design 3.4 software (Sequenom, San Diego, CA, USA), and sequences will be provided upon request. SNP genotyping was performed using the MassARRAY genotyping system (Sequenom, San Diego, CA, USA). Of the 25 SNPs, one SNP failed the design, and two others were not included in the analysis because they had genotyping efficiencies <95%.
Following the first round of genotyping, a small number of SNPs were modestly associated in and around exon 4. Exon 4 and the surrounding intronic regions were sequenced, and an additional 12 SNPs were identified. These SNPs were also genotyped in the 317 non-DM ESRD cases and 354 controls for a total of 34 genotyped SNPs.
In addition to the SNPs in the klotho gene, we also genotyped 70 ancestry informative markers (AIMS) in all non-DM ESRD cases and controls to estimate the percentage of African ancestry for each individual [13,14]. Individuals with <30% African ancestry as determined by the AIMS were removed from the study.
DNA sequencing
Exon 4 and the surrounding intronic junctions (~1751 bp) were sequenced to confirm SNP genotyping calls and identify SNPs in high linkage disequilibrium (LD) with tagged SNPs. The region was sequenced in 96 non-DM ESRD cases and 96 controls from the first round of genotyping. PCR primers were designed to amplify regions ~500 bases at a time and nested to provide a complete coverage of the region. Each 500 base region was PCR amplified, and the product was purified. DNA sequencing was performed using Big Dye Ready Reaction Mix on an ABI3730xl sequencer (Applied Biosystems, Foster City, CA). Sequence data was visualized using Sequencher software version 4.6 (GeneCodes Corporation, Ann Arbor, MI, USA). Primer sequences are available upon request.
Statistical analysis
Age, BMI, and percentage of African ancestry for the non-DM ESRD cases and healthy controls were compared using an unpaired t-test or a Mann–Whitney rank-sum test where appropriate (SigmaStat 3.5, SYSTAT software, San Jose, CA, USA). LD was determined using Haploview [12], with haplotypes defined using the method described by Gabriel [15]. Departures from Hardy–Weinberg equilibrium (HWE) were tested for each SNP using chi-square goodness-of-fit statistics in our association analysis programme SNPGWA [16]. A SNP was considered out of HWE if the P-value was less than the Bonferroni-corrected P = 0.05/34 = 0.0015. The percentage of African ancestry was calculated for each individual from the 70 genotyped AIMs using the statistical analysis programme Frappe [17]. Tests for genotypic association were performed on each SNP individually and included adjustment for the proportion of African ancestry for each individual. The overall two degrees of freedom genotype test of association and the three a priori genetic models (i.e. dominant, additive and recessive) were computed using SNPGWA with percentage of African ancestry as a covariate.
We computed a series of Cox proportional hazard models and the corresponding likelihood ratio statistics to test for associations between klotho polymorphisms and age at ESRD onset (age of initiation of dialysis) in the cases. Here, age at ESRD onset was contrasted with the age of the controls at the time of enrollment, and controls had ‘age at onset of ESRD’ censored at age at enrollment. The hazards ratio (HR) and corresponding 95% confidence interval (CI) were computed. Tests for association and the estimates for the HR were computed with adjustment for gender, BMI and percentage of African ancestry. As discussed, the genetic models are defined relative to the minor allele frequency.
Expression data
Klotho gene expression data from Yoruban HapMap (www.hapmap.org) individuals were obtained from the publicly available database at the Sanger Institute (http://www.sanger.ac.uk/humgen/genevar) [18]. Genotype calls for the SNPs (rs526906, rs525014 and rs571118) were obtained from the HapMap website. We compared the relative klotho expression levels from individuals possessing the protective alleles (T/T/A) versus all other individuals using an unpaired t-test (Sigma Stat).
Results
Biometric data
As summarized in Table 1, the controls were younger, had higher BMI and had a higher percentage of females compared to the non-DM ESRD cases. Mean percentage of African ancestry ranged from 78 ± 11 to 82 ± 10, and was slightly higher in the replicate and combined collections of non-DM ESRD cases.
Table 1.
Biometric data for African American non-DM ESRD cases and healthy controls
| Subjects | n | Age | BMI | % female | % African ancestry | AO ESRD |
|---|---|---|---|---|---|---|
| Initial genotyping | ||||||
| Controls | 354 | 49.9 ± 10.0 | 29.4 ± 6.7 | 46% | 78 ± 11 | - |
| Non-diabetic ESRD | 317 | 53.5 ± 14.8* | 26.8 ± 7.6* | 42% | 80 ± 10 | 47.5 ± 15.6 |
| Replicate genotyping | ||||||
| Controls | 187 | 52.0 ± 13.7 | 29.5 ± 6.8 | 58% | 79 ± 10 | - |
| Non-diabetic ESRD | 557 | 54.7 ± 14.4* | 27.0 ± 6.8* | 45% | 82 ± 10* | 49.3 ± 15.4 |
| Combined | ||||||
| Controls | 541 | 50.7 ± 11.6 | 29.5 ± 6.7 | 50% | 78 ± 11 | - |
| Non-diabetic ESRD | 874 | 54.2 ± 14.5* | 26.9 ± 7.1* | 44% | 81 ± 10* | 48.7 ± 15.5 |
AO ESRD, age of ESRD onset.
*P < 0.05 between non-DM ESRD cases and controls.
SNP genotyping
Initial genotyping consisted of 22 SNPs genotyped in 317 non-DM ESRD cases and 354 healthy controls. There was a modest evidence of an association between rs650439 and non-diabetic ESRD under the recessive model [P = 0.0135, odds ratio (OR) = 2.07, CI = 1.16–3.69, Table 2]. As this SNP is located near exon 4, this exon and its intronic junctions were sequenced to confirm genotyping calls and identify additional SNPs in the region (~1750 bp). Twelve additional SNPs were identified in the region of exon 4 after sequencing and were genotyped in the same set of 317 non-DM ESRD cases and 354 controls. These SNPs were chosen to increase the coverage of the 3′ region of the klotho gene and were in linkage disequilibrium with the associated SNP based on HapMap genotyping of the Yoruban samples (www.hapmap.org). One of these SNPs, rs643780, was modestly associated with non-DM ESRD under the recessive model (P = 0.0131, OR = 2.08, CI = 1.17–3.70). The other SNP, rs17643689, approached significance under the two degrees of freedom test (P = 0.0953). A walking window two-marker haplotype analysis was also performed, and no significant association was detected at any two consecutive SNPs (data not shown).
Table 2.
Minor allele frequency (MAF), Hardy–Weinberg equilibrium (HWE) and genotypic association for 34 SNPs genotyped in the klotho gene in 317 African American non-DM ESRD cases and 354 healthy African American controls
| SNP | Allele | MAF |
HWE |
Genotypic association |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r2 | Controls | Cases | Controls | Cases | 2 DF | Recessive | OR | CI | ||
| G-395A | G/A | 0.03 | 0.097 | 0.082 | 0.7545 | 1.0000 | 0.7021 | 0.8268 | 1.25 | 0.17–9.27 |
| rs2772364 | C/T | 0.04 | 0.193 | 0.190 | 0.2272 | 1.0000 | 0.6916 | 0.6916 | 1.2 | 0.48–3.02 |
| rs385564 | G/C | 0.03 | 0.259 | 0.268 | 0.2022 | 0.7711 | 0.7485 | 0.9643 | 0.99 | 0.55–1.77 |
| rs526906 | T/C | 0.55 | 0.496 | 0.483 | 0.6629 | 0.8153 | 0.8443 | 0.5939 | 0.9 | 0.62–1.31 |
| rs525014 | T/C | 0.19 | 0.347 | 0.337 | 0.0957 | 0.3662 | 0.1407 | 0.2160 | 1.37 | 0.83–2.24 |
| rs571118 | A/G | 0.17 | 0.333 | 0.343 | 0.7187 | 0.5267 | 0.8571 | 0.9051 | 0.97 | 0.59–1.60 |
| rs685417 | G/A | 0.18 | 0.392 | 0.372 | 0.5693 | 0.9014 | 0.6872 | 0.9718 | 0.99 | 0.63–1.55 |
| rs9526992 | G/A | 0.09 | 0.234 | 0.209 | 0.2955 | 0.6037 | 0.3444 | 0.7799 | 1.11 | 0.53–2.32 |
| rs2516571 | A/G | 0.13 | 0.224 | 0.247 | 0.4372 | 0.0834 | 0.0852 | 0.2483 | 0.65 | 0.31–1.35 |
| rs2320762 | T/G | 0.02 | 0.291 | 0.294 | 0.6945 | 0.4787 | 0.8778 | 0.7405 | 0.91 | 0.50–1.63 |
| rs9536282 | C/T | 0.08 | 0.295 | 0.277 | 0.3649 | 1.0000 | 0.6325 | 0.9769 | 1.01 | 0.56–1.83 |
| rs520103 | A/T | 0.22 | 0.174 | 0.162 | 0.5712 | 0.2890 | 0.2824 | 0.1198 | 0.43 | 0.15–1.24 |
| rs657049 | A/G | 0.20 | 0.458 | 0.422 | 0.5014 | 0.8113 | 0.3296 | 0.1368 | 0.74 | 0.49–1.10 |
| rs9527023 | T/C | 0.99 | 0.180 | 0.168 | 0.7127 | 0.2985 | 0.8167 | 0.9073 | 1.05 | 0.45–2.43 |
| rs9536314 | T/G | 1.00 | 0.187 | 0.168 | 0.7277 | 0.8411 | 0.7452 | 0.6000 | 0.79 | 0.33–1.89 |
| rs9527025 | G/C | 0.29 | 0.184 | 0.166 | 1.0000 | 0.8378 | 0.7393 | 0.7309 | 0.86 | 0.35–2.07 |
| rs2149860 | A/G | 0.00 | 0.439 | 0.408 | 0.8271 | 1.0000 | 0.4759 | 0.3644 | 0.83 | 0.55–1.25 |
| rs522796 | T/C | 0.20 | 0.455 | 0.488 | 0.6626 | 0.1633 | 0.4754 | 0.2260 | 1.26 | 0.87–1.82 |
| rs9527032a | G/C | 0.23 | 0.189 | 0.173 | 0.5966 | 1.0000 | 0.7487 | 0.4790 | 0.73 | 0.31–1.73 |
| rs9596717a | A/G | 0.00 | 0.056 | 0.044 | 1.0000 | 1.0000 | 0.8250 | - | - | - |
| T5266Aa | T/A | 0.00 | 0.014 | 0.008 | 1.0000 | 1.0000 | - | - | - | - |
| rs564481 | C/T | 0.00 | 0.088 | 0.104 | 0.0922 | 0.0029 | 0.8206 | - | - | - |
| C365Ta | C/T | 0.01 | 0.007 | 0.003 | 1.0000 | 1.0000 | - | - | - | - |
| rs648202 | C/T | 0.12 | 0.393 | 0.440 | 0.4999 | 0.9069 | 0.2497 | 0.1108 | 1.4 | 0.93–2.13 |
| rs649964 | C/T | 0.25 | 0.079 | 0.087 | 0.7083 | 0.4903 | 0.5785 | 0.2957 | 3.36 | 0.35–32.5 |
| rs650439 | A/T | 0.02 | 0.257 | 0.292 | 0.5706 | 0.0172 | 0.0413 | 0.0135 | 2.07 | 1.16–3.69 |
| C113Ta | C/T | 0.00 | 0.044 | 0.039 | 0.4998 | 1.0000 | 0.9412 | - | - | - |
| rs11840531a | T/C | 1.00 | 0.045 | 0.029 | 0.1395 | 1.0000 | 0.4966 | - | - | - |
| rs11840946a | T/C | 0.02 | 0.046 | 0.029 | 0.1478 | 1.0000 | 0.4359 | - | - | - |
| rs643780a | G/A | 0.32 | 0.257 | 0.297 | 0.4839 | 0.0380 | 0.0450 | 0.0131 | 2.08 | 1.17–3.70 |
| rs17643689a | T/G | 0.03 | 0.104 | 0.118 | 0.5594 | 0.0124 | 0.0953 | 0.0327 | 5.37 | 1.15–25.1 |
| rs677332a | A/G | 0.17 | 0.175 | 0.161 | 0.5774 | 0.1354 | 0.2676 | 0.1160 | 0.4 | 0.13–1.26 |
| rs582524a | A/G | 0.15 | 0.441 | 0.477 | 0.8252 | 0.1703 | 0.2461 | 0.0966 | 1.38 | 0.94–2.01 |
| rs534184a | G/T | - | 0.212 | 0.175 | 0.0762 | 1.0000 | 0.2540 | 0.1076 | 0.52 | 0.23–1.16 |
Genotypic association is shown after adjustment for African ancestry. Results from the two degrees of freedom test (2 DF) and recessive model are shown. Inter-SNP r2 is shown for the designated SNP and the SNPs immediately following.
OR, odds ratio; CI, confidence interval.
aSNPs that were identified in exon 4 and surrounding introns through sequencing.
The LD structure of the klotho gene reveals eight haplotype blocks, all of which are relatively small, and the largest consisting of four SNPs (Supplementary Figure 1, see online supplementary material for a colour version of this figure). The modestly associated SNP, rs650439, is not within any of the defined haplotype blocks, and there is no indication of strong LD with any other SNPs. The other two SNPs, rs643780 and rs17643689, are in a haplotype block with one other SNP, rs677332, at the 3′ end of the gene. Association analysis with this 3′ SNP haplotype did not reveal any evidence of association (P = 0.476).
Because the detected association and our sample sizes were moderate, we genotyped the most associated SNPs in a replicate collection of 557 non-DM ESRD cases and 187 controls. A total of 8 SNPs were genotyped in the replicate samples (Table 3). One SNP, rs582524, deviated from Hardy–Weinberg equilibrium in the cases (P < 0.0001), but not controls, and none of the SNPs were associated with non-DM ESRD in these samples. We combined the genotype data from the initial set of samples as well as the replicate set and analysed for association in all 874 non-DM ESRD cases and 541 controls. None of the SNPs were associated with non-DM ESRD.
Table 3.
Hardy–Weinberg equilibrium (HWE), minor allele frequency (MAF) and genotypic association (GA) for the most significant SNPs replicated in 557 African American non-DM ESRD cases and 187 healthy African American controls
| SNP | Replicate non-DM ESRD cases (557) and controls (187) |
Combined non-DM ESRD cases (874) and controls (541) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAF |
HWE |
GA | MAF |
HWE |
GA | |||||
| Controls | Cases | Controls | Cases | 2 DF | Controls | Cases | Controls | Cases | 2 DF | |
| rs2516571 | 0.246 | 0.231 | 0.413 | 1.000 | 0.684 | 0.232 | 0.236 | 0.902 | 0.288 | 0.606 |
| rs2149860 | 0.389 | 0.446 | 0.422 | 0.794 | 0.179 | 0.422 | 0.433 | 0.528 | 0.833 | 0.896 |
| rs564481 | 0.049 | 0.059 | 1.000 | 0.245 | 2.000 | 0.076 | 0.076 | 0.103 | 0.081 | 0.843 |
| rs648202 | 0.457 | 0.398 | 0.875 | 0.787 | 0.133 | 0.413 | 0.413 | 0.522 | 0.721 | 0.771 |
| rs650439 | 0.253 | 0.259 | 1.000 | 0.739 | 0.946 | 0.255 | 0.271 | 0.565 | 0.224 | 0.376 |
| rs643780 | 0.269 | 0.256 | 0.068 | 1.000 | 0.622 | 0.263 | 0.279 | 0.093 | 1.000 | 0.507 |
| rs17643689 | 0.078 | 0.093 | 0.601 | 0.445 | 0.781 | 0.096 | 0.102 | 0.298 | 0.023 | 0.122 |
| rs582524 | 0.380 | 0.387 | 0.122 | 0.000 | 0.759 | 0.422 | 0.423 | 0.308 | 0.047 | 0.863 |
We also analysed an association at these SNPs using the original and replicate non-diabetic ESRD cases (n = 874) and controls (n = 541) combined. Genotypic association is shown after adjustment for proportion of African ancestry.
2 DF, two degrees of freedom test.
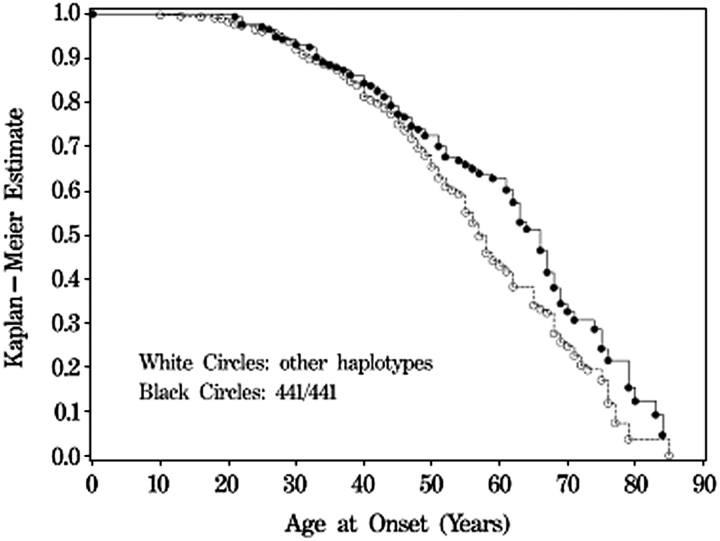
We tested each SNP for association with age at onset of ESRD using a Cox proportional hazard model. Tests for association with individual SNPs revealed significance at three SNPs (rs526906, rs525014 and rs571118; P = 0.004, 0.021 and 0.004, respectively) under the dominant model and modest significance at 5 SNPs (rs522796, rs9527032, rs564481, rs17643689 and rs582524; P = 0.034, 0.011, 0.036, 0.019 and 0.020, respectively) under the recessive model (Table 4). The proximity and high significance of the three SNPs associated under the dominant model led us to perform the haplotype analysis for an association with earlier age at onset of ESRD (Table 5). Haplotype analysis revealed significant association with the T/T/A haplotype under the recessive (P = 0.007, HR = 0.70) and additive (P = 0.009, HR = 0.80) models. We attempted to replicate this association in the expanded set of non-DM ESRD cases (n = 557), using two of the three SNPs which compose the haplotype, rs526906 and rs525014 (rs571118 was not able to be genotyped in the replicate samples). This two SNP haplotype (T/T) was not associated with age at onset of ESRD in the replicate population (P = 0.89, HR = 1.01 and P = 0.95, HR = 1.00, recessive and additive models, respectively). When the case samples were combined, the two SNP haplotype was still modestly associated with age at onset of ESRD, (P = 0.042, HR = 0.838 recessive model; P = 0.058, HR = 0.903 additive model). When the Kaplan–Meier estimates are plotted versus the age at onset of ESRD, it is clear that cases that are homozygous for the T/T/A (4/4/1) haplotype have an average age at onset that is 4 years later compared to cases with the other four haplotypes, indicating later onset or slowed progression of kidney disease (Figure 1). Kaplan–Meier plots were performed for the individual SNPs, and show a similar delay in onset of ESRD for individuals homozygous for the protective alleles (Supplementary Figure 2, see online supplementary material for a colour version of this figure).
Table 4.
Survival analysis to estimate risk of early age at onset of non-diabetic end stage renal disease in 317 non-DM ESRD cases and 354 healthy controls
| SNP | Alleles | Dominant model |
Recessive model |
Additive model |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P-value | HR | CI | P-value | HR | CI | P-value | HR | CI | ||
| G-395T | G/A | 0.968 | 0.99 | 0.820 | 1.18 | 0.993 | 1.00 | |||
| rs2772364 | C/T | 0.100 | 0.81 | 0.632 | 1.18 | 0.185 | 0.86 | |||
| rs385564 | G/C | 0.166 | 1.18 | 0.633 | 1.11 | 0.194 | 1.13 | |||
| rs526906 | T/C | 0.004 | 1.51 | (1.14, 1.99) | 0.152 | 1.24 | 0.006 | 1.27 | (1.07, 1.51) | |
| rs525014 | T/C | 0.021 | 1.33 | (1.04, 1.69) | 0.124 | 1.34 | 0.014 | 1.25 | (1.05, 1.49) | |
| rs571118 | A/G | 0.004 | 1.41 | (1.11, 1.79) | 0.413 | 1.18 | 0.010 | 1.26 | (1.06, 1.51) | |
| rs685417 | G/A | 0.078 | 1.24 | 0.214 | 1.25 | 0.057 | 1.18 | |||
| rs9526992 | G/A | 0.274 | 0.87 | 0.597 | 1.15 | 0.464 | 0.93 | |||
| rs2516571 | A/G | 0.582 | 0.93 | 0.204 | 0.66 | 0.347 | 0.91 | |||
| rs2320762 | T/G | 0.062 | 1.26 | 0.454 | 1.20 | 0.067 | 1.20 | |||
| rs9536282 | C/T | 0.118 | 0.83 | 0.274 | 0.78 | 0.092 | 0.85 | |||
| rs520103 | A/T | 0.866 | 0.98 | 0.069 | 0.35 | 0.451 | 0.92 | |||
| rs657049 | A/G | 0.697 | 0.95 | 0.189 | 0.81 | 0.314 | 0.92 | |||
| rs9527023 | T/C | 0.929 | 1.01 | 0.272 | 1.41 | 0.671 | 1.05 | |||
| rs9536314 | T/G | 0.979 | 1.00 | 0.401 | 1.33 | 0.824 | 1.03 | |||
| rs9527025 | G/C | 0.839 | 0.97 | 0.294 | 1.43 | 0.907 | 1.01 | |||
| rs2149860 | A/G | 0.649 | 1.06 | 0.347 | 1.17 | 0.418 | 1.07 | |||
| rs522796 | T/C | 0.300 | 1.15 | 0.034 | 1.35 | (1.02, 1.77) | 0.061 | 1.17 | ||
| rs648202 | G/C | 0.966 | 1.01 | 0.385 | 1.34 | 0.771 | 1.03 | |||
| rs649964 | A/G | 0.756 | 1.07 | 0.969 | 0.00 | 0.812 | 1.05 | |||
| rs650439 | T/A | 0.182 | 0.55 | . | . | 0.182 | 0.55 | |||
| rs9527032 | C/T | 0.142 | 1.26 | 0.011 | 2.62 | (1.24, 5.53) | 0.043 | 1.32 | (1, 1.72) | |
| rs9596717 | C/T | 0.372 | 0.53 | . | . | 0.372 | 0.53 | |||
| T5266A | C/T | 0.584 | 0.93 | 0.198 | 1.21 | 0.727 | 1.03 | |||
| rs564481 | C/T | 0.738 | 0.95 | 0.036 | 3.40 | (1.08, 10.69) | 0.991 | 1.00 | ||
| C365T | A/T | 0.997 | 1.00 | 0.103 | 1.36 | 0.476 | 1.07 | |||
| C113T | C/T | 0.918 | 0.98 | 0.980 | 0.00 | 0.896 | 0.97 | |||
| rs11840531 | T/C | 0.224 | 0.73 | 0.972 | 0.00 | 0.192 | 0.72 | |||
| rs11840946 | T/C | 0.199 | 0.72 | 0.972 | 0.00 | 0.171 | 0.71 | |||
| rs643780 | G/A | 0.667 | 1.05 | 0.076 | 1.40 | 0.270 | 1.11 | |||
| rs17643689 | T/G | 0.568 | 1.09 | 0.019 | 2.48 | (1.16, 5.28) | 0.268 | 1.16 | ||
| rs677332 | A/G | 0.873 | 1.02 | 0.239 | 0.43 | 0.875 | 0.98 | |||
| rs582524 | A/G | 0.823 | 1.03 | 0.020 | 1.38 | (1.05, 1.8) | 0.139 | 1.13 | ||
| rs534184 | G/T | 0.976 | 1.00 | 0.665 | 0.85 | 0.874 | 0.98 | |||
P-values and hazard ratios (HR) are reported for the dominant, additive and recessive models both before and after adjustment for sex, BMI and African ancestry. Confidence intervals (CI) are reported for significant P-values. Alleles are shown with the major allele, followed by the minor allele (the minor allele was the reference allele for the association tests).
Table 5.
Survival analysis to determine the association with a three-SNP haplotype, rs526906, rs525014 and rs571118, with early age at onset of non-diabetic ESRD
| Haplotype |
Freq | Dominant |
Recessive |
Additive |
|||||
|---|---|---|---|---|---|---|---|---|---|
| rs526906 | rs525014 | rs571118 | P-value | HR | P-value | HR | P-value | HR | |
| T | T | A | 0.52 | 0.143 | 0.81 | 0.007 | 0.70 | 0.009 | 0.80 |
| C | T | G | 0.016 | 0.365 | 1.13 | 0.967 | 0.98 | 0.420 | 1.10 |
| C | C | A | 0.12 | 0.584 | 1.08 | 0.386 | 1.66 | 0.494 | 1.09 |
| C | C | G | 0.22 | 0.013 | 1.36 | 0.374 | 1.28 | 0.017 | 1.27 |
P-values and hazard ratios (HR) are reported for the dominant, recessive and additive genetic models.
Fig. 1.
Kaplan–Meier plot for age of onset of ESRD for the three-SNP haplotype (rs526906, rs525014 and rs571118) under the recessive model for association. Shown are values for individuals who are homozygous for the T/T/A (4/4/1) haplotype versus all other haplotypes (317 non-DM ESRD cases and 354 controls).
Gene expression
In the HapMap individuals, klotho gene expression was increased in individuals homozygous for the protective alleles (T/T/A) at each of the three SNPs, rs526906 (n = 24, P = 0.003), rs525014 (n = 37, P = 0.022) and rs571118 (n = 31 P = 0.075) (Figure 2), supporting a protective role for this SNP. The difference in gene expression was similar when individuals with the T/T/A haplotype (n = 24) compared to all other genotypes (P = 0.003; Supplementary Figure 3, see online supplementary material for a colour version of this figure).
Fig. 2.
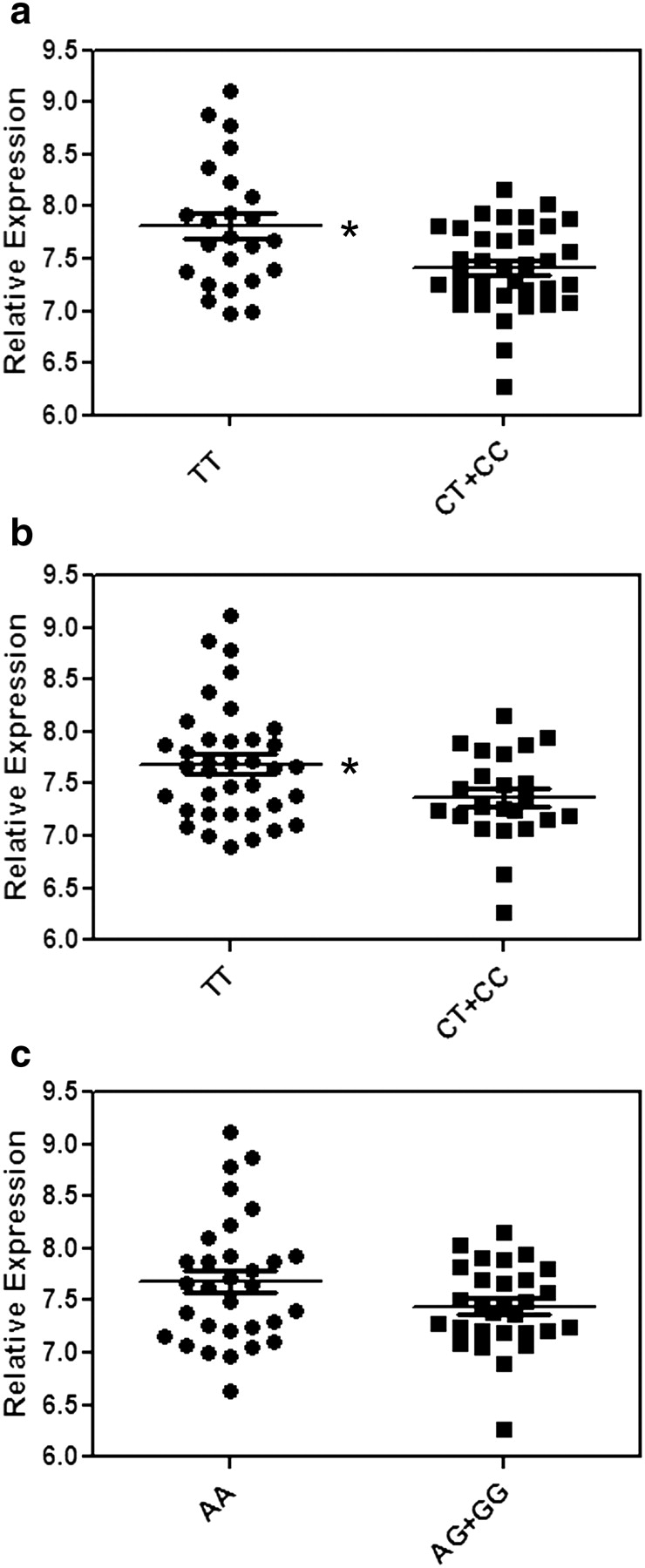
Relative klotho mRNA expression from EBV-transformed lymphoblastoid cell lines from 60 unrelated Yoruba people of Ibadan, Nigeria from HapMap, segregated by genotype. Data for the three SNPs are shown, rs526906 (P = 0.003) (a), rs525014 (P = 0.022) (b) and rs571118 (P = 0.075) (c), comparing individuals homozygous for the protective allele to individuals with all other genotypes. Expression data were normalized using Illumina BeadStudio (Illumina Inc, San Diego, CA, USA) output as described in [18].
Discussion
In this study, we tested SNPs in the positional and functional candidate gene klotho for an association with non-DM ESRD in African Americans. A total of 34 SNPs were genotyped in the initial collection of 317 non-DM ESRD cases and 354 controls. Three SNPs were modestly associated under the recessive model. We tested seven of the most associated SNPs in a replicate sample of 557 non-DM ESRD cases and 187 healthy controls. None of these SNPs was associated in the replicate set of samples, nor were they associated when data from the two collections were combined. In addition, haplotype analysis did not reveal any combination of SNPs which was associated with risk. The total sample size of 874 cases and 541 controls provided adequate power (>80%) to detect a significant association for odds ratios in the range of 1.35–1.63.
Three of the SNPs chosen for analysis, G-395A, rs564481 (C1818T) and rs9536314 (KL-VS), were genotyped because they have been previously associated with a variety of phenotypes, including longevity, cardiovascular disease and osteoporosis. KL-VS is a haplotype variant which consists of two amino acid substitutions (F352V and C370S) and can be defined by a single SNP, rs9536314. Several groups have associated KL-VS status with longevity [5,9], coronary artery disease [10] and bone mineral density [19,20]. C1818T (rs564481) is a silent mutation located in exon 4. This variant is less well studied, but the T allele has been shown to be associated with a reduced risk of coronary artery disease in Korean women [11] and with bone density in European-derived and Japanese women [21]. G-395A is a promoter SNP that has been shown to be associated with cardiovascular disease in Korean women [11] and bone density in post-menopausal European-derived and Asian women [21,22]. As bone mineral metabolism could be affected by renal calcium homeostasis, and cardiovascular disease has several vascular similarities to renal disease, these three markers were included in the study. Despite the ample evidence for an association of these markers and other phenotypes, we did not detect an association with any of these polymorphisms and non-DM ESRD in African Americans.
Despite the lack of evidence for a direct association of klotho polymorphisms with non-diabetic ESRD, it is possible that polymorphisms in the klotho gene may affect the severity of ESRD. Recently, Friedman et al. [23] demonstrated an association between the klotho SNP rs577916 and increased risk for mortality after initiation of haemodialysis in European Americans and Asians [23], indicating that klotho may play a role in the severity of the disease. Therefore, we tested the 34 genotyped SNPs in klotho for an association with age at onset of ESRD in our initial case population (n = 317) and detected significant association at several SNPs. The most interesting association was with the three SNPs located in intron 1 of the klotho gene (rs526906, rs525014 and rs571118). The three SNPs are 1.25 kb apart and the first SNP is 7.6 kb 3′ of exon 1 (Supplementary Figure 1, see online supplementary material for a colour version of this figure). The first two SNPs are in a small LD block, and the first and third SNP are in the high LD. The association of the individual SNP, rs526906, is stronger than the haplotype association, indicating that this SNP is driving the significant P-value. One weakness of this study, however, is that neither the individual SNP nor the haplotype association survives a strict Bonferroni-corrected association (P = 0.05/34 = 0.001), and the results, therefore, must be interpreted with caution.
We do not have klotho gene expression data for individuals in our study; however, there is data available from Epstein–Barr virus (EBV)-transformed lymphoblastoid cell lines derived from HapMap samples as part of the Genevar Project at the Wellcome Trust Sanger Institute [18]. Using the genotype data from these individuals, we were able to show that individuals homozygous for the protective alleles at all three SNPs (rs526906, rs525014 and rs571118) have increased klotho expression compared to individuals who are heterozygous or homozygous for the alternative alleles at these SNPs. As the associated SNPs are intronic, the genetic mechanism behind the increased expression is unknown. Functionally, klotho acts as a cofactor, increasing the affinity of the FGF23 ligand for the FGF receptor. FGF receptor binding initiates a signalling cascade that ultimately leads to increased renal phosphate excretion and reduced phosphate intestinal absorption resulting from lower levels of 1,25 dihydroxyvitamin D, thereby maintaining phosphate homeostasis [24,25]. The expression data we have presented in this manuscript have been obtained from cell lines and not directly from kidney tissue, and therefore must be viewed with caution. However, we can hypothesize that patients with higher levels of klotho expression prior to the progression to ESRD may be protected by an enhanced ability to regulate phosphate homeostasis.
We and others have shown that polymorphisms in the non-muscle myosin gene (MYH9) are highly associated with non-diabetic forms of ESRD in African Americans [26–28] and account for 70% of the risk of non-diabetic renal disease in this population [26]. Because a high level of risk may mask additional association with other genes, we tested klotho SNPs for association with non-diabetic ESRD in high-risk cases and controls (individuals homozygous for the E1 risk haplotype in MYH9). We also tested cases and controls that were protected (had fewer than four E1 risk alleles). No evidence of an association was detected with non-diabetic ESRD in either of these groups (data not shown). Additionally, tests for interaction between the associated klotho SNPs and the MYH9 risk SNPs (rs4821480, rs2032487, rs4821481 and rs3752462) revealed no evidence of interaction between the two genes.
In summary, we have systematically tested the functional and positional candidate gene klotho for an association with non-DM ESRD in African Americans. While we failed to detect a direct association with the presence of non-diabetic ESRD, we were able to detect association of a three-SNP haplotype with age at onset of ESRD, indicating that klotho may be involved in the rate of progression of this family of kidney diseases. We were also able to show that this haplotype is associated with increased klotho expression, supporting a protective role of this gene in progression of ESRD. Although we were unable to specifically identify the causal variant in the klotho gene, this work provides important information in understanding how renal disease progresses to end-stage kidney failure in African Americans.
Supplementary Material
Acknowledgments
This study was supported in part by NIH grants R01 DK 070941 (BIF) and R01 DK53591 (DWB), and by the NIDDK and NCI Intramural Research Programs. M.A.B. was supported by F32 DK080617 from the NIDDK. We would like to gratefully acknowledge the contributions of the participants as well as the physicians who were part of the study and the work of our study coordinators Joyce Byers, Carrie Smith, Mitzie Spainhour, Cassandra Bethea and Sharon Warren.
Supplimentary Data
Supplementary data is available online at http://ndt.oxfordjournals.org.
Conflict of interest statement. None declared.
References
- 1.Freedman BI, Soucie JM, Stone SM, et al. Familial clustering of end-stage renal disease in blacks with HIV-associated nephropathy. Am J Kidney Dis. 1999;34:254–258. doi: 10.1016/s0272-6386(99)70352-5. [DOI] [PubMed] [Google Scholar]
- 2.Freedman BI, Langefeld CD, Rich SS, et al. A genome scan for ESRD in black families enriched for nondiabetic nephropathy. J Am Soc Nephrol. 2004;15:2719–2727. doi: 10.1097/01.ASN.0000141312.39483.4F. [DOI] [PubMed] [Google Scholar]
- 3.Dusel JA, Burdon KP, Hicks PJ, et al. Identification of podocin (NPHS2) gene mutations in African Americans with nondiabetic end-stage renal disease. Kidney Int. 2005;68:256–262. doi: 10.1111/j.1523-1755.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 5.Arking DE, Atzmon G, Arking A, et al. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 6.Torres PU, Prie D, Molina-Bletry V, et al. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 2007;71:730–737. doi: 10.1038/sj.ki.5002163. [DOI] [PubMed] [Google Scholar]
- 7.Koh N, Fujimori T, Nishiguchi S, et al. Severely reduced production of klotho in human chronic renal failure kidney. Biochem Biophys Res Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 8.Mitani H, Ishizaka N, Aizawa T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension. 2002;39:838–843. doi: 10.1161/01.hyp.0000013734.33441.ea. [DOI] [PubMed] [Google Scholar]
- 9.Arking DE, Krebsova A, Macek M, Sr., et al. Association of human aging with a functional variant of klotho. Proc Natl Acad Sci USA. 2002;99:856–861. doi: 10.1073/pnas.022484299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arking DE, Becker DM, Yanek LR, et al. KLOTHO allele status and the risk of early-onset occult coronary artery disease. Am J Hum Genet. 2003;72:1154–1161. doi: 10.1086/375035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhee EJ, Oh KW, Yun EJ, et al. Relationship between polymorphisms G395A in promoter and C1818T in exon 4 of the KLOTHO gene with glucose metabolism and cardiovascular risk factors in Korean women. J Endocrinol Invest. 2006;29:613–618. doi: 10.1007/BF03344160. [DOI] [PubMed] [Google Scholar]
- 12.Barrett JC, Fry B, Maller J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 13.Keene KL, Mychaleckyj JC, Leak TS, et al. Exploration of the utility of ancestry informative markers for genetic association studies of African Americans with type 2 diabetes and end stage renal disease. Hum Genet. 2008;124:147–154. doi: 10.1007/s00439-008-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keene KL, Mychaleckyj JC, Smith SG, et al. Association of the distal region of the ectonucleotide pyrophosphatase/phosphodiesterase 1 gene with type 2 diabetes in an African-American population enriched for nephropathy. Diabetes. 2008;57:1057–1062. doi: 10.2337/db07-0886. [DOI] [PubMed] [Google Scholar]
- 15.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 16.Matarin M, Brown WM, Scholz S, et al. A genome-wide genotyping study in patients with ischaemic stroke: initial analysis and data release. Lancet Neurol. 2007;6:414–420. doi: 10.1016/S1474-4422(07)70081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keene K, Mychaleckyj J, Leak T, et al. Exploration of the utility of ancestry informative markers for genetic association studies of African Americans with type 2 diabetes. 2008;124:197–198. doi: 10.1007/s00439-008-0532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stranger BE, Nica AC, Forrest MS, et al. Population genomics of human gene expression. Nat Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riancho JA, Valero C, Hernandez JL, et al. Association of the F352V variant of the Klotho gene with bone mineral density. Biogerontology. 2007;8:121–127. doi: 10.1007/s10522-006-9039-5. [DOI] [PubMed] [Google Scholar]
- 20.Zarrabeitia MT, Hernandez JL, Valero C, et al. Klotho gene polymorphism and male bone mass. Calcif Tissue Int. 2007;80:10–14. doi: 10.1007/s00223-006-0233-x. [DOI] [PubMed] [Google Scholar]
- 21.Kawano K, Ogata N, Chiano M, et al. Klotho gene polymorphisms associated with bone density of aged postmenopausal women. J Bone Miner Res. 2002;17:1744–1751. doi: 10.1359/jbmr.2002.17.10.1744. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Ando F, Niino N, et al. Association of polymorphisms of the androgen receptor and klotho genes with bone mineral density in Japanese women. J Mol Med. 2005;83:50–57. doi: 10.1007/s00109-004-0578-4. [DOI] [PubMed] [Google Scholar]
- 23.Friedman DJ, Afkarian M, Tamez H, et al. Klotho variants and chronic hemodialysis mortality. J Bone Miner Res. 2009;24:1847–1855. doi: 10.1359/JBMR.090516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drueke TB, Prie D. Klotho spins the thread of life–what does Klotho do to the receptors of fibroblast growth factor-23 (FGF23)? Nephrol Dial Transplant. 2007;22:1524–1526. doi: 10.1093/ndt/gfm122. [DOI] [PubMed] [Google Scholar]
- 25.Kuro-o M. Klotho in chronic kidney disease—what’s new? Nephrol Dial Transplant. 2009;24:1705–1708. doi: 10.1093/ndt/gfp069. [DOI] [PubMed] [Google Scholar]
- 26.Kopp JB, Smith MW, Nelson GW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao WH, Klag MJ, Meoni LA, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman BI, Hicks PJ, Bostrom MA, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.