Abstract
Background
The survival of Ewing sarcoma (ES) patients has improved since the 1970s but is associated with considerable future health risks.
Methods
The study population consisted of long-term (≥5-year) survivors of childhood ES diagnosed before age 21 from 1970 to 1986. Cause-specific mortality was evaluated in eligible survivors (n = 568), and subsequent malignant neoplasms, chronic health conditions, infertility, and health status were evaluated in the subset participating in the Childhood Cancer Survivor Study (n = 403). Outcomes were compared with the US population and sibling control subjects (n = 3899). Logistic, Poisson, or Cox proportional hazards models, with adjustments for sex, age, race/ethnicity, and potential intrafamily correlation, were used. Statistical tests were two-sided.
Results
Cumulative mortality of ES survivors was 25.0% (95% confidence interval [CI] = 21.1 to 28.9) 25 years after diagnosis. The all-cause standardized mortality ratio was 13.3 (95% CI = 11.2 to 15.8) overall, 23.1 (95% CI = 17.6 to 29.7) for women, and 10.0 (95% CI = 7.9 to 12.5) for men. The nonrecurrence-progression non-external cause standardized mortality ratio (subsequent non-ES malignant neoplasms and cardiac and pulmonary causes potentially attributable to ES treatment) was 8.7 (95% CI = 6.2 to 12.0). Twenty-five years after ES diagnosis, cumulative incidence of subsequent malignant neoplasms, excluding nonmelanoma skin cancers, was 9.0% (95% CI = 5.8 to 12.2). Compared with siblings, survivors had an increased risk of severe, life-threatening, or disabling chronic health conditions (relative risk = 6.0, 95% CI = 4.1 to 9.0). Survivors had lower fertility rates (women: P = .005; men: P < .001) and higher rates of moderate to extreme adverse health status (P < .001).
Conclusion
Long-term survivors of childhood ES exhibit excess mortality and morbidity.
CONTEXTS AND CAVEATS
Prior knowledge
Advances in therapy for pediatric Ewing sarcoma (ES) patients have improved long-term survival, but the health risks for adult survivors have not been systematically studied.
Study design
Cause-specific mortality and health status of a cohort of long-term (≥5 years) survivors of childhood ES from the US Childhood Cancer Survivor Study (CCSS) were compared with those of sibling control subjects and the US population.
Contribution
Compared with siblings and the US population, ES survivors had higher mortality and higher risk of morbidity because of treatment, second malignancies, chronic health conditions, and functional impairment.
Implications
Long-term follow-up and late effect interventions are needed to improve the health of all ES survivors.
Limitations
The CCSS cohort is institution based rather than population based, so it is possible that the study survivor population was not representative of the overall survivor population. Health status was self-reported, which may introduce reporting bias. Treatments for ES have changed since 1986; thus, treatment-related morbidity may be different for more recent survivors.
From the Editors
The Ewing family of tumors is the second most common primary osseous malignancy in childhood and adolescence (1). Classically, these tumors originate in bone, although they can also occur in soft tissue. The annual incidence of Ewing sarcoma (ES) in the United States is 2.93 per million children (2). Since 1970, the survival of patients with ES has increased substantially. Presently, the 5-year overall survival rate for localized ES is about 75% (3). These tumors are aggressive, and multimodality therapy is always required, involving the use of chemotherapy and some form of local therapy (surgery and/or radiation).
With recent advances in treatment and a substantial increase in survival, oncologists have begun to focus on treatment-related complications in patients who are long-term survivors. Therapy-related morbidities include outcomes such as second (and subsequent) malignant neoplasms, cardiac toxicity, and infertility. Our understanding of the long-term outcomes among childhood ES survivors is based on small single-institution studies (4–8) or studies focused solely on second malignant neoplasms (9–15). The small number of patients diagnosed with ES, and an even smaller number of survivors, complicates the study of disease-specific late effects. To expand our understanding, this study aimed to assess selected key outcomes (late mortality, subsequent malignant neoplasms, chronic health conditions, fertility, and health status) among long-term ES survivors from the Childhood Cancer Survivor Study (CCSS) (www.stjude.org/ccss).
Methods
Childhood Cancer Survivor Study
The CCSS is a retrospectively ascertained and prospectively followed cohort of long-term survivors who were treated for childhood cancer between 1970 and 1986 at one of the 26 participating institutions in the United States and Canada (listed in Appendix 1). All participants were diagnosed before 21 years of age with leukemia, brain tumor, Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, or bone tumor and survived at least 5 years following diagnosis (16,17). To enable comparisons with a population that had not been treated for cancer, a random sample of CCSS survivors was asked to identify a nearest-age living sibling for inclusion in the CCSS sibling cohort. The Human Subjects Committee at each participating institution approved the CCSS protocol, and all participants (or a parent or guardian of participants below age 18) provided informed consent before the study.
The CCSS baseline questionnaire, administered to the majority of the cohort by mail and telephone interview between 1994 and 1996, collected self-reported information about demographic data, medical care, medical conditions, health behaviors, and second or subsequent cancers. For each survivor in the CCSS, details of the primary cancer diagnosis and treatment were abstracted from medical records. Cumulative dose data were collected for 22 specific chemotherapy agents, and qualitative (yes/no) data were collected for 20 additional agents. Exposure to anthracyclines was expressed as the cumulative dose received. Exposure to alkylating agents was expressed as a total score based on the tertiles of various alkylating agents received, according to methods reported previously (18). Radiation therapy records were photocopied and sent to the CCSS Radiation Physics Center to abstract relevant data and assess patient exposures in terms of field size, site, and dose. Copies of all questionnaires and medical abstract forms are available at www.stjude.org/ccss.
ES Study Population
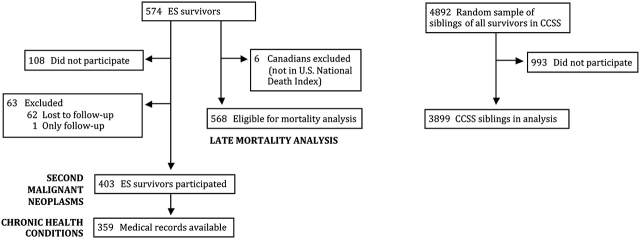
Of the 574 ES survivors eligible for the CCSS cohort, 62 (10.8%) were not available for follow-up despite extensive efforts to locate them (Figure 1). Among the remaining 512 subjects, 403 (78.7%) ES survivors enrolled in the CCSS, 108 (21.1%) declined participation, and one (<1%) participated only in the follow-up surveys. A comparison group of nearest-age siblings was identified from randomly selected survivors from the entire CCSS cohort. Of 4782 eligible siblings, 3899 (81.5%) participated. To define the potential for introducing bias among the studied cohort, we previously compared demographic and cancer-related characteristics among participants, nonparticipants, and those who could not be located. These three groups were found to be very similar with regard to sex, age at diagnosis, age when asked to participate in the study (or for those lost to follow-up, age when cohort was assembled), and type of cancer treatment (16,17,19).
Figure 1.
Flow diagram of Ewing sarcoma (ES) survivors and siblings in the Childhood Cancer Survivor Study (CCSS).
Assessment of late mortality included 568 of the 574 eligible ES survivors after excluding six Canadians who were not listed in the US National Death Index (http://www.cdc.gov/nchs/ndi.htm). Analysis of subsequent malignant neoplasms, chronic health conditions, fertility, and health status was restricted to the 403 ES survivors who completed the baseline questionnaire.
Statistical Analysis
Demographic and treatment characteristics were described for the 403 ES survivors who completed the baseline questionnaire, and noncancer-related factors were compared with the 3899 CCSS siblings.
Overall and cause-specific mortality before January 1, 2003, was ascertained for the cohort of eligible ES survivors (n = 568) using the National Death Index (http://www.cdc.gov/nchs/ndi.htm) and information from the CCSS follow-up surveys (administered every several years by mail and telephone interview). Cause of death was obtained from death certificates and a review of CCSS survey responses according to methods previously described (20). Overall survival was estimated by the Kaplan–Meier method from cohort entry (5 years after diagnosis) until censoring of subjects at the date of last contact or death. Standardized mortality ratios (SMRs) for overall and cause-specific mortality were computed as the observed number of deaths in the cohort divided by the expected number of deaths in the general population. To obtain expected numbers, person-years (PY) at risk for death were calculated from the time of cohort entry to the date of death or censoring, stratified by age, sex, and calendar year, and multiplied by age-, sex-, and year-specific US mortality rates reported by the National Center for Health Statistics (21). A 95% confidence interval (CI) for each standardized mortality ratio was calculated using Poisson probability models. For ES survivors with treatment information, mortality rates were assessed according to radiation therapy (yes or no) using Cox proportional hazards models with age as the timescale and adjusting for sex and race/ethnicity. On the baseline and follow-up questionnaires, participants were asked to self-report second and subsequent cancers diagnosed after their original childhood cancer. Self-reports were verified and confirmed by the CCSS Pathology Center according to methods reported previously (22). Nonmelanoma skin cancers, noninvasive meningiomas, and other neoplasms with an International Classification of Diseases for Oncology (23) behavior code other than malignant code 3 were excluded from the analysis of subsequent malignant neoplasms. Cumulative incidence probabilities of subsequent malignant neoplasms were estimated using death as a competing risk event (24). Neoplasms that occurred before the baseline questionnaire was completed were considered prevalent at the time of cohort entry in the cumulative incidence curves. Standardized incidence ratios and excess absolute risk (EAR) of overall and specific types of second and subsequent malignancies were calculated in the same manner as the standardized mortality ratios using the US Surveillance, Epidemiology, and End Results (25) cancer incidence rates.
The occurrence and severity of chronic health conditions were determined following our previously described methods (26). Severity of conditions was scored using the Common Terminology Criteria for Adverse Events, version 3 (27): grade 1 (mild), 2 (moderate), 3 (severe), 4 (life threatening or disabling), or 5 (fatal). Severity of conditions, the presence of multiple conditions, specific types of conditions, and interval between cancer diagnosis and condition onset (calculated by age at onset of the condition and age at ES diagnosis) were assessed. The prevalence of chronic conditions among ES survivors and siblings at study entry (condition onset <5 years after diagnosis) was compared using a prevalence ratio, calculated by dividing the observed number of events among survivors by the expected number of events, assuming the survivors had the same age-, sex-, and race/ethnicity-adjusted rates as the siblings. Poisson probability models were used for these comparisons, adjusting for age, sex, and race/ethnicity (28). Chronic conditions during follow-up (onset ≥5 years after diagnosis) were described by the relative risk of each condition among survivors compared with siblings, as computed by age-, sex-, and race/ethnicity-adjusted Poisson probability models.
For the fertility analysis, 31 survivors (16 women and 15 men) were excluded because they were surgically sterile. In addition, 15 survivors (nine women and six men) were excluded because of the age restriction (age at baseline <15 years). Lastly, five men sired a pregnancy before cohort entry, and 11 women were pregnant before cohort entry and were excluded. Among 3899 siblings from baseline, 497 siblings (324 women and 173 men) were eliminated because of surgical sterility and 468 siblings (241 women and 227 men) were eliminated because of the age restriction (inclusion, age 15–44 at baseline). In addition, 56 female siblings were eliminated because they were pregnant before cohort entry. Thus, fertility was assessed among 341 ES survivors and compared with 2878 siblings. Cox proportional hazard models that used age as the timescale were used to compare hazards of a pregnancy, as previously described (29,30). Participants entered the risk set for regression analyses at the age at which they entered the CCSS cohort (5 years after date of diagnosis of primary cancer) or at age 15 years, whichever was greater, and were observed until the minimum age of first pregnancy, death, completion of baseline questionnaire, or age of 44 years, whichever came first. To create a similar age-based follow-up period, siblings were assigned a pseudo-diagnosis date that corresponded to the age of their survivor sibling at diagnosis of their primary cancer, and identical methods were used to define their time-to-event variables. Multiple-imputation methodology for event-time imputations was used for those who reported one or more pregnancies but who did not report age at first pregnancy (ES survivors, 3.5%; siblings, 3.1%). Relative risks were estimated for fertility among survivors vs siblings adjusted for education level, marital status, age at diagnosis, race/ethnicity, and smoking status.
Self-reported health status was evaluated for survivors and siblings who were age 18 or older at baseline. This study included four domains of health status: general health, mental health, functional impairment, and activity limitations (31,32). For general health, participants were asked, “Would you say that your health is excellent, very good, good, fair, or poor?” For the mental health domain, the 18-item Brief Symptom Inventory (33) was used. Participants who had a value above 63 (upper 10th percentile cutoff) on any of the three symptom-specific subscales (depression, somatization, and anxiety) were classified as having adverse mental health, and this outcome was used as the primary mental health outcome for this analysis. Questions assessing general health, functional status, and limitations of activity were adapted from the National Health Interview Survey (http://www.cdc.gov/nchs/nhis.htm) and the Behavioral Risk Factor Surveillance System Survey Questionnaire (http://www.cdc.gov/brfss/). Functional status was determined from three questions that asked respondents if they had any impairment or health problem that resulted in 1) needing “help with personal care needs, such as eating, bathing, dressing, or getting around your home”; 2) needing “help in handling routine needs, such as everyday household chores, doing necessary business, shopping, or getting around for other purposes”; or 3) “keeping you from holding a job or attending school.” Activity status was determined from three questions that asked respondents if in the past 2 years their health was limited for more than 3 months in 1) “the kinds or amounts of moderate activities you can do, like moving a table, carrying groceries, or bowling”; 2) “walking uphill or climbing a few flights of stairs”; or 3) “walking 1 block.” For each of the four domains of health status, the proportion of survivors and siblings reporting moderate to extreme adverse health was compared using odds ratios (ORs) derived from generalized linear models with a logistic link function adjusted for age, sex, and race/ethnicity.
Statistical inference was based on a bootstrap analysis with 1000 repeated samplings based on selecting families to account for potential within-family correlation between survivors and siblings (34). For all other comparisons between survivors and siblings using logistic, Poisson, or Cox proportional hazards models, adjustments were made for potential intrafamily correlation with robust sandwich variance estimates (35,36), which accounts for nonindependence of observations between siblings. For Cox regression models, proportionality of hazards was evaluated for the key variables by testing for time-dependent changes in hazard ratios (ie, a test for interaction between variable of interest and the time-scale variable) and no departure from proportionality was observed. All analyses were conducted using two-sided statistical inferences and SAS version 9.1 (SAS Institute, Inc, Cary, NC).
Results
ES survivors and siblings were 52.6% and 48.1% male, respectively. Mean age at diagnosis for survivors was 11.6 years (range 0–20 years). Mean age of survivors and siblings at the time of completing the baseline questionnaire was identical at 26 years. Mean follow-up time from diagnosis to last contact was 23 years (range 16–33 years) for surviving participants, and time to death was 11 years (range 5–28 years) for those participants who died (Table 1).
Table 1.
Characteristics of Ewing sarcoma (ES) survivors and sibling control subjects
| Characteristic | Survivors, n = 403 | Siblings, n = 3899 | P* |
| Demographic and diagnostic data | |||
| Sex, No. (%) | |||
| Male | 212 (52.6) | 1875 (48.1) | .09 |
| Female | 191 (47.4) | 2024 (51.9) | |
| Race/ethnicity, No. (%) | |||
| White, non-Hispanic | 362 (89.8) | 3414 (87.5) | .19 |
| Others | 40 (10.0) | 485 (12.4) | |
| Missing | 1 (0.2) | ||
| Age at study, y, No. (%) | |||
| 0–17 | 37 (9.2) | 816 (20.9) | <.001 |
| 18–29 | 232 (57.6) | 1651 (42.3) | |
| ≥30 | 134 (33.3) | 1432 (36.7) | |
| Mean (range) | 26.3 (9–45) | 26.0 (1–55) | |
| Age at diagnosis, y, No. (%) | |||
| 0–4 | 29 (7.2) | ||
| 5–9 | 108 (26.8) | ||
| 10–14 | 147 (36.5) | ||
| 15–20 | 119 (29.5) | ||
| Mean (range) | 11.6 (0–20) | ||
| Follow-up since diagnosis, y† | |||
| Surviving subjects, mean (range) | 23.0 (16–33) | ||
| Deceased subjects, mean (range) | 11.2 (5–28) | ||
| Tumor type, No. (%) | |||
| Osseous ES | 332 (82.4) | ||
| Extraosseous ES | 47 (11.7) | ||
| Ill-defined | 24 (5.9) | ||
| Cancer therapy‡ | |||
| Patients treated with anthracyclines, No. (%) | |||
| Any | 311 (86.6) | ||
| <300 mg/m2 | 52 (18.5) | ||
| ≥300 mg/m2 | 229 (81.5) | ||
| None | 48 (13.4) | ||
| Patients treated with alkylating agents§, No. (%) | |||
| Any | 350 (97.5) | ||
| Low | 39 (12.9) | ||
| Moderate | 205 (67.7) | ||
| High | 59 (19.5) | ||
| None | 9 (2.5) | ||
| Patients treated with ifosfamide, No. (%) | 34 (9.5) | ||
| Patients treated with etoposide, No. (%) | 41 (11.4) | ||
| Patients treated with radiation, No. (%) | |||
| Any | 288 (80.2) | ||
| None | 71 (19.8) | ||
P values from Wald test for associations in a logistic regression model with robust variance estimates to account for correlations between survivors and siblings. All statistical tests were two-sided.
Mean time until study or date of last contact for surviving subjects or mean time until death for deceased subjects.
Excludes subjects without treatment data.
Low, moderate, or high for cumulative alkylating agent dose scores (18).
Survival and Late Mortality
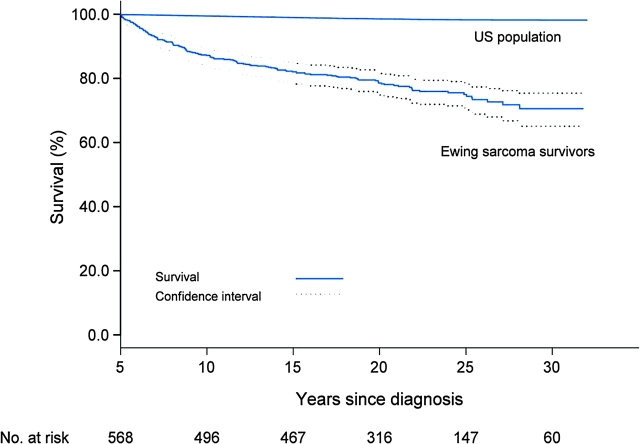
Overall survival at 20 years from diagnosis was 78.5% for all ES survivors (95% CI = 74.9 to 81.8) (Figure 2). There were 136 deaths among this cohort of 568 eligible ES survivors of five or more years. Importantly, for individuals who survived their ES at least 5 years from the time of the diagnosis, 75% remained alive at 25 years after diagnosis, that is, the cumulative mortality was 25% at 25 years following entry into the CCSS (95% CI = 21.1 to 28.9). The all-cause standardized mortality ratio, including recurrence, was 13.3 (95% CI = 11.2 to 15.8) overall, 23.1 for women (95% CI = 17.6 to 29.7), and 10.0 for men (95% CI = 7.9 to 12.5).
Figure 2.
Overall survival from Kaplan–Meier estimates, with 95% confidence intervals among 5-year survivors of Ewing sarcoma compared with expected survival using age, sex, and year standardized to the US population.
Disease recurrence/progression of the primary ES accounted for 82 (60.3%) deaths. Other causes of death included subsequent malignant neoplasms (n = 19; SMR = 20.0, 95% CI = 12.0 to 31.6), cardiac disease (n = 8; SMR = 12.0, 95% CI = 5.2 to 23.6), other medical causes (n = 10; SMR = 4.0, 95% CI = 1.9 to 7.3), and pulmonary disease (n = 1). External nonmedical causes of death occurred in five survivors, and cause of death could not be determined in 11 survivors. The cumulative mortality because of nonrecurrence-progression non-external causes (subsequent malignant neoplasms not related to the primary ES and cardiac and pulmonary disease potentially attributable to treatment) was 8.3% (95% CI = 5.4 to 11.3) at 25 years after ES diagnosis (Figure 3, A). The nonrecurrence-progression non-external cause standardized mortality ratio was 8.7 (95% CI = 6.2 to 12.0). Cumulative mortality because of nonrecurrence-progression non-external causes was similar for survivors treated with and without radiation therapy (Figure 3, B); adjusting for age, sex, and race/ethnicity, survivors who received radiation therapy were not more likely to die of nonrecurrence-progression non-external causes compared with those who did not receive radiation (hazard ratio = 1.2, 95% CI = 0.4 to 3.5, P = .76). With respect to subsequent malignant neoplasms, for those patients who received radiation therapy and those who did not, the standardized mortality ratios were 26.4 (95% CI = 14.4 to 44.7) and 10.0 (95% CI = 0.1 to 55.9), respectively.
Figure 3.
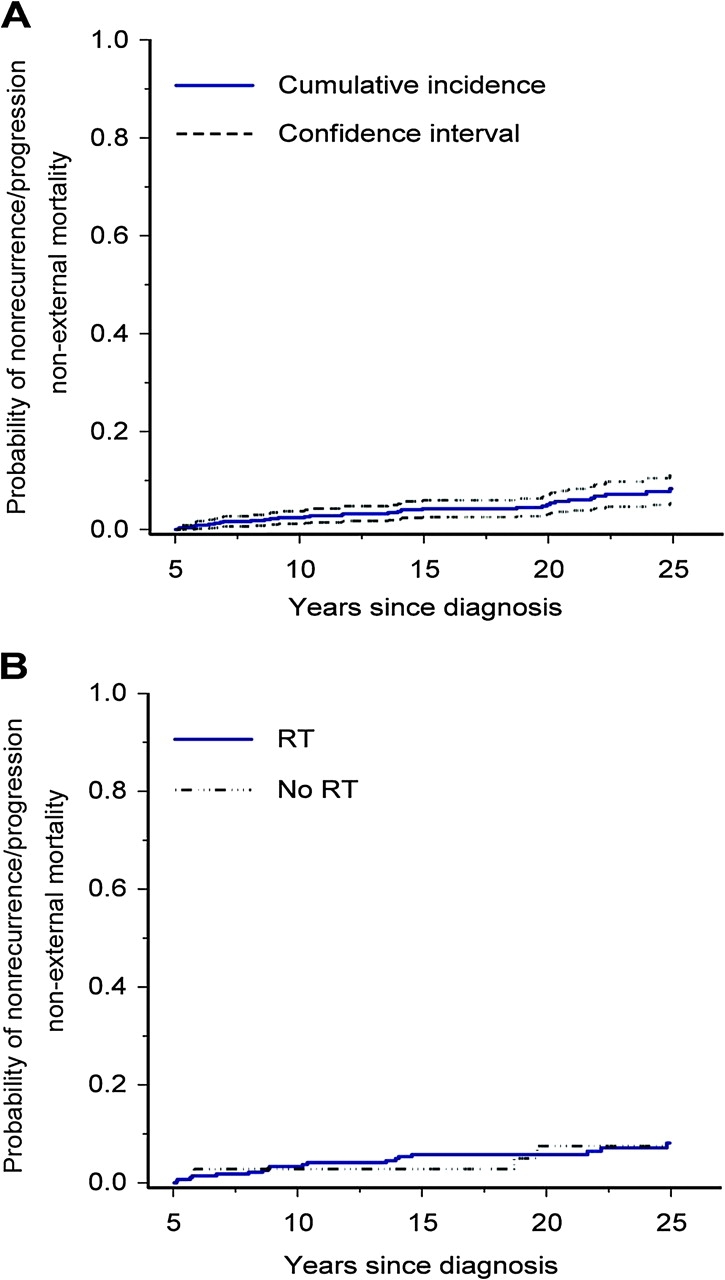
Cumulative incidence of mortality attributable to nonrecurrence-progression non-external causes (subsequent non-Ewing sarcoma (ES) malignant neoplasms and cardiac and pulmonary causes) among 5-year survivors of ES, treating death from other causes as competing risk events. A) Mortality for entire ES cohort. B) Mortality by radiation therapy (RT).
Subsequent Malignant Neoplasms
Excluding 11 nonmelanoma skin cancers, there were 36 subsequent malignant neoplasms reported among 34 participants. Of these, 26 (86.7%) had received radiation therapy. The standardized incidence ratio for all subsequent malignant neoplasms was 5.9 (95% CI = 4.1 to 8.3) with an excess absolute risk of 48.1 per 10 000 PY of follow-up. First reported subsequent malignant neoplasms included breast cancer (n = 11, EAR = 17.7 per 10 000 PY), osteosarcoma (n = 8, EAR = 13.4 per 10 000 PY), papillary thyroid carcinoma (n = 4, EAR = 6.2 per 10 000 PY), acute myelogenous leukemia (n = 2, EAR = 3.3 per 10 000 PY), and one each of sarcoma (not otherwise specified), embryonal rhabdomyosarcoma, neuroblastoma, and others (n = 5). Median age at original diagnosis for those who developed subsequent malignant neoplasms was 13.5 years (range 6–20 years); median age at subsequent diagnosis of malignant neoplasm was 30 years (range 14–43 years). Median time from first diagnosis to first subsequent malignant neoplasm was 14.5 years (range 4–32 years).
For those survivors who received radiation, the standardized incidence ratio was 6.6 (95% CI = 4.5 to 9.6), and for those survivors who did not receive radiation, the standardized incidence ratio was 3.3 (95% CI = 1.1 to 10.2, P = .28). As expected, thyroid cancer and secondary sarcomas were found most frequently in or near the radiation field. Among the 11 women (13 neoplasms) with breast cancer, four women (five neoplasms) had tumors in the radiation field (whole-lung radiation, dose range 1200–1500 centigray [cGy] units), one woman had invasive breast cancer in the right breast near her right humerus radiation field (6500 cGy), followed by a ductal carcinoma in situ in the left breast, and six women with neoplasms had not had any radiation in the area. The standardized incidence ratio for breast cancer among women treated with whole-lung radiation was 36.0 (95% CI = 15.5 to 83.5) with an excess absolute risk of 5.6. Among women not treated with chest radiation (or radiation to the adjoining areas), the standardized incidence ratio was 17.0 (95% CI = 7.8 to 37.2) with an excess absolute risk of 3.2.
The cumulative incidence of subsequent malignant neoplasms in all ES survivors at 25 years from diagnosis was 9.0% (95% CI = 5.8 to 12.2) (Figure 4, A). The cumulative incidence for those who received radiation did not differ statistically significantly from those who did not receive radiation, although the number of survivors not receiving radiation was very small.
Figure 4.
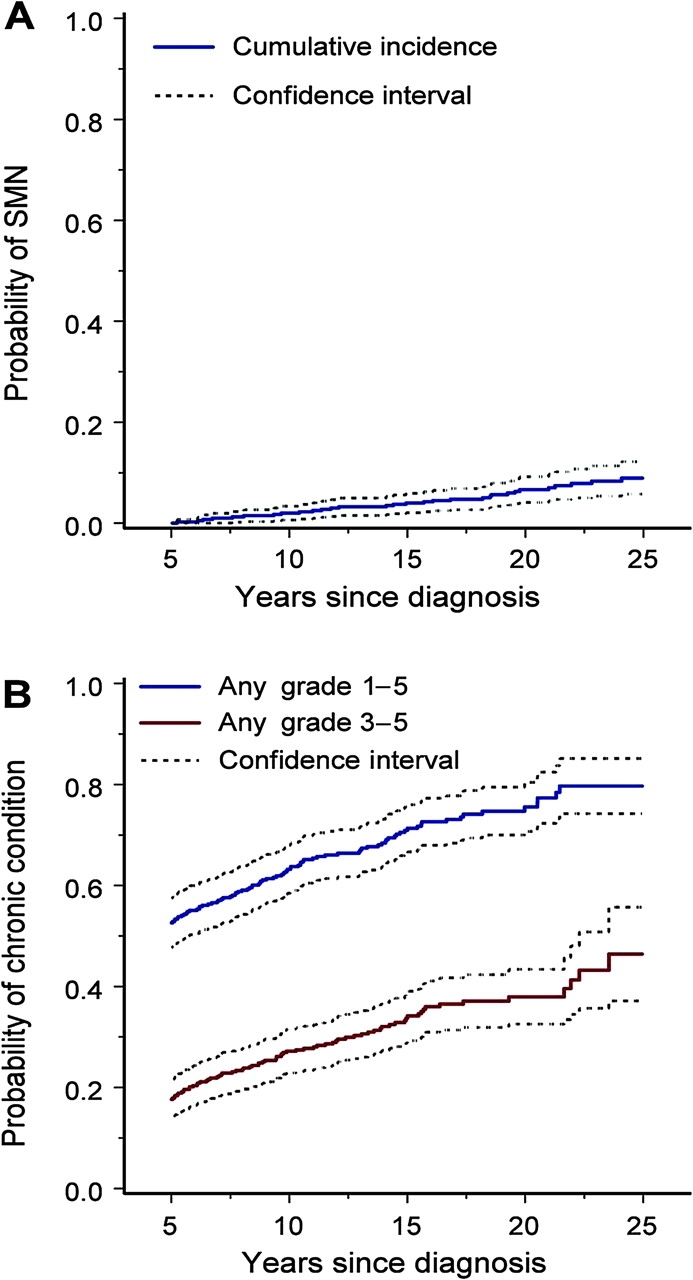
Subsequent malignant neoplasms (SMN) and chronic health conditions among 5-year survivors of Ewing sarcoma. A) Cumulative incidence with 95% confidence intervals of SMN (excluding nonmelanoma skin cancer) with death as a competing risk. B) Cumulative incidence with 95% confidence intervals of chronic health conditions according to Common Terminology Criteria for Adverse Events, version 3, severity grades (grades 1–5 vs grades 3–5).
Chronic Health Conditions
At least one chronic health condition was reported by 70.7% of survivors compared with 33.7% of siblings (Table 2). Among survivors, 32.0% reported either severe (grade 3) or life-threatening or disabling (grade 4) conditions compared with 5.0% of siblings. For ES survivors, about 70% of any chronic conditions and 50% of severe or life-threatening conditions were present within 5 years of the original cancer diagnosis. During this time (before cohort entry), the prevalence ratio for at least one chronic condition in survivors vs siblings was 11.3 (95% CI = 10.2 to 12.6) and the prevalence ratio for severe or life-threatening/disabling conditions was 66.3 (95% CI = 50.8 to 84.9). Strikingly, the prevalence ratio for three or more chronic conditions was 86.9 (95% CI = 69.8 to 108.7).
Table 2.
Prevalence ratio and relative risk of chronic health conditions among 5-year survivors of Ewing sarcoma compared with siblings of childhood cancer, adjusted for age*
| Chronic health condition | Siblings | Survivors† | ||||
| Total | <5 y after diagnosis | ≥5 y after diagnosis | ||||
| No. (%) | No. (%) | No. | PR (95% CI) | No. | RR (95% CI) | |
| Severity‡ | ||||||
| Any grade 1–4 | 1313 (33.7) | 285 (70.7) | 189 | 11.3 (10.2 to 12.6) | 77 | 2.0 (1.6 to 2.6) |
| Grade 3 or 4 | 194 (5.0) | 129 (32.0) | 64 | 66.3 (50.8 to 84.9) | 61 | 6.0 (4.1 to 9.0) |
| Multiple conditions | ||||||
| ≥2 | 478 (12.3) | 182 (45.2) | 135 | 34.5 (30.2 to 39.5) | 32 | 2.3 (1.6 to 3.5) |
| ≥3 | 195 (5.0) | 102 (25.3) | 83 | 86.9 (69.8 to 108.7) | 12 | 2.0 (1.0 to 3.3) |
| Specific conditions | ||||||
| Arrhythmia | 111 (2.9) | 30 (7.4) | 14 | § | 16 | 2.3 (1.4 to 3.9) |
| Cardiac‖ | 18 (0.5) | 18 (4.5) | 10 | § | 8 | 7.5 (3.1 to 18.7) |
| Pulmonary¶ | 146 (3.7) | 53 (13.2) | 30 | 95.2 (62.0 to 140.6) | 22 | 2.6 (1.6 to 4.2) |
| Problems with balance | 124 (3.2) | 28 (6.7) | 13 | 69.2 (36.6 to 114.3) | 13 | 1.8 (1.0 to 3.4) |
| Tremors | 30 (0.8) | 18 (4.5) | 12 | § | 6 | 3.4 (1.2 to 9.3) |
| Weakness | 39 (1.0) | 40 (9.9) | 30 | § | 7 | 3.1 (1.3 to 7.8) |
| Sensory neuropathy | 238 (6.1) | 114 (28.3) | 77 | 83.4 (65.4 to 106.6) | 32 | 2.5 (1.7 to 3.7) |
| Any neurological | 341 (8.8) | 128 (31.8) | 89 | 52.1 (42.3 to 63.8) | 31 | 2.0 (1.4 to 3.0) |
All confidence intervals are two-sided. CI = confidence interval based on bootstrap sampling methods for PR and generalized estimating equations methodology for RR; PR = prevalence ratio comparing survivor prevalence with that expected in an age-adjusted estimate from the sibling population; RR = relative risk from a Poisson regression model.
For survivors, the sum of the “<5 years after diagnosis” and “≥5 years after diagnosis” columns may not equal the total because of missing information regarding the age at onset of the condition.
Severity of chronic health conditions graded according to Common Terminology Criteria for Adverse Events, version 3 (27); to allow comparisons with siblings (all alive at the time of study), fatal conditions (grade 5) among survivors were represented by the maximum grade of a condition reported before death (grades 1–4).
Events among siblings too sparse to estimate expected number of events.
Heart attack, angina, congestive heart failure, heart transplant, or stroke.
Chronic cough, shortness of breath, or lung fibrosis not requiring oxygen.
The number of chronic health conditions that developed five or more years after diagnosis was not insubstantial. During this time (after cohort entry), ES survivors were twice as likely to report at least one chronic condition (relative risk [RR] = 2.0, 95% CI = 1.6 to 2.6) compared with siblings, six times more likely to report a severe or life-threatening chronic condition (RR = 6.0, 95% CI = 4.1 to 9.0), and two times more likely to report three or more chronic conditions (RR = 2.0, 95% CI = 1.0 to 3.3) (Table 2). Twenty-five years after diagnosis, the cumulative incidence of any chronic health condition (grades 1–5) in ES survivors was 79.7% (95% CI = 74.2 to 85.1) and the cumulative incidence of a severe, life-threatening or disabling, or fatal chronic condition was 46.4% (95% CI = 37.1 to 55.7) (Figure 4, B). Note that the difference between the curves in Figure 4, B, depicts the proportion of subjects who experienced chronic conditions of grades 1 and 2.
Survivors of ES were more likely than siblings to have arrhythmias (7.4% vs 2.9%) and other serious cardiac conditions (4.5% compared with 0.5%) (Table 2). After adjusting for age, sex, and race/ethnicity, the relative risk of a survivor having an arrhythmia or serious cardiac event five or more years after diagnosis was 2.3 (95% CI = 1.4 to 3.9) and 7.5 (95% CI = 3.1 to 18.7), respectively. Chronic pulmonary conditions (chronic cough, shortness of breath, or lung fibrosis not requiring oxygen) were also more common in survivors five or more years after diagnosis, relative to siblings (RR = 2.6, 95% CI = 1.6 to 4.2). During this period, survivors were twice as likely as siblings to report chronic neurological problems (RR = 2.0, 95% CI = 1.4 to 3.0). Specifically, tremors (RR = 3.4, 95% CI = 1.2 to 9.3) and weakness (RR = 3.1, 95% CI = 1.3 to 7.8) were statistically significantly more common among ES survivors compared with siblings, adjusting for age, sex, and race/ethnicity.
Fertility
Among the 341 ES survivors who were evaluated for fertility status, 29.7% of women reported a pregnancy and 11.3% of men reported siring a pregnancy. In contrast, among the 2878 siblings, 40.1% of women reported a pregnancy and 33.2% of men reported siring a pregnancy. After adjusting for marital status, race/ethnicity, educational attainment, smoking status, and age at diagnosis, female ES survivors were less likely than siblings to report a pregnancy (RR = 0.65, 95% CI = 0.48 to 0.88, P = .005) (Table 3). Similarly, male ES survivors were less likely to report siring a child than male siblings (RR = 0.38, 95% CI = 0.24 to 0.59, P < .001). Survivors with younger age at diagnosis had an increased likelihood of reporting a pregnancy.
Table 3.
Multivariable relative risk (RR) of pregnancy among 5-year survivors of Ewing sarcoma and siblings of childhood cancer survivors*
| Variable | Men | Women | ||
| RR (95% CI) | P | RR (95% CI) | P | |
| Siblings | 1.00 (referent) | 1.00 (referent) | ||
| Survivors | 0.38 (0.24 to 0.59) | <.001 | 0.65 (0.48 to 0.88) | .005 |
| Marital status | ||||
| Never married | 1.00 (referent) | 1.00 (referent) | ||
| Currently married | 7.07 (4.85 to 10.31) | <.001 | 6.21 (4.55 to 8.46) | <.001 |
| Formerly married | 4.48 (2.87 to 6.99) | <.001 | 4.41 (2.76 to 7.05) | <.001 |
| Race/ethnicity | ||||
| White, non-Hispanic | 1.00 (referent) | 1.00 (referent) | ||
| Black, non-Hispanic | 2.53 (1.30 to 4.91) | .006 | 1.31 (0.71 to 2.44) | .38 |
| Hispanic | 2.11 (1.09 to 4.09) | .027 | 1.08 (0.61 to 1.91) | .78 |
| Other | 1.75 (1.06 to 2.90) | .03 | 1.29 (0.87 to 1.92) | .21 |
| Educational attainment | ||||
| No high school/GED | 1.00 (referent) | 1.00 (referent) | ||
| High school/GED | 0.80 (0.48 to 1.34) | .4 | 0.97 (0.60 to 1.55) | .89 |
| Some college | 0.48 (0.29 to 0.79) | .004 | 0.67 (0.43 to 1.06) | .089 |
| Bachelor’s or higher | 0.39 (0.23 to 0.65) | <.001 | 0.38 (0.24 to 0.59) | <.001 |
| Smoking status | ||||
| Never smoked | 1.00 (referent) | 1.00 (referent) | ||
| Current smoker | 0.78 (0.59 to 1.04) | .097 | 0.84 (0.63 to 1.11) | .22 |
| Former smoker | 0.71 (0.55 to 0.91) | .008 | 0.68 (0.54 to 0.86) | .002 |
| Age at cancer diagnosis, y | ||||
| 0–4 | 1.99 (1.43 to 2.76) | <.001 | 1.83 (1.36 to 2.45) | <.001 |
| 5–9 | 1.05 (0.80 to 1.38) | .72 | 1.64 (1.29 to 2.08) | <.001 |
| 10–14 | 0.84 (0.66 to 1.07) | .16 | 1.31 (1.05 to 1.64) | .018 |
| 15–20 | 1.00 (referent) | 1.00 (referent) | ||
Both RR and confidence interval (CI) were estimated from Cox proportional hazards parameters. Two-sided P values were based on likelihood ratio tests. GED = general education development (high school equivalency).
Health Status
Among ES survivors who were age 18 or older at the time of the baseline questionnaire, 13.6% reported moderate to extreme adverse general health compared with 5.1% of siblings (OR = 2.9, 95% CI = 2.0 to 4.1, P < .001) (Table 4). A modestly greater proportion of survivors reported moderate to extreme adverse mental health (16.3%) than did siblings (10.2%) (OR = 1.8, 95% CI = 1.3 to 2.5, P < .001). Activity limitations were reported by 27.5% of survivors and 5.8% of siblings (OR = 6.5, 95% CI = 4.9 to 8.6, P < .001); functional impairment was reported by 14.7% of survivors and 2.6% of siblings (OR = 6.3, 95% CI = 4.3 to 9.4, P < .001).
Table 4.
Moderate to extreme adverse health status in adult survivors of Ewing sarcoma compared with siblings of childhood cancer survivors*
| Health status domains | Survivors, n = 366, No. (%) | Siblings, n = 3899, No. (%) | OR† (95% CI) | P |
| Adverse general health | 42 (13.6) | 157 (5.1) | 2.9 (2.0 to 4.1) | <.001 |
| Adverse mental health | 49 (16.3) | 302 (10.2) | 1.8 (1.3 to 2.5) | <.001 |
| Functional impairment | 44 (14.7) | 79 (2.6) | 6.3 (4.3 to 9.4) | <.001 |
| Activity limitations | 97 (27.5) | 178 (5.8) | 6.5 (4.9 to 8.6) | <.001 |
| Any of the above | 179 (55.8) | 547 (18.7) | 3.5 (2.7 to 4.5) | <.001 |
CI = confidence interval; OR = odds ratio for survivors vs siblings.
†OR was adjusted for age, sex, and race/ethnicity and evaluated in logistic regression models. Two-sided P values were calculated from likelihood ratio tests.
Discussion
Advances in multimodal therapy for pediatric ES have led to gradually improving 5-year survival rates, increasing from 42% in 1975–1984 to 58% in 1985–1994 to 60% in 1996–2004 (2,37). With this improvement in 5-year survival, the numbers of long-term survivors of ES have gradually increased. This study provides a comprehensive description of long-term survival and key health outcomes from a relatively large and geographically diverse cohort of 5-year ES survivors treated in the 1970s and 1980s. To our knowledge, this is the first study that provides detailed information regarding survival beyond 10 years for patients diagnosed with ES. Importantly, for individuals who survived their ES at least 5 years after diagnosis, 75% remained alive at 25 years after diagnosis. Whereas 60% of late mortality (≥5 years after diagnosis) was attributable to recurrence/progression of the primary disease, it is important to note that 40% of late mortality was attributable to other causes, most notably second cancers (14%) and cardiac disease (6%). Pediatric oncologists must continue to engage in large randomized controlled therapeutic trials in an effort to improve the primary treatment of ES because 60% of the late mortality in this cohort was attributable to recurrent disease.
As noted, a major contributing factor to late mortality and to diminished health status of ES survivors was a subsequent malignant neoplasm. Previous studies with shorter follow-up reported an increased risk of secondary cancers following therapy for ES, primarily therapy-induced acute myelogenous leukemia (t-AML) or radiation-induced sarcomas (osteosarcoma and soft tissue sarcoma) (9,12,15). To our knowledge, our sample from the CCSS cohort is the largest number, to date, of case patients with subsequent malignant neoplasms following ES (excluding nonmelanoma skin cancer: 36 malignancies among 34 survivors). By 25 years following the ES diagnosis, the cumulative incidence of a second malignant neoplasm among survivors in our cohort was 9%. It is important to understand that to be eligible for the CCSS, participants had to have survived at least 5 years from the date of their primary cancer diagnosis. Thus, patients who died from t-AML within 5 years of ES diagnosis would not have been included. The small number of patients with t-AML in our cohort (n = 2) likely reflects this eligibility criterion. Notably, breast and thyroid cancer represented 32% and 12% of the second cancers, respectively. Indeed, the highest absolute excess risk of a second cancer was for breast cancer, which confirms two previous reports (11,15). In addition, to our knowledge, this study is the first to report an excess risk among both ES survivors treated with low-dose (1200–1500 cGy) whole-lung irradiation and women not treated with radiation to the chest area. With respect to the former group, the Children's Oncology Group (38) recommends early initiation for breast cancer screening among women treated with at least 2000 cGy chest radiation. However, as noted by Inskip et al. (39), the risk of breast cancer among women treated with therapeutic radiation during their childhood years is linearly related to the radiation dose; thus, screening among women treated with lower-dose whole-lung irradiation should be considered. Surprisingly, we also found an excess of risk among women who were not treated with radiation to the chest or breast area, suggesting a possible underlying genetic predisposition and warranting further investigation.
Few studies have focused on serious morbidities other than second cancers. The generalizability of these studies is limited by smaller cohorts of ES survivors (<100), shorter follow-up, and/or lack of a comparison population (4–8). The prevalence of chronic conditions in ES survivors in our study (70.7%) is modestly higher than the 62% reported for the entire CCSS cohort (26). This outcome is not surprising given the routine use of moderate- to high-dose anthracyclines, alkylating agents, musculoskeletal surgeries, and radiation in the treatment of ES. For these survivors, the burden of chronic conditions remained high from diagnosis through follow-up. Although many conditions developed within 5 years of diagnosis, it is important to recognize that about half of the most serious conditions did not occur until later. In particular, arrhythmias and other cardiac conditions, pulmonary conditions, and problems with balance were approximately evenly split between the two time intervals (onset <5 and ≥5 years from diagnosis). The importance of long-term follow-up to monitor for these and other late-occurring conditions cannot be overemphasized.
Infertility was common among both male and female ES survivors. Female survivors were 35% less likely to report a pregnancy than siblings of childhood cancer survivors, even after excluding women who were surgically sterile. As reported by Green et al. (29), infertility is associated with ovarian irradiation and high-dose alkylating agent chemotherapy. Contemporary therapy for ES includes high-dose cyclophosphamide and ifosfamide (40,41), and so we can anticipate that the rate of infertility will not be much different in women treated in the CCSS era compared with women treated today. Thus, it is imperative that methods of fertility conservation be further studied (42).
Musculoskeletal problems are relatively common late complications of treatment in ES survivors (5,7,43,44). The ES survivors in our cohort reported substantial functional impairment. Moderate to extreme adverse mental health was more common among ES survivors in this study than among siblings but was similar to the entire CCSS cohort (16.3% of ES survivors in this study vs 17.2% of all survivors in the CCSS) (32). This result for mental health suggests that despite having considerable functional impairment and high rates of infertility, second cancers, and serious chronic health problems, long-term survivors of ES cope remarkably well.
One of the potential limitations of this study is that the CCSS cohort is institutionally based rather than population based; because institutions may have specific referral patterns, the potential exists for enrollment of a nonrepresentative survivor population. At the time the cohort was constructed, it was estimated that the institutions selected to participate in CCSS diagnosed and treated approximately 50% of pediatric cancer patients in the United States. It is notable that results from CCSS have been remarkably consistent for outcomes, including late mortality and chronic health conditions derived from population-based series (45,46). A second potential limitation of this study is that participants were treated between 1970 and 1986, so the results may not be relevant to current treatment modalities, although contemporary therapy for ES still includes the frequent use of high-dose anthracycline and alkylating agents (3,40,41), as well as radiation or surgery for local control (47). Although radiation is still an important modality for these patients, the use of lower doses (4500–5500 cGy rather than 6000–7000 cGy), smaller fields (1–2 cm margin rather than whole bone or muscle compartment) and conformal techniques (intensity-modulated, three-dimensional, limited area radiation therapy) will potentially decrease morbidity in currently treated patients. The CCSS is currently expanding the cohort to include patients diagnosed between 1987 and 1999. This expansion will provide important information regarding late effects of more contemporary therapeutic protocols. Nevertheless, the present results are relevant in underscoring the critical importance of long-term risk-based health care for this high-risk population of cancer survivors. A third potential limitation is that although mortality and subsequent malignant neoplasms were externally verified, most chronic health conditions and health status outcomes in this study were self-reported, introducing the possibility of reporting bias. Survivors may be more likely to be aware of asymptomatic health conditions simply because of more frequent medical checkups; however, detection bias is unlikely to account for the magnitude of difference between survivors and siblings. Radiation has been implicated as the primary treatment modality associated with late mortality. However, this observation is confounded by the use of radiation in patients with more advanced disease or when the tumor is centrally located (ie, pelvis, spine, head, and neck) and where surgery may not be a viable option for local control. Indeed, more than three-quarters of the ES survivors in this study were treated with radiation, thus limiting our subanalysis of some outcomes. Notably, whereas the incidence of second malignancies was higher among irradiated survivors vs nonirradiated survivors, mortality attributable to subsequent neoplasms and cardiac or pulmonary disease (ie, excluding progression or recurrence of ES) was similar for the two groups. Clinicians who treat ES appear to have incorporated the recommendation to use surgery for local control more frequently in clinical practice. In a recent trial conducted by the Children's Oncology Group (3), which enrolled patients from 1995 through 1998, 65% of patients were treated with surgery alone for local control; the remainder were treated with radiotherapy alone or a combination of surgery and radiotherapy.
In summary, this retrospective cohort of ES survivors and siblings of childhood cancer provides clear evidence of long-term negative sequelae in ES survivors. In addition to increased mortality following ES, survivors are at substantially higher risk of morbidity because of second malignancies, chronic health conditions, and functional impairment, all of which require extensive clinical management. Enrollment and long-term follow-up in late effect programs is clearly indicated in this cohort.
Funding
This research was supported by National Cancer Institute Grant (U24 55727 to L.L.R.), the Intramural Research Program of the National Institutes of Health and the National Cancer Institute, and funding to St Jude Children's Research Hospital from the American Lebanese Syrian Associated Charities (ALSAC). The Childhood Cancer Survivor Study is funded by the National Cancer Institute as a resource to promote and facilitate research among long-term survivors of cancer diagnosed during childhood and adolescence.
Appendix 1
The Childhood Cancer Survivor Study (CCSS) is a collaborative multi-institutional project, funded as a resource by the National Cancer Institute (NCI), of individuals who survived five or more years after diagnosis of childhood cancer.
CCSS is a retrospectively ascertained cohort of 20 346 childhood cancer survivors diagnosed before age 21 between 1970 and 1986 and approximately 4000 siblings of survivors, who serve as a control group. The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and Canada. The study is currently funded by a U24 resource grant (NCI grant U24 CA55727) awarded to St Jude Children's Research Hospital. Currently, we are in the process of expanding the cohort to include an additional 14 000 childhood cancer survivors diagnosed before age 21 between 1987 and 1999. For information on how to access and utilize the CCSS resource, visit www.stjude.org/ccss.
CCSS Institutions and Investigators.
| St Jude Children's Research Hospital, Memphis, TN | Leslie L. Robison, PhD*,†, Melissa Hudson, MD†,‡ |
| Greg T. Armstrong, MD, MSCE†, Daniel M. Green, MD† | |
| Kevin R. Krull, PhD† | |
| Children's Healthcare of Atlanta/Emory University, Atlanta, GA | Lillian Meacham, MD‡, Ann Mertens, PhD† |
| Children's Hospitals and Clinics of Minnesota Minneapolis, St Paul, MN | Joanna Perkins, MD, MS‡ |
| Children's Hospital and Medical Center, Seattle, WA | Scott Baker, MD‡, Eric Chow, MD, MPH† |
| Children's Hospital, Denver, CO | Brian Greffe, MD‡ |
| Children's Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH‡ |
| Children's Hospital, Oklahoma City, OK | John Mulvihill, MD†,‡ |
| Children's Hospital of Orange County, Orange, CA | Leonard Sender, MD‡ |
| Children's Hospital of Philadelphia, Philadelphia, PA | Jill Ginsberg, MD‡, Anna Meadows, MD† |
| Children's Hospital of Pittsburgh, Pittsburgh, PA | Jean Tersak, MD‡ |
| Children's National Medical Center, Washington, DC | Gregory Reaman, MD‡, Roger Packer, MD† |
| Cincinnati Children's Hospital Medical Center, Cincinnati, OH | Stella Davies, MD, PhD†,‡ |
| City of Hope Medical Center, Los Angeles, CA | Smita Bhatia, MD†,‡ |
| Cook Children's Medical Center, Ft Worth, TX | Paul Bowman, MD, MPH‡ |
| Dana-Farber Cancer Institute/Children's Hospital, Boston, MA | Lisa Diller, MD†,‡ |
| Fred Hutchinson Cancer Research Center, Seattle, WA | Wendy Leisenring, ScD†,‡ |
| Hospital for Sick Children, Toronto, ON | Mark Greenberg, MBChB‡, Paul C. Nathan, MD†,‡ |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD†,‡ |
| Mayo Clinic, Rochester, MN | Vilmarie Rodriguez, MD‡ |
| Memorial Sloan-Kettering Cancer Center, New York, NY | Charles Sklar, MD†,‡, Kevin Oeffinger, MD† |
| Miller Children's Hospital, Long Beach, CA | Jerry Finklestein, MD‡ |
| National Cancer Institute, Bethesda, MD | Roy Wu, PhD†, Nita Seibel, MD† |
| Preetha Rajaraman, PhD†, Peter Inskip, ScD† | |
| Julia Rowland, PhD† | |
| Nationwide Children's Hospital, Columbus, OH | Amanda Termuhlen, MD‡, Sue Hammond, MD† |
| Northwestern University, Chicago, IL | Kimberley Dilley, MD, MPH‡ |
| Riley Hospital for Children, Indianapolis, IN | Terry A. Vik, MD‡ |
| Roswell Park Cancer Institute, Buffalo, NY | Martin Brecher, MD‡ |
| St Louis Children's Hospital, St Louis, MO | Robert Hayashi, MD‡ |
| Stanford University School of Medicine, Stanford, CA | Neyssa Marina, MD‡, Sarah S. Donaldson, MD† |
| Texas Children's Hospital, Houston, TX | Zoann Dreyer, MD‡ |
| University of Alabama, Birmingham, AL | Kimberly Whelan, MD, MSPH‡ |
| University of Alberta, Edmonton, AB | Yutaka Yasui, PhD†,‡ |
| University of California-Los Angeles, CA | Jacqueline Casillas, MD, MSHS‡, Lonnie Zeltzer, MD† |
| University of California-San Francisco, CA | Robert Goldsby, MD‡ |
| University of Chicago, Chicago, IL | Tara Henderson, MD, MPH‡ |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson, MD‡ |
| University of Minnesota, Minneapolis, MN | Joseph Neglia, MD, MPH†,‡ |
| University of Southern California, Los Angeles, CA | Dennis Deapen, DrPH†,‡ |
| University of Texas Southwestern Medical Center, Dallas, TX | Daniel C. Bowers, MD‡ |
| University of Texas MD Anderson Cancer Center, Houston, TX | Louise Strong, MD†,‡, Marilyn Stovall, MPH, PhD† |
Project Principal Investigator (U24 CA55727)
Member of CCSS Steering Committee
Institutional Principal Investigator.
Footnotes
Investigators interested in potential uses of this resource are encouraged to visit http://www.stjude.org/ccss. Other investigators and institutions participating in the Childhood Cancer Survivor Study are listed in Appendix 1.
The funders did not have any involvement in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
References
- 1.Bernstein M, Kovar H, Paulussen M, et al. Ewing sarcoma family of tumors: Ewing sarcoma of bone and soft tissue and the peripheral primitive neuroectodermal tumors. In: Pizzo P, Poplack D, editors. Principles and Practice of Pediatric Oncology. 5th ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2005. pp. 1002–1032. [Google Scholar]
- 2.Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: SEER data. J Pediatr Hematol Oncol. 2008;30(6):425–430. doi: 10.1097/MPH.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 3.Granowetter L, Womer R, Devidas M, et al. Dose-intensified compared with standard chemotherapy for nonmetastatic Ewing sarcoma family of tumors: a Children's Oncology Group Study. J Clin Oncol. 2009;27(15):2536–2541. doi: 10.1200/JCO.2008.19.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fuchs B, Valenzuela RG, Inwards C, Sim FH, Rock MG. Complications in long-term survivors of Ewing sarcoma. Cancer. 2003;98(12):2687–2692. doi: 10.1002/cncr.11891. [DOI] [PubMed] [Google Scholar]
- 5.Mansky P, Arai A, Stratton P, et al. Treatment late effects in long-term survivors of pediatric sarcoma. Pediatr Blood Cancer. 2007;48(2):192–199. doi: 10.1002/pbc.20871. [DOI] [PubMed] [Google Scholar]
- 6.Marinsek ZP, Kavalar R, Jereb B. Ewing sarcoma/PNET: 27 years of experience in Slovenia. Pediatr Hematol Oncol. 2006;23(4):355–367. doi: 10.1080/08880010600632001. [DOI] [PubMed] [Google Scholar]
- 7.Novakovic B, Fears TR, Horowitz ME, Tucker MA, Wexler LH. Late effects of therapy in survivors of Ewing's sarcoma family tumors. J Pediatr Hematol Oncol. 1997;19(3):220–225. doi: 10.1097/00043426-199705000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Paulino AC, Nguyen TX, Mai WY. An analysis of primary site control and late effects according to local control modality in non-metastatic Ewing sarcoma. Pediatr Blood Cancer. 2007;48(4):423–429. doi: 10.1002/pbc.20754. [DOI] [PubMed] [Google Scholar]
- 9.Bacci G, Longhi A, Barbieri E, et al. Second malignancy in 597 patients with Ewing sarcoma of bone treated at a single institution with adjuvant and neoadjuvant chemotherapy between 1972 and 1999. J Pediatr Hematol Oncol. 2005;27(10):517–520. doi: 10.1097/01.mph.0000183270.28785.33. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia S, Krailo MD, Chen Z, et al. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: a report from the Children's Oncology Group. Blood. 2007;109(1):46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji J, Hemminki K. Incidence of multiple primary malignancies among patients with bone cancers in Sweden. J Cancer Res Clin Oncol. 2006;132(8):29–535. doi: 10.1007/s00432-006-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing's sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14(10):2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 13.Navid F, Billups C, Liu T, Krasin MJ, Rodriguez-Galindo C. Second cancers in patients with the Ewing sarcoma family of tumours. Eur J Cancer. 2008;44(7):983–991. doi: 10.1016/j.ejca.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paulussen M, Ahrens S, Lehnert M, et al. Second malignancies after Ewing tumor treatment in 690 patients from a cooperative German/Austrian/Dutch study. Ann Oncol. 2001;12(11):1619–1630. doi: 10.1023/a:1013148730966. [DOI] [PubMed] [Google Scholar]
- 15.Curtis R, Freedman D, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 16.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 18.Tucker MA, D’Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 19.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breslow NE, Day NE. Fitting Models to Grouped Data, Statistical Methods in Cancer Research. Vol. II—The Design and Analysis of Cohort Studies. Lyon, France: IARC; 1987. Report No.: 82. [PubMed] [Google Scholar]
- 22.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Based on the Recommendations of the Ninth Revision Conference, 1975. Geneva, Switzerland: World Health Organization; 1977. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. [Google Scholar]
- 24.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 25. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database: Incidence—SEER 9 Regs Limited-Use, Nov 2008 Sub (1973-2006) <Katrina/Rita Population Adjustment>—Linked To County Attributes—Total U.S., 1969-2006 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, Released April 2009, Based on the November 2008 Submission. www.seer.cancer.gov. [Google Scholar]
- 26.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 27.Common Terminology Criteria for Adverse Events, Version 3.0. Bethesda, MD: National Cancer Institute; 2003. Cancer Therapy Evaluation Program. http://ctep.cancer.gov/reporting/ctc_v30.html. Accessed March 1, 2010. [Google Scholar]
- 28.Breslow N, Day N. Statistical Methods in Cancer Research: The Design and Analysis of Cohort Studies. New York: Oxford University Press; 1994. [PubMed] [Google Scholar]
- 29.Green DM, Kawashima T, Stovall M, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27(16):2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158(11):1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 31.Castellino SM, Casillas J, Hudson MM, et al. Minority adult survivors of childhood cancer: a comparison of long-term outcomes, health care utilization, and health-related behaviors from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23(27):6499–6507. doi: 10.1200/JCO.2005.11.098. [DOI] [PubMed] [Google Scholar]
- 32.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 33.Derogatis L. BSI-18 Administration, Scoring and Procedures Manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 34.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman & Hall; 1993. [Google Scholar]
- 35.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 36.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, NY: Springer-Verlag; 2000. [Google Scholar]
- 37.Gurney J, Swensen A, Bulterys M. Malignant bone tumors. In: Ries LA, Smith MAS, Gurney JG, editors. Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program 1975-1995. Bethesda, MD: National Cancer Institute; 1999. pp. 99–110. [Google Scholar]
- 38.Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 3.0. Arcadia, CA: Children's Oncology Group; 2008. http://www.survivorshipguidelines.org. Accessed November 4, 2009. [Google Scholar]
- 39.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(24):3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carvajal R, Meyers P. Ewing's sarcoma and primitive neuroectodermal family of tumors. Hematol Oncol Clin North Am. 2005;19(3):501–525. doi: 10.1016/j.hoc.2005.03.004. vi–vii. [DOI] [PubMed] [Google Scholar]
- 41.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348(8):694–701. doi: 10.1056/NEJMoa020890. [DOI] [PubMed] [Google Scholar]
- 42.Lee SJ, Schover LR, Partridge AH, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 43.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87(12):1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 44.Ness KK, Mertens AC, Hudson MM, et al. Limitations on physical performance and daily activities among long-term survivors of childhood cancer. Ann Intern Med. 2005;143(9):639–647. doi: 10.7326/0003-4819-143-9-200511010-00007. [DOI] [PubMed] [Google Scholar]
- 45.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297(24):2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 46.Moller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence: a population-based study in the Nordic countries. J Clin Oncol. 2001;19(13):3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 47.La TH, Meyers PA, Wexler LH, et al. Radiation therapy for Ewing's sarcoma: results from Memorial Sloan-Kettering in the modern era. Int J Radiat Oncol Biol Phys. 2006;64(2):544–550. doi: 10.1016/j.ijrobp.2005.07.299. [DOI] [PubMed] [Google Scholar]