Abstract
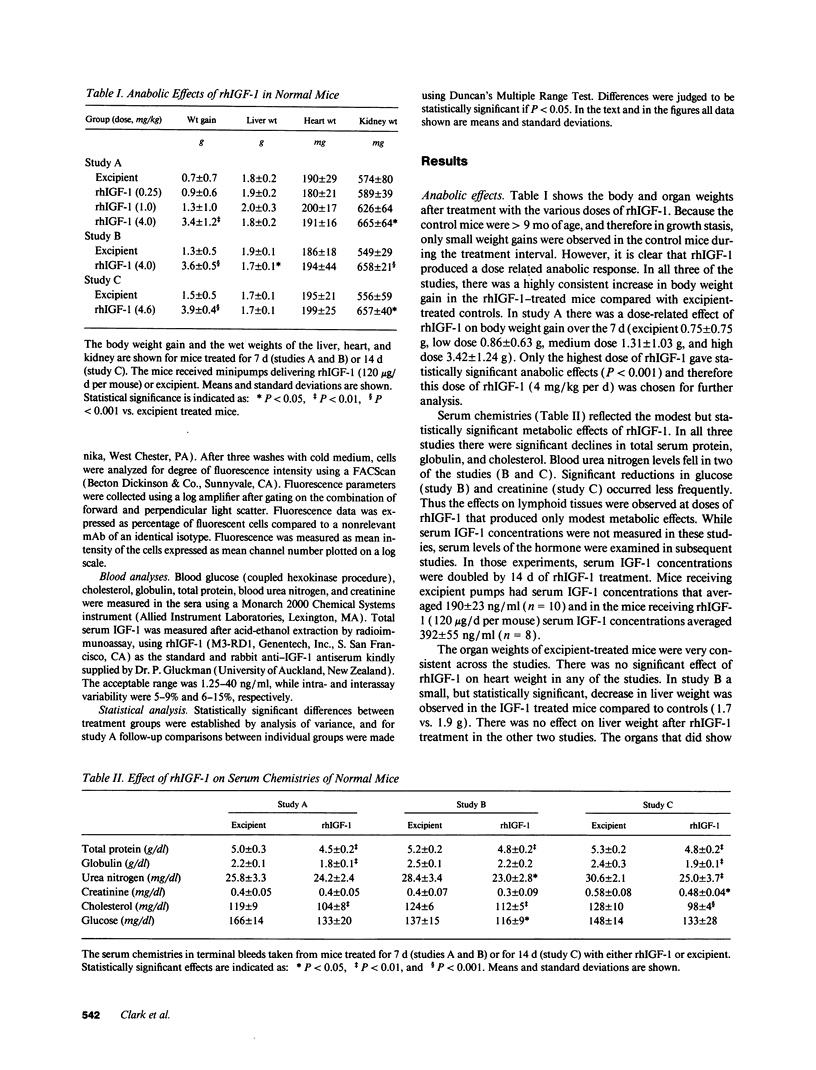
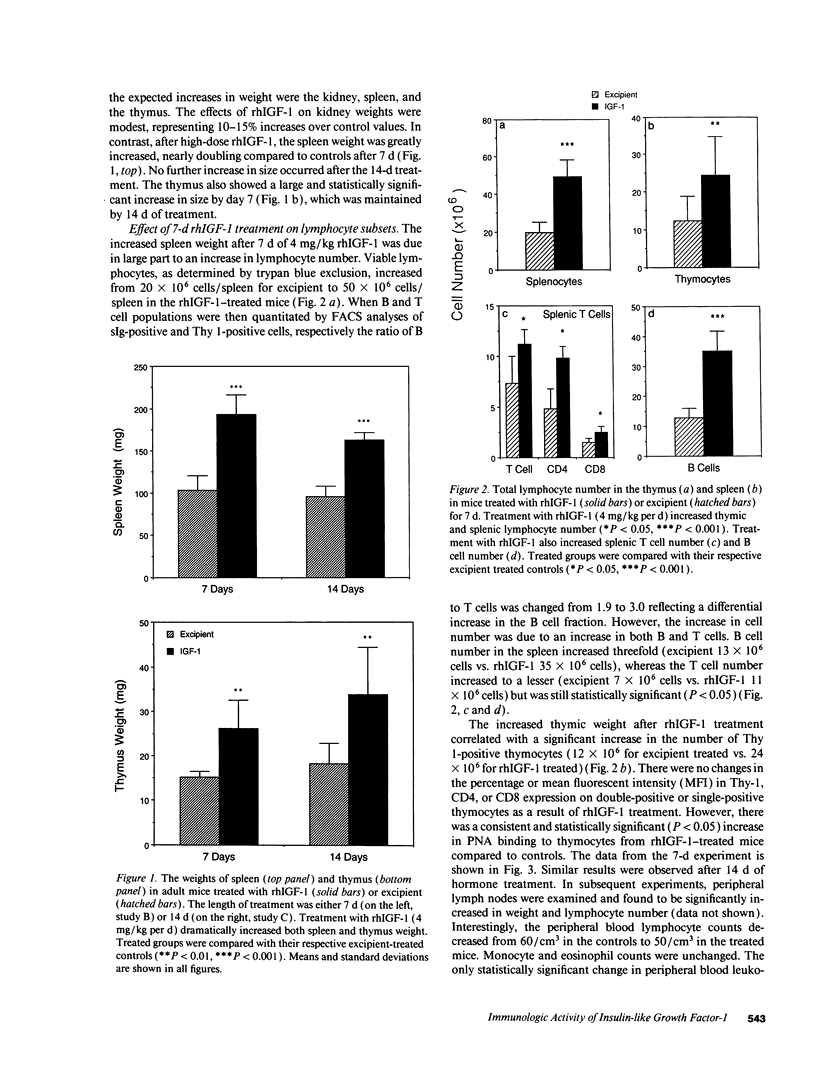
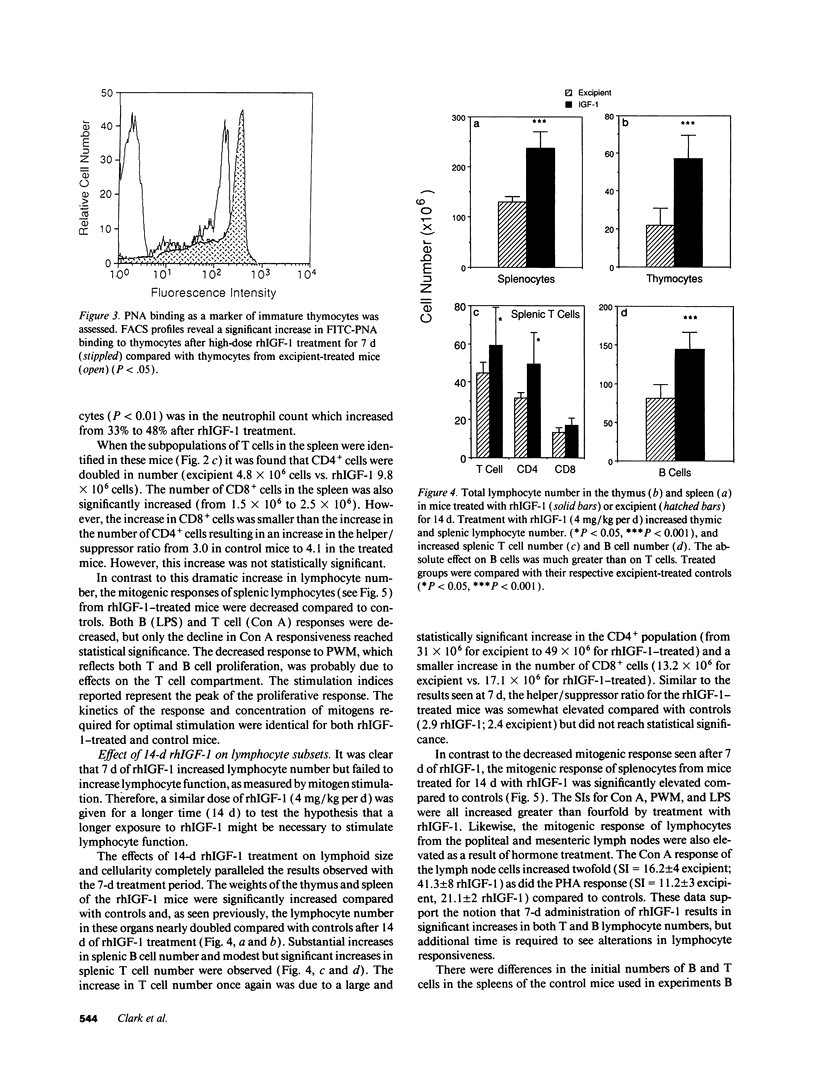
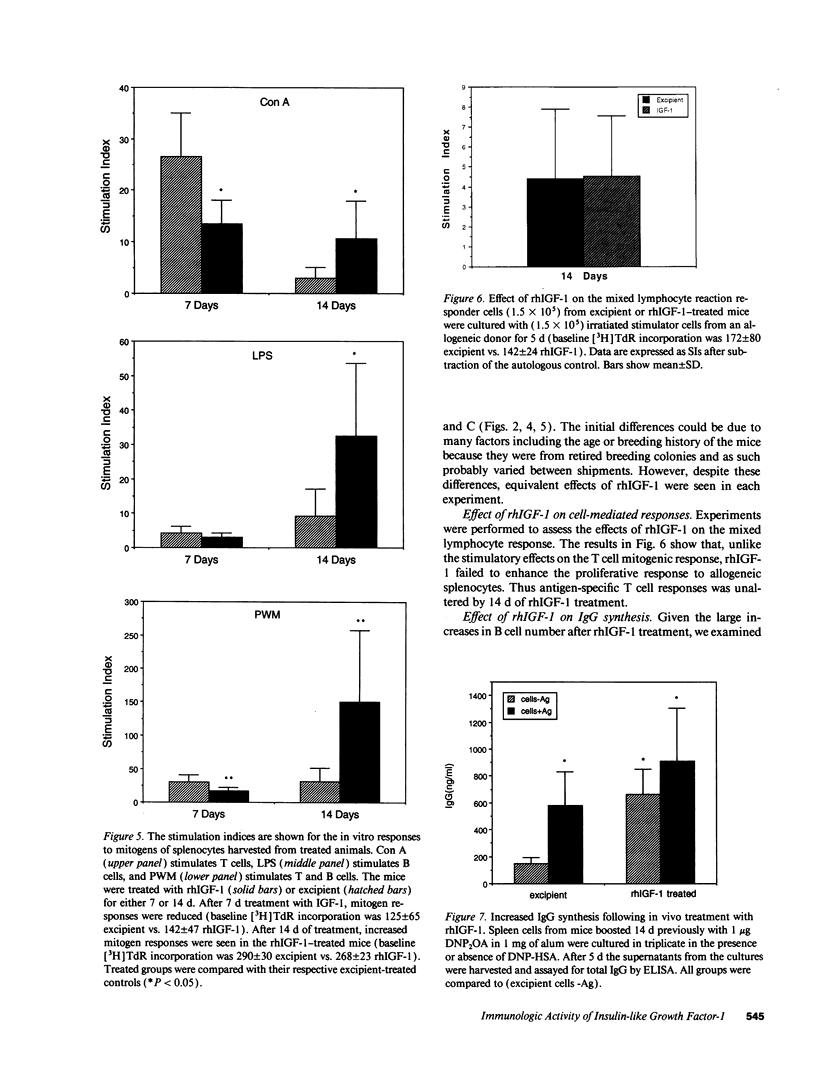
We show that treatment of adult mice with recombinant human insulin-like growth factor 1 (rhIGF-1) induces striking modifications in lymphocyte number and function. 9-mo-old male mice received rhIGF-1 (4 mg/kg per d) or its excipient by subcutaneous infusion from osmotic minipumps for 7 or 14 d. Mice were weighed daily and bled at sacrifice; the spleen and thymus were harvested and single cell suspensions were made for analysis of cell phenotype and cell number. The responses of splenocytes to mitogens (concanavalin A, lipopolysaccharide, and pokeweed mitogen), alloantigens and dinitrophenyl ovalbumin were measured. After either 7 or 14 d of treatment, rhIGF-1 had an overall whole-body anabolic effect, resulting in increased body and organ weights with prominent increases in the weight of the spleen and thymus. Furthermore, the rhIGF-1 treated mice were normoglycemic but had reduced blood urea nitrogens, again reflecting the anabolic activity of rhIGF-1. The increased spleen and thymus weights were associated with a large increase in the number of lymphocytes in both organs. In addition to an increase in T cells, specifically CD4+ T cells, a dramatic increase in splenic B cells was also observed. This increase was accompanied by an enhanced responsiveness to dinitrophenyl ovalbumin resulting in increased immunoglobulin production. However, despite the increases in cellularity, there was a decrease in the in vitro responses of spleen cells to mitogens after 7 d of rhIGF-1 treatment. In contrast, treatment with rhIGF-1 for 14 d increased both the cell number and mitogenic responses of splenocytes suggesting that some time is required for the cells populating the peripheral organs to gain mitogenic responsiveness. It is clear from these data that rhIGF-1, at doses that have whole-body anabolic activity, can expand cell number in lymphoid tissue in a normal adult mouse. These dual effects of rhIGF-1, of increasing lymphocyte number and activity, indicate that, in a normal adult animal, rhIGF-1 can cause major changes in lymphoid tissues that are of potential benefit to the functioning of the immune system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Matias C., Guiffre A., Seymour R., Cooley M., Biggs J., Munro V., Gillis S. In vivo administration of granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage CSF, interleukin-1 (IL-1), and IL-4, alone and in combination, after allogeneic murine hematopoietic stem cell transplantation. Blood. 1991 Mar 15;77(6):1376–1382. [PubMed] [Google Scholar]
- Beschorner W. E., Divic J., Pulido H., Yao X., Kenworthy P., Bruce G. Enhancement of thymic recovery after cyclosporine by recombinant human growth hormone and insulin-like growth factor I. Transplantation. 1991 Nov;52(5):879–884. doi: 10.1097/00007890-199111000-00024. [DOI] [PubMed] [Google Scholar]
- Binz K., Joller P., Froesch P., Binz H., Zapf J., Froesch E. R. Repopulation of the atrophied thymus in diabetic rats by insulin-like growth factor I. Proc Natl Acad Sci U S A. 1990 May;87(10):3690–3694. doi: 10.1073/pnas.87.10.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charak B. S., Choudhary G. D., Tefft M., Mazumder A. Interleukin-2 in bone marrow transplantation: preclinical studies. Bone Marrow Transplant. 1992 Aug;10(2):103–111. [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Hall K., Salmon W. D., Jr, Van den Brande J. L., Van Wyk J. J. On the nomenclature of the somatomedins and insulin-like growth factors. J Clin Endocrinol Metab. 1987 Nov;65(5):1075–1076. doi: 10.1210/jcem-65-5-1075. [DOI] [PubMed] [Google Scholar]
- Dziarski R. Ontogenic development of proliferative and polyclonal antibody and autoantibody responses to staphylococcal peptidoglycan, protein A and cell walls in mice. Dev Comp Immunol. 1985 Winter;9(1):119–130. doi: 10.1016/0145-305x(85)90065-5. [DOI] [PubMed] [Google Scholar]
- Fabris N., Pierpaoli W., Sorkin E. Hormones and the immunological capacity. 3. The immunodeficiency disease of the hypopituitary Snell-Bagg dwarf mouse. Clin Exp Immunol. 1971 Aug;9(2):209–225. [PMC free article] [PubMed] [Google Scholar]
- Geffner M. E., Bersch N., Lippe B. M., Rosenfeld R. G., Hintz R. L., Golde D. W. Growth hormone mediates the growth of T-lymphoblast cell lines via locally generated insulin-like growth factor-I. J Clin Endocrinol Metab. 1990 Aug;71(2):464–469. doi: 10.1210/jcem-71-2-464. [DOI] [PubMed] [Google Scholar]
- Groesbeck M. D., Parlow A. F., Daughaday W. H. Stimulation of supranormal growth in prepubertal, adult plateaued, and hypophysectomized female rats by large doses of rat growth hormone: physiological effects and adverse consequences. Endocrinology. 1987 May;120(5):1963–1975. doi: 10.1210/endo-120-5-1963. [DOI] [PubMed] [Google Scholar]
- Guler H. P., Zapf J., Scheiwiller E., Froesch E. R. Recombinant human insulin-like growth factor I stimulates growth and has distinct effects on organ size in hypophysectomized rats. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4889–4893. doi: 10.1073/pnas.85.13.4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder A. T., Spencer E. M., Preece M. A. Effect of bovine growth hormone and a partially pure preparation of somatomedin on various growth parameters in hypopituitary dwarf mice. J Endocrinol. 1981 May;89(2):275–282. doi: 10.1677/joe.0.0890275. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. Growth hormone, lymphocytes and macrophages. Biochem Pharmacol. 1989 Mar 1;38(5):705–713. doi: 10.1016/0006-2952(89)90222-0. [DOI] [PubMed] [Google Scholar]
- Kelley K. W. The role of growth hormone in modulation of the immune response. Ann N Y Acad Sci. 1990;594:95–103. doi: 10.1111/j.1749-6632.1990.tb40471.x. [DOI] [PubMed] [Google Scholar]
- Khansari D. N., Gustad T. Effects of long-term, low-dose growth hormone therapy on immune function and life expectancy of mice. Mech Ageing Dev. 1991 Jan;57(1):87–100. doi: 10.1016/0047-6374(91)90026-v. [DOI] [PubMed] [Google Scholar]
- Leshem B., Dekel R., Bercovier H., Tchakirov R., Polacheck I., Zakay-Rones Z., Schlesinger M., Kedar E. Cytokine-induced resistance to microbial infections in normal, immunosuppressed and bone marrow transplanted mice. Bone Marrow Transplant. 1992 Jun;9(6):471–477. [PubMed] [Google Scholar]
- Mackensen A., Galanos C., Engelhardt R. Treatment of cancer patients with endotoxin induces release of endogenous cytokines. Pathobiology. 1991;59(4):264–267. doi: 10.1159/000163659. [DOI] [PubMed] [Google Scholar]
- Merchav S., Tatarsky I., Hochberg Z. Enhancement of human granulopoiesis in vitro by biosynthetic insulin-like growth factor I/somatomedin C and human growth hormone. J Clin Invest. 1988 Mar;81(3):791–797. doi: 10.1172/JCI113385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Human growth hormone promotes engraftment of murine or human T cells in severe combined immunodeficient mice. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4481–4485. doi: 10.1073/pnas.89.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Durum S. K., Longo D. L. Role of neuroendocrine hormones in murine T cell development. Growth hormone exerts thymopoietic effects in vivo. J Immunol. 1992 Dec 15;149(12):3851–3857. [PubMed] [Google Scholar]
- Ranges G. E., Bombara M. P., Aiyer R. A., Rice G. G., Palladino M. A., Jr Tumor necrosis factor-alpha as a proliferative signal for an IL-2-dependent T cell line: strict species specificity of action. J Immunol. 1989 Feb 15;142(4):1203–1208. [PubMed] [Google Scholar]
- Ransom D. T., Neuberg D., Loprinzi C. L., Tormey D. C., Blum R. H., Harris J. E., Asbury R. F., Falkson G. A pilot study of three sequential chemotherapeutic regimens in metastatic breast cancer. Am J Clin Oncol. 1991 Feb;14(1):45–48. doi: 10.1097/00000421-199102000-00010. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tarduchy G., Collins M. K., García I., López-Rivas A. Insulin-like growth factor-I inhibits apoptosis in IL-3-dependent hemopoietic cells. J Immunol. 1992 Jul 15;149(2):535–540. [PubMed] [Google Scholar]
- Schimpff R. M., Repellin A. M., Salvatoni A., Thieriot-Prevost G., Chatelain P. Effect of purified somatomedins on thymidine incorporation into lectin-activated human lymphocytes. Acta Endocrinol (Copenh) 1983 Jan;102(1):21–26. doi: 10.1530/acta.0.1020021. [DOI] [PubMed] [Google Scholar]
- Schuurman H. J., Brekelmans P., Daemen T., Broekhuizen R., Kater L. T-cell maturation in the human thymus and tonsil: peanut agglutinin binding T lymphocytes in thymus and tonsil differ in maturation stage. Clin Immunol Immunopathol. 1983 Nov;29(2):271–281. doi: 10.1016/0090-1229(83)90029-6. [DOI] [PubMed] [Google Scholar]
- Shannon K. Genetic alterations in leukemia: events on a grand scale. Blood. 1992 Jul 1;80(1):1–2. [PubMed] [Google Scholar]
- Skottner A., Clark R. G., Fryklund L., Robinson I. C. Growth responses in a mutant dwarf rat to human growth hormone and recombinant human insulin-like growth factor I. Endocrinology. 1989 May;124(5):2519–2526. doi: 10.1210/endo-124-5-2519. [DOI] [PubMed] [Google Scholar]
- Stuart C. A., Meehan R. T., Neale L. S., Cintron N. M., Furlanetto R. W. Insulin-like growth factor-I binds selectively to human peripheral blood monocytes and B-lymphocytes. J Clin Endocrinol Metab. 1991 May;72(5):1117–1122. doi: 10.1210/jcem-72-5-1117. [DOI] [PubMed] [Google Scholar]
- Tapson V. F., Boni-Schnetzler M., Pilch P. F., Center D. M., Berman J. S. Structural and functional characterization of the human T lymphocyte receptor for insulin-like growth factor I in vitro. J Clin Invest. 1988 Sep;82(3):950–957. doi: 10.1172/JCI113703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit J., Savino W., Safieh B., Chanson P., Gagnerault M. C., Bach J. F., Dardenne M. Growth hormone and insulin-like growth factor-I stimulate hormonal function and proliferation of thymic epithelial cells. J Clin Endocrinol Metab. 1992 Jul;75(1):183–188. doi: 10.1210/jcem.75.1.1619008. [DOI] [PubMed] [Google Scholar]
- Woodall S. M., Breier B. H., O'Sullivan U., Gluckman P. D. The effect of the frequency of subcutaneous insulin-like growth factor-1 administration on weight gain in growth hormone deficient mice. Horm Metab Res. 1991 Dec;23(12):581–584. doi: 10.1055/s-2007-1003760. [DOI] [PubMed] [Google Scholar]
- van Buul-Offers S., Ueda I., Van den Brande J. L. Biosynthetic somatomedin C (SM-C/IGF-I) increases the length and weight of Snell dwarf mice. Pediatr Res. 1986 Sep;20(9):825–827. doi: 10.1203/00006450-198609000-00002. [DOI] [PubMed] [Google Scholar]