Abstract
Background
Information that enhances expectations about drug effectiveness improves the response to placebos for pain. Although asthma often improves with placebo, it is not known whether the response to placebo or active treatment can be augmented by increasing expectation of benefit.
Objective
The study objective was to determine if response to placebo or a leukotriene antagonist (montelukast) can be augmented by messages that increase expectation of benefit.
Methods
A randomized 20-center controlled trial enrolled 601 asthmatics with poor symptom control were assigned to one of five study groups. Participants were randomly assigned to either four treatment groups in a factorial design: placebo with enhanced messages, placebo with neutral messages, montelukast with enhanced messages, or montelukast with neutral messages; or to usual care. Assignment to study drug was double-masked; assignment to message content was single-masked; usual care was not masked. The enhanced message aimed to increase expectation of benefit from the drug. The primary outcome was mean change in daily peak-flow over 4 weeks. Secondary outcomes included lung function and asthma symptom control.
Results
Peak flow and other lung function measures were not improved in participants assigned to the enhanced message groups versus neutral messages groups for either montelukast or placebo; no differences were noted between neutral placebo and usual care groups. Placebo-treated participants had improved asthma control with the enhanced message, but not montelukast-treated participants; neutral placebo did have improved asthma control compared to usual care after adjusting for baseline difference. Headaches were more common in participants provided messages that mentioned headache as a montelukast side effect.
Conclusions
Optimistic drug presentation augments the placebo effect for patient-reported outcomes (asthma control), but not lung function. However, the effect of montelukast was not enhanced by optimistic messages regarding treatment effectiveness.
INTRODUCTION
Delivery of information that alters a patient’s expectation regarding the effectiveness of the treatment appears to be one of the most important and consistently effective components of the placebo response. 1 Strategies that affect patients’ expectation of benefit have included direct and indirect messages to the participants,2,3,4,5,6,7,8,9 method of drug administration,10 and color and branding of oral drugs.11,12,13 Such strategies have been reported most often for pain treatment. However, in asthma, placebo effects have been observed on both patient-reported and physiologic outcomes, but there is variability in the magnitude of response.14,15,16,17,18 If it is possible to enhance the effect of placebo for treatment of asthma, it also might be possible to improve the effectiveness of active treatments through such strategies.
We wanted to know whether altered expectation of benefit might account for variability in placebo responses in asthma and whether such interventions might also improve responses to pharmacologically active treatments. Such findings could help physicians to understand whether drug presentation, branding and advertising can improve clinical outcomes for active drugs or inactive compounds used in the treatment of asthma. Moreover, because patient-reported asthma control as well as improvement in lung function is increasingly used to guide to asthma therapy, we sought to learn whether these outcomes might be differentially affected by presentation of the treatment.
Accordingly, we conducted a clinical trial to evaluate the effect of manipulating expectations regarding drug effectiveness on both physiologic and patient-reported responses to an active drug (montelukast) or placebo. We hypothesized that the effects of enhancing expectancy would be different in an active treatment group compared to a placebo group and that physiologic outcomes measures would be less susceptible to expectancy effects than subjective measures.
METHODS
Study Design
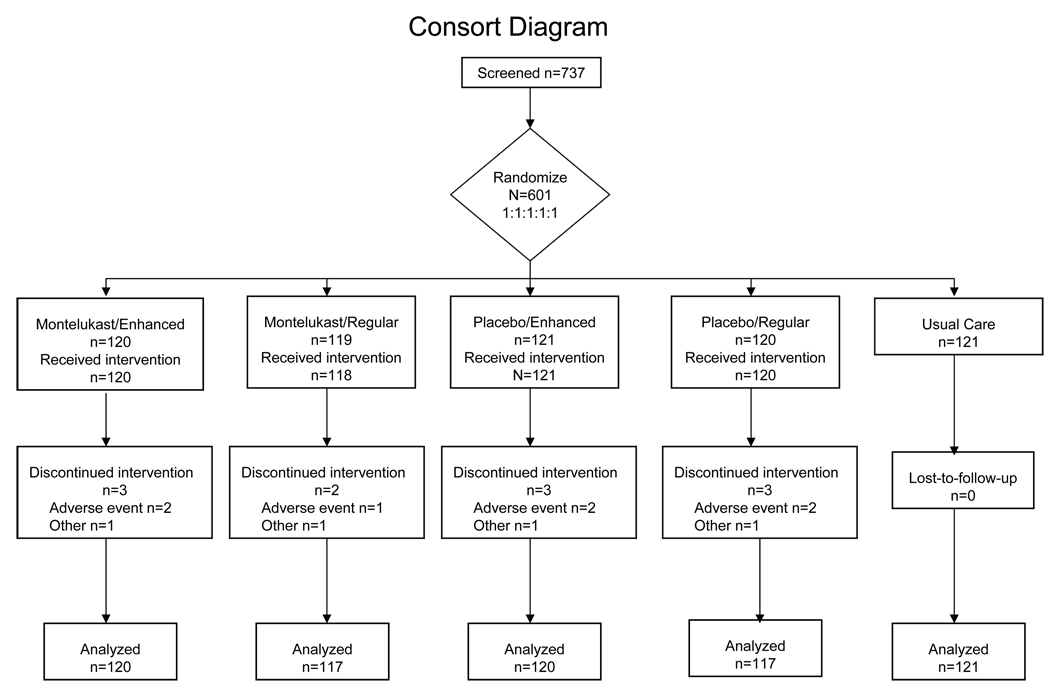
The study design was a 2 × 2 factorial design with an additional group for a total of 5 groups. The two main factors were study drug (montelukast versus placebo) and treatment presentation (neutral versus enhanced expectancy); the additional group was usual care. Participants at each center were randomly assigned with equal chance of allocation to one of 5 treatments (Figure 1). Randomization was stratified by clinical center and utilized a permuted block design with variable block sizes. The factorial design permitted us to assess the effect of the treatment presentation on response to placebo as well as the response to montelukast. After a 2-week run-in period for collection of baseline data, participants took study drug for 4 weeks. Study visits occurred every 2 weeks. We also studied a concurrent group of asthmatics treated with usual care who were randomized to receive no study treatment to determine whether the placebo capsules had an effect beyond enrollment in a research study,19,20,21.
Figure 1. Enrollment, Randomization, and Follow-up of Study Participants.
All patients for whom follow-up data were available were included in the analysis, regardless of whether or not they discontinued treatment.
The 20 centers of the American Lung Association Asthma Clinical Research Centers (ALA-ACRC) conducted the trial from December 2003 to December 2005. The ALA-ACRC Coordinating Center collected and analyzed the data. The trial was sponsored by the American Lung Association and NIH. All centers obtained and maintained IRB approval throughout the study. The trial was registered at ClinicalTrials.gov, NCT00148408 under the acronym Trial of Asthma Patient Education (TAPE).
Participants
Eligible participants had inadequately-controlled asthma. Inclusion criteria were: non-smokers 15 years or older, a history of physician-diagnosed asthma with regular use of asthma medication in the preceding year, post-bronchodilator FEV1 ≥ 75% of predicted,22 and one or more indicators of poor asthma control [Asthma Control Questionnaire (ACQ)23 ≥ 1.5, use of beta-agonists for asthma symptoms ≥ 2 times per week, or nocturnal awakening ≥ once/week]. Participants taking or intolerant of montelukast or participants with other serious health problems were excluded. Participants signed IRB-approved informed consent. No deception was employed in the conduct of the trial. The informed consent document stated that “The purpose of this research study is to investigate the way that educational approaches and presentation of a drug may affect the response to montelukast and placebo (an inactive medication) in subjects with asthma. You are being asked to join this research study because you have asthma that causes you symptoms with your current treatment.”
Drug Treatments
The active treatment was montelukast 10 mg orally (Singulair®, Merck & Co. Whitehouse Station, N.J.). Tablets were over-encapsulated to be indistinguishable from placebo. All drugs supplies were purchased by the ALA-ACRC. Participants and research staff were masked to drug treatment assignment. Participants assigned to usual care did not receive study drug and their assignment was unmasked.
Drug Presentation
Presentation of treatments to participants was done either with enhanced messages to increase expectation of benefit or with neutral messages about treatment benefit. Participants assigned to usual care did not receive a presentation; they received a NIH booklet on controlling asthma.24 The presentation occurred shortly after randomization and after two weeks. Clinical center staff were not masked to the presentation assignment. The drug presentation had 3 components: a scripted message, a computer presentation, and the appearance of the capsules. The research coordinator gave a scripted description of the treatment. (See online repository). The enhanced expectancy script emphasized the benefits of treatment and the probability of improved asthma symptoms; the neutral presentation expressed uncertainty about improvement. Participants then viewed an interactive computer-based educational presentation at randomization and after 2-weeks. The computer presentations provided education in basic asthma self-care, including information about asthma pathophysiology, self-monitoring, trigger avoidance, peak flow monitoring, and an asthma action plan for both groups. The program consisted of 16 to 21 screens, some with interactive and animated features, and a narration. The interactive presentations took about 10–20 minutes to view at participants’ own pace. Each message group viewed 2 presentations. The first was viewed at the randomization visit after baseline data were collected; the second presentation extended and reinforced the first presentation and was viewed at the week 2 visit after data collection was complete. The presentations are available for downloading and viewing at: https://jshare.johnshopkins.edu/xythoswfs/webui/_xy-2406068_1-t_uakZ2QFb
The enhanced computer presentation emphasized the value and potency of the treatment (see online repository) including a television commercial for montelukast. The commercial included positive messages showing attractive young adults with asthma leading apparently active healthy and fulfilling lives. The commercial also described potential side effects of the medication. The neutral computer presentation showed the same basic education but did not show positive messages about the expected benefits of montelukast and did not contain the television commercial.
Because different colors of capsules cause different expectation of drug effect and potency, the capsules given to the enhanced group were two-tone blue, while the neutral presentation group received off-white capsules.25 Because brand-names are associated with increased perceived potency, the enhanced group’s active treatment was referred to as Singulair® while the neutral group’s active treatment was referred to as montelukast
Visit Schedule
Each participant had four visits scheduled over six weeks. The first visit was for enrollment, and the second was for collection of baseline data, randomization of eligible participants, distribution of drug, and the first educational session. Participants were assigned randomly to a study group at the time of the second visit, with prior concealment of treatment assignment via an online randomization system. At the third visit, 2 weeks after randomization, there was an additional educational session and collection of interim data; the fourth visit, 4 weeks after randomization, was for data collection and return of unused drug. Participants were instructed to record morning peak flow, asthma medication use, urgent care for asthma, and asthma symptoms on diary cards each day; one diary card was used per week.
Outcome measures
The primary outcome measure was change in morning peak-expiratory-flow (PEF) from baseline. Other measures included spirometry, the Asthma Control Questionnaire (ACQ),23 the Asthma Quality of Life Questionnaire,26 and the Asthma Symptom Utility Index (ASUI),27 and the Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire KASE-AQ.28 Participants also completed a questionnaire on their perceptions of asthma treatment that included four Likert-scale questions about the efficacy of montelukast in treating asthma. This questionnaire was completed prior to randomization and again at the final visit.
Statistical Analysis
The planned enrollment of 120 participants per group had 98% power to detect a difference of 30 L/min between the neutral versus enhanced presentation with a two-sided type I error of 0.025.29 The power to detect a similar difference between the effect of presentation on placebo and the effect of presentation on active drug (i.e. an interaction effect between factorial components) was estimated by Monte Carlo simulation to be 0.83, but was highly sensitive to the assumptions regarding the effect of presentation on the placebo group. Because montelukast is known to be superior to placebo,30 comparison of montelukast to placebo was not an aim of the trial. Power for the comparison of usual care versus neutral placebo was 76% with a two-sided type I error rate of 0.025.
Continuous outcome measures were analyzed as change from baseline values assessed during the initial two-week run-in period. Categorical event type outcomes were evaluated as the percent of participants experiencing the event. Statistical analysis of the outcomes was performed based on treatment assignment (intention-to-treat) using a linear regression or logistic model with robust estimates of variance (GEE) and adjusted for visit (2 or 4 weeks post randomization) and clinic.31 32 Post hoc sub group analyses were performed for groups based on lung function (bronchodilator response and percent predicted FEV1) and ACQ score at baseline. Analyses were performed with SAS V8.33
RESULTS
Participant Characteristics (Table 1)
Table 1.
Baseline Characteristics of Participants
| Montelukast | Placebo | ||||
|---|---|---|---|---|---|
| Enhanced | Neutral | Enhanced | Neutral | Usual Care | |
| N | 120 | 119 | 121 | 120 | 121 |
| Demographic Characteristics | |||||
| Mean age at randomization, yr ± SD | 37 ± 14 | 37 ± 13 | 39 ± 14 | 39 ± 14 | 37 ± 14 |
| Males (% of group) | 19 | 29 | 27 | 32 | 30 |
| Race or ethnic group (% of group) | |||||
| White | 57 | 59 | 60 | 62 | 54 |
| Black | 34 | 30 | 34 | 28 | 37 |
| Hispanic | 7 | 8 | 4 | 5 | 7 |
| Other | 2 | 3 | 2 | 5 | 2 |
| Former smoker (% of group) | 14 | 13 | 13 | 21 | 12 |
| Education (% of group) | |||||
| High school | 20 | 24 | 23 | 23 | 22 |
| Some college | 46 | 44 | 40 | 41 | 46 |
| College graduate | 33 | 33 | 37 | 36 | 31 |
| Employment status (% of group) | |||||
| Full-time employment | 50 | 50 | 50 | 46 | 49 |
| Party-time employment | 12 | 12 | 15 | 17 | 9 |
| Student | 16 | 18 | 14 | 17 | 18 |
| Not employed outside home | 14 | 14 | 10 | 11 | 14 |
| Retired/disabled | 7 | 5 | 12 | 10 | 10 |
| Asthma Characteristics | |||||
| Mean age of asthma onset yr ± SD | 18 ± 18 | 21 ± 24 | 21 ± 22 | 21 ± 20 | 19 ± 18 |
| Use of inhaled short-acting beta-agonist (MDI/Neb) ≥ 2 times/week (% of group) |
83 | 82 | 87 | 83 | 86 |
| Daily use of ICS (% of group) | 63 | 57 | 55 | 58 | 57 |
| Asthma Questionnaires* (mean ± SD) | |||||
| ACS (↓) (score range: 0–6) | 1.4 ± 0.7 | 1.6 ± 0.8 | 1.6 ± 0.7 | 1.5 ± 0.7 | 1.7 ± 0.7 |
| ASUI (↑) (score range: 0–1) | 0.78 ± 0.15 | 0.76 ± 0.17 | 0.78 ± 0.15 | 0.79 ± 0.14 | 0.76 ± 0.16 |
| AQL (↑) (score range: 1–7) | 5.1 ± 1.0 | 5.0 ± 1.1 | 5.0 ± 1.1 | 5.1 ± 1.1 | 4.7 ± 1.2 |
| KASE-AQ (↑) | |||||
| Knowledge (range: 1–20) | 12 ± 3 | 11 ± 3 | 12 ± 3 | 11 ± 3 | 11 ± 3 |
| Attitude (range:5–100) | 85 ± 7 | 86 ± 7 | 86 ± 7 | 86 ± 7 | 84 ± 7 |
| Self-efficacy (range:5–100) | 82 ± 9 | 82 ± 10 | 82 ± 8 | 81 ± 9 | 81 ± 10 |
| Pulmonary Function Measures(mean ± SD) | |||||
| PEF (L/min) † | 380 ± 83 | 380 ± 99 | 378 ± 98 | 401 ± 104 | 380 ± 975 |
| FEV1 Pre-BD (L) | 2.7 ± 0.7 | 2.8 ± 0.7 | 2.7 ± 0.8 | 2.8 ± 0.8 | 2.7 ± 0.7 |
| Pre-BD FEV1 (%predicted) ‡ | 87 ± 13 | 86 ± 13 | 87 ± 15 | 87 ± 13 | 86 ± 14 |
| FVC Pre-BD | 2.9 ± 0.7 | 3.0 ± 0.7 | 3.0 ± 0.9 | 3.0 ± 0.8 | 2.9 ± 0.7 |
| Pre-BD FVC (%predicted)‡ | 94 ± 13 | 95 ± 14 | 95 ± 14 | 94 ± 12 | 92 ± 12 |
| FEV1 %change post-BD | 9 ± 11 | 10 ± 13 | 11 ± 15 | 8 ± 9 | 9 ± 9 |
| FVC %change post-BD | 4 ± 8 | 4 ± 10 | 6 ± 12 | 3 ± 6 | 3 ± 79 |
↑ = higher score is better, ↓ = lower score is better
Mean value based on 10 to 14 day period prior to randomization
Predicted values for FEV1 and FVC are taken from: Hankinson JL et al.22.
Abbreviations: Enhanced = Enhanced expectancy message about study drug. Neutral = Neutral message about study drug. SD = standard deviation. MDI = metered dose inhaler. Neb = nebulizer. ACS = Asthma Control Score. ASUI = Asthma Symptom Utility Index. AQL = Asthma Quality of Life. FEV1 = Forced expiratory volume in 1 second, FVC = Forced vital capacity, BD = bronchodilator. KASE-AQ = Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire.
The trial enrolled participants between December 2003 and December 2005. A total of 601 participants were randomized to the five study groups arms at 20 academic clinical centers; clinics enrolled from 11 to 56 patients with a median of 27. Participants were recruited from the clinic patient populations and local advertisements; there was heterogenity among sites for several baseline characteristics. Of the 601 enrolled participants, 480 were randomly assigned to the factorial component of the trial: treatment with placebo or montelukast and enhanced or standard messages about treatment and 121 were assigned to usual care. (Figure 1) Most participants were females, and about one-third African-Americans (Table 1). Overall, the characteristics of the population were balanced among the groups with the exception that more women were in the montelukast-enhanced presentation group. By design, participants had poor asthma control as shown by an elevated ACQ score34 frequent use of beta-agonists to control asthma symptoms, or frequent nocturnal awakenings due to asthma. The mean pre-bronchodilator FEV1 was at the lower range of normal; 27% of participants demonstrated bronchodilator reversibility of 12% or greater and 33% had a pre-bronchodilator FEV1.that was 80% or less of predicted at baseline..
Follow-up (Figure 1)
Eleven participants terminated treatment early (2%): 7 due to adverse events (3 in montelukast, 4 in placebo), and 4 for other reasons. Six participants did not have follow-up data on the primary outcome. Diary card completion rates were high in all groups; 96 to 100% of participants recorded at least some diary entries across the 5 study groups. Comparably high rates of adherence to study treatment and diary card completion across treatment groups indicate that there were no effects of the presentation on compliance with study procedures.
Adverse Effects
Headaches were reported by 37% of participants assigned montelukast with enhanced presentation versus only 29% who took montelukast with neutral presentation. In the placebo group, 28% of participants assigned to the enhanced presentation reported headaches versus 19% of those assigned the neutral presentation. Thus, both montelukast (P=0.02) and the enhanced presentation (P=0.03) were associated with 8 to 9% increase in frequency of reports of headache. The percentages of participants reporting other side effects including lethargy, gastrointestinal distress, fever, rhinitis, cough, “flu”, and skin rash were similar among the four treatment groups. Adverse events were similar in the neutral placebo and usual care group with the exception of dizziness (11% versus 22%, respectively, P = 0.02).
Pulmonary Function (Table 2)
Table 2.
Physiologic Outcomes – change from baseline
| Treatment Groups | P- values* | ||||||
|---|---|---|---|---|---|---|---|
| Montelukast | Placebo | Message Effect |
Drug Effect |
Interaction Drug x Message |
|||
| Enhanced | Neutral | Enhanced | Neutral | ||||
| N | 120 | 117 | 120 | 116 | |||
| Mean change from baseline (95% Confidence Interval) | |||||||
| PEF (L/Min) | 15 (9, 20) | 14 (8, 21) | 7 (1, 13) | 3 (−3, 9) | 0.42 | 0.001 | 0.55 |
| FEV1 Pre-BD (L) | 0.09 (0.05, 0.13) | 0.05 (0.01, 0.10) | 0.00 (−0.04,0.03) | −0.01 (−0.04, 0.02) | 0.25 | <0.001 | 0.42 |
| FEV1 Post-BD (L) | 0.02 (−0.02, .07) | 0.00 (−0.04, 0.04) | −0.09 (−0.17, −0.01) | −0.05 (−0.09, −0.01) | 0.66 | <0.001 | 0.16 |
| FVC Pre-BD (L) | 0.06 (0.01, 0.11) | 0.05 (0.00, 0.10) | −0.01 (−0.05, 0.03) | −0.03 (−0.06, 0.01) | 0.50 | 0.001 | 0.93 |
| FVC Post-BD (L) | 0.00 (−0.05,0.05) | −0.02 (−0.06, 0.03) | −0.10 (−0.18, −0.01) | −0.06 (−0.10, −0.01) | 0.63 | 0.003 | 0.25 |
P-values presented are adjusted for visit and the variance estimates were adjusted for repeated measures. Message effect = Effect of enhanced message independent of drug. Drug Effect = Effect of montelukast independent of message type. The interaction P-value evaluates for whether the drug effect is modified by the message effect
Abbreviations: Enhanced = Enhanced expectancy message for study drug. Neutral = Neutral message about study drug. PEF = Peak expiratory flow. FEV1 = Forced expiratory volume in 1 second. FVC = Forced vital capacity. BD = bronchodilator
PEF and other measures of lung function significantly improved with montelukast compared to placebo. However, PEF and other spirometry measures did not significantly improve with the enhanced expectancy message compared to the neutral message. Participants who demonstrated bronchodilator reversibility of 12% or greater or had pre-bronchodilator FEV1 of 80% or less at baseline had greater improvements in lung function during follow-up, however there was no evidence to support that either the drug effect or message effect was stronger in those subgroups (data not shown). A baseline ACQ score of 2 or greater was not associated with differences in lung function outcomes (data not shown). There were no differences in pulmonary function outcomes between participants assigned to neutral placebo versus usual care (Table 5).
Table 5.
Outcomes for the Usual Care and Neutral Placebo group
| Group | |||
|---|---|---|---|
| Usual Care | Placebo-Neutral | P-value* | |
| Pulmonary Function | Mean change from baseline | ||
| (95% Confidence Interval) | |||
| N | 121 | 116 | |
| PEF (L/Min) | −4 (−8, 0) | 3 (−3, 9) | 0.04 |
| FEV1 Pre-BD (L) | −0.05 (−0.09, 0.00) | −0.01 (−0.04, 0.02) | 0.16 |
| FEV1 Post-BD (L) | −0.05 (−0.10, −0.01) | −0.05 (−0.09, −0.01) | 0.81 |
| FVC Pre-BD (L) | −0.07 (−0.11, −0.03) | −0.03 (−0.06, 0.01) | 0.07 |
| FVC Post-BD (L) | −0.04 (−0.09, 0.01) | −0.06 (−0.10, −0.01) | 0.64 |
| Baseline mean (SD) | |||
| Treatment perceptions† | |||
| Mean change from baseline | |||
| Statement | |||
| (95% Confidence Interval) | |||
| N | 121 | 117 | |
| Singulair (Montelukast) is an effective drug in helping control asthma. |
6.3 (1.8) 0.1 (−0.2, 0.4) |
6.4 (1.6) 0.2 (−0.1, 0.5) |
0.56 |
| If I were to take Singulair (Montelukast), it would help my asthma |
6.4 (1.7) −0.1 (−0.4, 0.2) |
6.3 (1.4) 0.0 (−0.3, 0.3) |
0.89 |
| Singulair (Montelukast) is a weak drug for asthma. | 4.0 (1.6) 0.0 (−0.3, 0.2) |
4.1 (1.7) −0.3 (−0.6, 0.0) |
0.19 |
| Singulair (Montelukast) is likely to help people with asthma |
6.7 (1.5) −0.1 (−4, 0.2) |
6.6 (1.4) 0.2 (0.1, 0.2) |
0.06 |
| Asthma Questionnaires‡ |
Mean change from baseline (95% Confidence Interval)† |
||
| N | 121 | 117 | |
| ACQ score (↓ score range: 0–6) | 0.−1 (−0.3, 0.0) | −0.2 (−0.3, −0.1) | 0.23 |
| Asthma QoL (↑score range: 1–7) | 0.3 (0.2, 0.5) | 0.4 (0.3, 0.5) | 0.64 |
| ASUI x 100 (↑score range: 0–100) | 3.3 (1.2, 5.5) | 1.7 (−0.4, 3.8) | 0.30 |
| KASE-AQ | |||
| Knowledge (↑score range: 1–20) | 0.2 (0.2, 0.6) | 0.3 (−0.1, 0.7) | 0.60 |
| Attitude (↑score range: 5–100) | 2 (1, 3) | 3 (2, 4) | 0.15 |
| Self-efficacy (↑score range: 5–100) | 2 (1, 4) | 4 (3, 5) | 0.04 |
| Other outcomes | |||
| %Symptom-free days (mean, 95% CI) § | 49 (43, 55) | 58 (52, 64) | 0.03 |
| Any nocturnal awakenings (n (%))¶ | 57 (47) | 36 (31) | 0.007 |
| Participants requiring urgent asthma care or steroid courses (n(%)) |
9 (7) | 7 (6) | 0.67 |
P-values presented are adjusted for clinic, visit and the variance estimates were adjusted for repeated measures and measures the difference between the Usual Care group and the Neutral Placebo group.
Baseline mean and difference from baseline are based on ordinal scale score of 1 to 9, strongly disagree to strongly agree, 5 is neutral.
↑ = higher score is better, ↓ = lower score is better.; the clinically important differences for the questionnaires are: 0.5 units for ACQ and QoL; 20 units for the ASUI x 100; and 5 units for the Knowledge componet of the KASE-AQ and 10 units for the Attitude and Self-efficacy components.
Symptom-free days are defined as a day with an Asthma Symptom score of 0, no nocturnal awakenings, not taking oral prednisone, and no need for urgent care.
Nocturnal awakenings are calculated as the percent of patients who experienced one or more nocturnal awakening due to asthma symptoms.
Abbreviations: Enhanced = Enhanced expectancy message for study drug. Neutral = Neutral message about study drug. PEF = Peak expiratory flow. FEV1 = Forced expiratory volume in 1 second. FVC = Forced vital capacity. BD = bronchodilator. ACQ = Asthma Control Questionnaire. ASUI = Asthma Symptom Utility Index. KASE-AQ = Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire
Patient Reported Outcomes (Tables 3 and 4)
Table 3.
Treatment perceptions
| Treatment Groups | |||||||
|---|---|---|---|---|---|---|---|
| Montelukast | Placebo | P-values* | |||||
| Enhanced | Neutral | Enhanced | Neutral | Message Effect |
Drug Effect |
Interaction Drug x Message |
|
| N | 119 | 116 | 115 | 117 | |||
| Statement | Mean value at baseline (standard deviation) † | ||||||
| Mean change from baseline (95% Confidence Interval) | |||||||
| Singulair (Montelukast) is an effective drug in helping control asthma. |
6.2 (1.7) 0.9 (0.5, 1.3) |
6.3 (1.6) 0.5 (0.2., 0.8) |
6.3 (1.6) 0.9 (0.6, 1.2) |
6.4 (1.6) 0.2 (−0.1, 0.5) |
0.001 | 0.35 | 0.47 |
| If I were to take Singulair (Montelukast), it would help my asthma |
6.2 (1.7) 1.0 (0.7, 1.4) |
6.2 (1.5) 0.5 (0.2, 0.8) |
6.2 (1.5) 0.7 (0.4, 1.0) |
6.3 (1.4) 0.0 (−0.3, 0.2) |
<0.001 | 0.01 | 0.40 |
| Singulair (Montelukast) is a weak drug for asthma. | 4.0 (1.5) −0.7 (−1.1, −0.4) |
4.1 (1.6) −0.5 (−0.8, −0.2)) |
4.0 (1.4) −0.7 (−1, −0.4) |
4.1 (1.7) −0.3 (−0.6, 0.0) |
0.04 | 0.56 | 0.73 |
| Singulair (Montelukast) is likely to help people with asthma |
6.6 (1.6) 0.7 (0.4, 1.1) |
6.8 (1.4) 0.2 (0.0, 0.5) |
6.6 (1.4) 0.6 (0.4, 0.9) |
6.6 (1.4) 0.2 (0.0, 0.5) |
0.001 | 0.73 | 0.71 |
P-values presented are adjusted for clinic. Message effect = Effect of enhanced message independent of drug. Drug Effect = Effect of montelukast independent of message type. The interaction P-value evaluates for whether the drug effect is modified by the message effect
Baseline mean and difference from baseline are based on ordinal scale score of 1 to 9, strongly disagree to strongly agree, 5 is neutral.
Table 4.
Patient Reported Outcomes
| Treatment Groups | |||||||
|---|---|---|---|---|---|---|---|
| Montelukast | Placebo | P- values* | |||||
| Enhanced | Neutral | Enhanced | Neutral | Message Effect |
Drug Effect |
Interaction Drug x Message |
|
| N | 120 | 117 | 120 | 117 | |||
| Asthma Questionnaires | Mean change from baseline (95% Confidence Interval) † | ||||||
| ACQ score (↓ score range: 0–6) | −0.40 (−0.52, −0.29) | −0.45 (−0.56,−0.34) | −0.45 (−0.55,−0.34) | −0.23 (−0.32, −0.14) | NA‡ | NA‡ | 0.01 |
| Asthma QoL (↑score range: 1–7) | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.7) | 0.4 (0.2, 0.5) | 0.24 | 0.16 | 0.13 |
| ASUI x 100 (↑score range: 0–100) | 6.0 (3.6, 8.5) | 5.6 (3.1, 8.0) | 6.5 (4.0, 9.0) | 1.7 (−0.4, 3.8) | 0.03 | 0.15 | 0.07 |
| KASE-AQ | |||||||
| Knowledge (↑score range: 1–20) | 0.4 (0.1, 0.8) | 0.8 (0.5, 1.2) | 0.3 (−0.1, 0.7) | 0.3 (−0.1, 0.7) | 0.25 | 0.09 | 0.26 |
| Attitude (↑score range: 5–100) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 3 (2, 4) | 0.82 | 0.81 | 0.87 |
| Self-efficacy (↑score range: 5–100) | 3 (2, 4) | 4 (2, 5) | 5 (4, 6) | 4 (3, 5) | 0.55 | 0.07 | 0.28 |
| Other outcomes | |||||||
| %Symptom-free days (mean, 95% CI)§ | 67 (61, 73) | 59 (53, 65) | 59 (53, 65) | 58 (52, 64) | 0.13 | 0.12 | 0.35 |
| Any nocturnal awakenings (n (%))¶ | 41 (34) | 32 (27) | 41 (34) | 36 (31) | 0.19 | 0.67 | 0.68 |
| Participants requiring urgent asthma care or steroid courses (n(%)) |
4 (3) | 3 (3) | 3 (3) | 7 (6) | 0.43 | 0.45 | 0.26 |
P-values presented are adjusted for visit and clinic and, where appropriate, the variance estimates were adjusted for repeated measures. Message effect = Effect of enhanced message independent of drug. Drug Effect = Effect of montelukast independent of message type. The interaction P-value evaluates for whether the drug effect is modified by the message effect
↑ = higher score is better, ↓ = lower score is better.; the clinically important differences for the questionnaires are: 0.5 units for ACQ and QoL; 20 units for the ASUI x 100; and 5 units for the Knowledge componet of the KASE-AQ and 10 units for the Attitude and Self-efficacy components.
Not Applicable - In the presence of an interaction, P-values for main effects are not reported; P-values for each level of the other covariate are reported in text.
Symptom-free days are defined as a day with an Asthma Symptom score of 0, no nocturnal awakenings, not taking oral prednisone, and no need for urgent care.
Nocturnal awakenings are calculated as the percent of patients who experienced one or more nocturnal awakening due to asthma symptoms.
Abbreviations and minimally important differences: ACQ = Asthma Control Questionnaire. ASUI = Asthma Symptom Utility Index. Enhanced = Enhanced expectancy message for study drug. Neutral = Neutral message about study drug. KASE-AQ = Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire.
On average, participants at baseline thought that montelukast was an effective drug for the treatment of asthma. Four weeks later, this perception was significantly improved for participants assigned to the enhanced expectancy presentation compared to the neutral presentation, but was not different for most perceptions in those assigned to montelukast versus placebo. The KASE-AQ28 questionnaire measured asthma self-efficacy and attitudes about asthma treatment. Neither the neutral nor the enhanced message had a significant impact on asthma attitudes or self-efficacy scores as measured by the KASE-AQ.
In addition to the daily asthma symptom diaries, participants completed three asthma symptom questionnaires, the ACQ, Asthma Quality of Life, and ASUI. The enhanced expectancy message was associated with a larger decrease (i.e. improvement) in the ACQ asthma control score in the placebo group (neutral-placebo versus enhanced-placebo: −0.23 versus −0.45, P = 0.001). This did not occur in the montelukast group (neutral-montelukast versus enhanced-montelukast: −0.45 versus −0.40, P = 0.54). A test for interaction between message type and drug treatment was significant (P = 0.01) indicating that the effect of the enhanced message was greater in the placebo group. After adjusting for baseline ACQ score, the enhanced presentation remained associated with improvements in ACQ scores for participants assigned to placebo (P = 0.002) but not in participants assigned to montelukast (P = 0.66). The ASUI showed a similar differential for the enhanced message effect, but evidence for the interaction was weaker (P = 0.07). Overall the presentation of an enhanced message was associated with an improvement in ASUI (P = 0.03) that was most evident in the patients receiving placebo. In subgroup analyses, participants who had more symptoms (ACQ scores ≥ 2) or worse lung function (FEV1 bronchodilator reversibility ≥ 12% or FEV1 or percent predicted ≤ 80%) at baseline had greater improvements in ACQ and ASUI scores in follow-up, however there was no strong evidence that message or drug effects were more pronounced in participants in these subgroups (data not shown). Other patient reported measures such as percent of days that were symptom-free or percent of participants with nocturnal awakenings or requiring urgent care or oral steroids for asthma were similar among the four treatment groups.
Participants assigned to neutral placebo had better outcomes for ACQ than the usual care group only after adjusting for baseline scores (P = 0.23 before adjustment versus P = 0.002 after adjustment); improvements in the ASUI or quality of life measures were not different between the usual care and neutral placebo group. Participants assigned to neutral placebo had more symptom-free days and fewer nocturnal awakenings than those assigned to usual care (Table 5).
DISCUSSION
This study was designed to address whether the response of poorly controlled asthmatics to placebo or montelukast could be improved by an optimistic message about drug effectiveness and, if so, whether the enhancement was similar in both groups. We used a multi-modality approach that included scripted messages delivered by the research staff, an interactive multimedia computer presentation including consumer commercials, and coloured over-encapsulation of the medication. The messages were designed to enhance the expectation that the treatment (if active) would be effective. This strategy did prove effective in increasing the perception that montelukast was an effective treatment for asthma, regardless of whether the patient was assigned to placebo or montelukast.
Although all of our participants had poorly controlled asthma, they demonstrated high scores for the asthma knowledge, attitude and self-efficacy at baseline, so the educational intervention was not associated with further improvement of these measures. However, we found that patient-reported outcomes that focused on asthma control (ACQ) and asthma symptoms (ASUI) were generally improved by the optimistic message that encouraged expectation of benefit in the placebo group. Furthermore, the effect of this optimistic mode of placebo presentation had the same magnitude of effect on asthma control as did the active drug montelukast. The enhanced message did not confer any added benefit to montelukast for improving symptom control. Importantly, although the enhanced message improved asthma control in the placebo group, it did not influence lung function measures. In contrast, montelukast improved lung function to the same extent regardless of how the treatments were presented. The lack of effect of an enhanced message on physiologic outcomes is consistent with the results of Kemeny et al, who found no effect of the communication positive expectancies by physicians on bronchoprovocation test results.18
We also evaluated the existence of a placebo effect in asthma by comparing a group randomized to usual care versus the neutral placebo group. Our results confirmed prior research that demonstrates that placebo effects are largely confined to patient-reported subjective outcomes and do not influence physiologic measures.
The effects on ACQ score were statistically significant, however, no effects of the enhanced message or the placebo were observed on Quality of Life scores. ACQ and Quality of Life questionnaires differ in that the former focuses on symptoms where as the later addresses activities of daily living. Hence the lack of effect on the Quality of Life scores may indicate that ACQ differences observed were minor and did not impact participants’ activities.
This trial is the largest and most comprehensive study to evaluate the manipulation of placebo effects in asthma, and the results of this study have implications for care of asthma patients as well as asthma clinical research. In placebo controlled randomized clinical trials there is an implicit assumption that the active treatment effect is additive to the placebo effect so that the treatment effect is estimated without bias19. If clinical trial participants are treated similarly in all respects other than provision of active drug or placebos, it is assumed that the “true” drug effect is ascertained by subtracting out the placebo response. Our study suggests that this is not necessarily true for asthma control measures. Presentation of the treatments with a neutral message about drug effectiveness (montelukast-neutral versus placebo-neutral) demonstrated the efficacy of montelukast for asthma control. However, when the treatment was presented with an optimistic bias (montelukast-enhanced versus placebo-enhanced) the placebo group improved so much that there was no measurable benefit of montelukast. Furthermore, after adjustment for baseline imbalances, we demonstrated a placebo effect on asthma control and, without adjustment, a placebo effect on symptom-free days and nocturnal awakenings. Thus, participants’ expectation of benefit from an experimental treatment could affect the outcome of a clinical trial that uses asthma control measures as a primary outcome.
Asthma control measures have been incorporated and given more importance in current treatment guidelines and there is recent interest among investigators in assessing the effectiveness of asthma treatments on control of asthma symptoms. 35 This is especially true for treatments with anti-inflammatory properties but without bronchodilator properties because their effectiveness may be better assessed by asthma symptom control rather than changes in lung function. Enrollment of participants with strong preconceptions of a new therapy’s value might produce an augmented placebo effect reducing the possibility of demonstrating the drug’s efficacy. Therefore, insuring that all participants and investigators are at equipoise may be an important component in clinical trial design, execution, and interpretation.
Although increasing expectancy improved the response to placebo, we were unable to improve the active treatment effect (of montelukast) on asthma control by increasing expectancy of benefit. One explanation for our inability to increase the response to montelukast with an enhanced presentation is a possible “ceiling” effect for this treatment. However, we cannot explain this ceiling effect as the result of achieving complete remission of asthma, as these participants still had residual asthma symptoms.
Neither the presence of a placebo or the enhanced expectancy presentation had any effect on lung function. Asthma care providers may be somewhat reassured that perception of the effectiveness of a treatment does not alter the bronchodilator response to treatment. On the other hand, our study shows that ineffective treatments for asthma can lead to better asthma symptom control if there is an optimistic message delivered with the treatment. Direct-to-consumer advertising for asthma products such as herbal or nutritional supplements, even if pharmacologically inactive, might lead to better asthma control as a consequence of enhanced expectation of benefit. The mechanism of this improvement is not known. It may reflect a change in reporting or perception of symptoms36, but could also reflect immune modulation due to suggestion.37 Moreover, because we used several strategies to increase expectation of benefit, we cannot determine how much was due to each component. A further limitation of our study is that we cannot extrapolate our results to other diseases or methods of expectancy enhancement.
The enhanced presentation increased the report of headaches as an adverse effect for those taking both montelukast and placebo. Since headache was not mentioned in any other components of the trial, we interpret that this was the consequence of viewing television commercials suggesting the possibility of headaches as an adverse effect. Thus, direct-to-consumer advertising may increase the reporting of adverse effects, a nocebo effect.
One limitation is the four week duration of the study, which limits insight into the duration of any observed effect. The placebo effect has been reported to wane over time for other classes of drugs, and this may also occur with asthma.38 However, no degradation of the effect of treatment presentation on ACQ was observed between weeks 2 and 4 raising the possibility that such effects could be durable.
In summary, this study showed that messages enhancing the expectation of benefit from a drug for asthma do not affect lung function but can augment the placebo effect on asthma symptoms. Because we could not induce a change in response to montelukast treatment with increased expectancy, such messages could mask the demonstration of benefit in any clinical trial of an effective treatment. Although it is unlikely that investigators would present a message as optimistic as we did in this trial, more subtle messages may be delivered by researchers, consumer advertising, internet or news media. Our results show that the way that a placebo is administered for asthma, and messages enhancing expectation of effectiveness, can influence the symptomatic response of patients despite a lack of efficacy for lung function.
Supplementary Material
Acknowledgments
This study is supported by grants from the NIH-NHLBI R01HL073494 and the American Lung Association.
List of abbreviations
- ACQ
Asthma Control Questionnaire
- ALC-ACRC
American Lung Association Asthma Clinical Research Centers
- ASUI
Asthma Symptom Utility Index
- AQL
Asthma Quality of Life
- BD
bronchodilator
- Enhanced
Enhanced expectancy message about study drug
- FEV1
Forced expiratory volume in 1 second
- FVC
Forced vital capacity
- KASE-AQ
Knowledge, Attitude, and Self-Efficacy Asthma Questionnaire
- MDI
metered dose inhaler
- Neb
nebulizer
- ACS
Asthma Control Score
- Neutral
Neutral message about study drug
- SD
standard deviation
Credit Roster: American Lung Association Asthma Clinical Research Centers
The following persons participated in the TAPE study
Baylor College of Medicine, Houston: N. A. Hanania (principal investigator), M. Sockrider (co-principal investigator), L. Giraldo (principal clinic coordinator), R. Valdez (coordinator);
Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla, N.Y.: A. Dozor (principal investigator), N. Amin and Y. C. Kim (co-principal investigator), I. Gherson (principal clinic coordinator), M. Heydendael and M. Key (coordinators);
Columbia University–New York University Consortium, New York: J. Reibman (principal investigator), E. DiMango (co-principal investigator), W. Hoerning (clinic coordinator at New York University), J. Sormillon (clinic coordinator at Columbia University);
Duke University Medical Center, Durham, N.C.: L. Williams (principal investigator), J. Sundy (co-principal investigator), G. Dudek (principal clinic coordinator), R. Newton (coordinator);
Emory University School of Medicine, Atlanta: W.G. Teague (principal investigator), S. Khatri (co-principal investigator), J. Costolnick, (principal clinic coordinator), J. Peabody, R. Patel, E Hunter (coordinators);
Illinois Consortium, Chicago: L. Smith (principal investigator), J. Moy, E Naureckas, C.S. Olopade (co-principal investigators), J. Hixon (principal clinic coordinator), A. Brees, G. Rivera, S. Sietsema, V. Zagaja (coordinators);
Indiana University, Asthma Clinical Research Center, Indianapolis: M. Busk (principal investigator), F. Leickly, C. Williams (co-principal investigators), S. Lynch (principal clinic coordinator); P. Puntenney (coordinator);
Jefferson Medical College, Philadelphia: F. Leone (principal investigator), M. Hayes-Hampton (principal clinic coordinator);
Louisiana State University Health Sciences Center, Ernest N. Morial Asthma, Allergy, and Respiratory Disease Center, New Orleans: W.R. Summer (principal investigator), C. Glynn (principal clinic coordinator);
National Jewish Medical and Research Center, Denver: S. Wenzel (principal investigator), P Silkoff (co-principal investigator), R. Gibbs (principal clinic coordinator), L. Lopez, C. Ruis, B. Schoen (coordinators);
Nemours Children’s Clinic–University of Florida Consortium, Jacksonville: J. Lima (principal investigator), K. Blake (co-principal investigator), A. Santos (principal clinic coordinator), L. Duckworth, D. Schaeffer (coordinators);
North Shore–Long Island Jewish Health System, New Hyde Park, N.Y.: J. Karpel (principal investigator), R. Cohen (co-principal investigator), R. Ramdeo (principal clinic coordinator);
Northern New England Consortium formerly Vermont Lung Center at the University of Vermont), Colchester, Vt.: C.G. Irvin (principal investigator), A.E. Dixon, D.A. Kaminsky, E. Kent, T. Lahiri, P. Shapiro (co-principal investigators), S. Lang (principal clinic coordinator), J. Allen, A. Coote, L.M. Doucette, K. Girard, J. Lynn, L. Moon, T. Viola (coordinators);
The Ohio State University Medical Center/Columbus Children’s Hospital, Columbus: J. Mastronarde (principal investigator), K. McCoy (co-principal investigator) J. Drake (principal clinic coordinator), R. Compton, L. Raterman (coordinators);
University of Alabama at Birmingham, Birmingham: L.B. Gerald (principal investigator), W.C. Bailey (co-principal investigator), S. Erwin (principal clinic coordinator), H. Young, A. Kelley, D. Laken, B. Martin (coordinators);
University of Miami, Miami–University of South Florida, Tampa: A. Wanner (principal investigator), R. Lockey (principal investigator), E. Mendes (principal clinic coordinator for University of Miami), M. Grandstaff (principal clinic coordinator for University of South Florida) B Fimbel (coordinator);
University of Minnesota, Minneapolis: M.N. Blumenthal (principal investigator), G. Brottman, J. Hagen (co-principal investigators), A. Decker, D. Lascewski, S. Kelleher (principal clinic coordinators), K. Bachman, M. Sneen (coordinators);
University of Missouri, Kansas City School of Medicine, Kansas City: G. Salzman (principal investigator), D. Pyszczynski (co-principal investigator), P. Haney (principal clinic coordinator);
St. Louis Asthma Clinical Research Center: Washington University, St. Louis University, and Clinical Research Center, St. Louis: M. Castro (principal investigator), L. Bacharier, K. Sumino (co-investigators), M.E. Scheipeter (principal clinic coordinator), J. Tarsi (coordinator);
University of California San Diego: S Wasserman (principal investigator), J Ramsdell (co-principal investigator)
Chairman’s Office, Respiratory Hospital, Winnipeg, Man., Canada: N. Anthonisen (research group chair);
Data Coordinating Center, Johns Hopkins University Center for Clinical Trials, Baltimore: R. Wise (center director), J. Holbrook (deputy director), E. Brown (principal coordinator), M. Dale, M. Daniel, G. Leatherman, C. Levine, J. Jones, R. Masih, S Modak, D. Nowakowski, N. Prusakowski, D. Shade, E. Sugar;
Data and Safety Monitoring Board: L. Hudson (chair), V. Chinchilli, P. Lanken, B. McWilliams, C. Rinaldo, D. Tashkin;
Project Office, American Lung Association, New York: R. Vento (project officer), N. Edelman (scientific consultant), S. Rappaport, G. Pezza,;
ALA Scientific Advisory Committee: G. Snider (chair), N. Anthonisen, M. Castro, J. Fish, D. Ingbar, S. Jenkinson, D. Mannino, H. Perlstadt, L. Rosenwasser, J. Samet, T. Standiford, J. Smith, L. Smith, D. Schraufnagel, A. Wanner, T. Weaver.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The trial was registered at ClinicalTrials.gov, NCT00148408
Clinical Implications
The result of our study showed that neither the presence of a placebo or the enhancing patients’ expectancy about the effectiveness of drug effect lung function. However, they may lead to better asthma symptom control.
Contributor Information
Susan J. Bartlett, Email: bartlett@jhmi.edu.
Ellen D. Brown, Email: ellbrown@jhsph.edu.
Mario Castro, Email: castrom@im.wustl.edu.
Rubin Cohen, Email: rcohen@lij.edu.
Janet T. Holbrook, Email: jholbroo@jhsph.edu.
Charles G. Irvin, Email: charles.irvin@uvm.edu.
Cynthia S. Rand, Email: crand@jhmi.edu.
Marianna M. Sockrider, Email: mmsockri@TexasChildrensHospital.org.
Elizabeth A. Sugar, Email: esugar@jhsph.edu.
REFERENCES
- 1.Price DD, Finniss DG, Benedetti F. A Comprehensive Review of the Placebo Effect: Recent Advances and Current Thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 2.De Pascalis V, Chiaradia C, Carotenuto E. The contribution of suggestibility and expectation to placebo analgesia phenomenon in an experimental setting. Pain. 2002;96:393–402. doi: 10.1016/S0304-3959(01)00485-7. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann JF, Chassany O, Gandiol J, et al. A randomised clinical trial of the effect of informed consent on the analgesic activity of placebo and naproxen in cancer pain. Clin Trials Metaanal. 1994;29:41–47. [PubMed] [Google Scholar]
- 4.Dahan R, Caulin C, Fiega L, Kanis JA, Caulin F, Segrestaa JM. Does informed consent influence therapeutic outcome? A clinical trial of the hypnotic activity of placebo in patients admitted to hospital. Br Med J. 1986;293:363–364. doi: 10.1136/bmj.293.6543.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MW, Taylor MA, Elliman NA, Rhodes O. Placebo expectancy effects in the relationship between glucose and cognition. Br J Nutr. 2001 Aug 86;(2):173–179. doi: 10.1079/bjn2001398. [DOI] [PubMed] [Google Scholar]
- 6.Gryll SL, Katahn M. Situational factors contributing to the placebo effect. Psychopharmacology. 1978;47:253–261. doi: 10.1007/BF00426747. [DOI] [PubMed] [Google Scholar]
- 7.Rose DA, Kahan TL. Melatonin and sleep qualities in healthy adults: pharmacological and expectancy effects. J Gen Psychol. 2001;128:401–421. doi: 10.1080/00221300109598918. [DOI] [PubMed] [Google Scholar]
- 8.Brody HB, Brody D. Placebo and health--II. Three perspectives on the placebo response: expectancy, conditioning, and meaning. Adv Mind Body Med. 2000;16:216–232. doi: 10.1054/ambm.2000.0183. [DOI] [PubMed] [Google Scholar]
- 9.Price DD, Milling LS, Kirsch I, Duff A, Montgomery GH, Nicholls SS. An analysis of factors that contribute to the magnitude of placebo analgesia in an experimental paradigm. Pain. 1999;83:147–156. doi: 10.1016/s0304-3959(99)00081-0. [DOI] [PubMed] [Google Scholar]
- 10.Levine JD, Gordon NC. Influence of the method of drug administration on analagesic response. Nature. 1984;312:755–756. doi: 10.1038/312755a0. [DOI] [PubMed] [Google Scholar]
- 11.Buckalew LW, Coffield KE. An investigation of drug expectancy as a function of capsule color and size and preparation form. J Clin Psychopharmacol. 1982;2:245–248. [PubMed] [Google Scholar]
- 12.Buckalew LW, Coffield KE. Drug expectations associated with perceptual characteristics: ethnic factors. Perceptual and Motor Skills. 1982;55:915–918. doi: 10.2466/pms.1982.55.3.915. [DOI] [PubMed] [Google Scholar]
- 13.Branthwaite A, Cooper P. Analgesic effects of branding in treatment of headaches. Br Med J. 1981;282(6276):1576–1578. doi: 10.1136/bmj.282.6276.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce DP, Jackevicius C, Chapman KR, McIvor RA, Kesten S. The placebo effect in asthma drug therapy trials: a meta-analysis. J Asthma. 2000;37:303–318. doi: 10.3109/02770900009055454. [DOI] [PubMed] [Google Scholar]
- 15.Spector S, Luparello TJ, Kopetzky MT, Souhrada J, Kinsman RA. Response of asthmatics to methacholine and suggestion. Am Rev Respir Dis. 1976;113:43–50. doi: 10.1164/arrd.1976.113.1.43. [DOI] [PubMed] [Google Scholar]
- 16.McFadden ER, Luparello T, Lyons HA, Bleecker E. The mechanism of action of suggestion in the induction of acute asthma attacks. Psychosom Med. 1969;31:134–143. doi: 10.1097/00006842-196903000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Luparello T, Lyons HA, Bleecker ER, McFadden ER., Jr Influences of suggestion on airway reactivity in asthmatic subjects. Psychosom Med. 1968;30:819–825. doi: 10.1097/00006842-196811000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Kemeny ME, Rosenwasser LJ, Panettieri RA, Rose RM, Berg-Smith SM, Kline JN. Placebo response in asthma: a robust and objective phenomenon. J Allergy Clin Immunol. 2007;119:1375–1381. doi: 10.1016/j.jaci.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Kleijnen J, de Craen AJ, van Everdingen J, Krol L. Placebo effect in double-blind clinical trials: a review of interactions with medications. Lancet. 1994;344(8933):1347–1349. doi: 10.1016/s0140-6736(94)90699-8. [DOI] [PubMed] [Google Scholar]
- 20.Marlatt GA, Rohsenow DJ. Cognitive processes in alcohol use: expectancy and the balancedplacebo design. In: Mello NK, editor. Advances in substance abuse: behavioral and biological research. Vol. 1. Greenwich, Conn.: JAI Press; 1980. pp. 159–199. [Google Scholar]
- 21.Mikalsen A, Bertelsen B, Flaten MA. Effects of caffeine, caffeine-associated stimuli, and caffeine-related information on physiological and psychological arousal. Psychopharmacology (Berl) 2001;157:373–380. doi: 10.1007/s002130100841. [DOI] [PubMed] [Google Scholar]
- 22.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 23.Juniper EF, O-Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 24.NIH Publication no. 97–2339, Sept 1997.
- 25.de Craen AJM, Roos PJ, de Vries AL, Kleijnen J. Effect of colour of drugs: systematic review of perceived effect of drugs and their effectiveness. BMJ. 1996;313:1624–1626. doi: 10.1136/bmj.313.7072.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 27.Revicki DA, Leidy NK, Brennan-Diemer F, Sorensen S, Togias A. Integrating patient preferences into health outcomes assessment. CHEST. 1998;114:998–1007. doi: 10.1378/chest.114.4.998. [DOI] [PubMed] [Google Scholar]
- 28.Wigal JK, Stout C, Brandon M, Winder JA, McConnaughy K, Creer TL, Kotses H. The Knowledge, Attitude, and Self-efficacy Asthma Questionnaire. Chest. 1993;104:1144–1148. doi: 10.1378/chest.104.4.1144. [DOI] [PubMed] [Google Scholar]
- 29.Elashoff J. N Query Advisor User Guide. Cork Ireland: Statistical Solutions; 2000. [Google Scholar]
- 30.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Piñeiro A, Wei LX, Seidenberg BC, Reiss TF. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Ann Intern Med. 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 31.Diggle PJ, Liang KY, Zeger SL. Analysis of Longitudinal Data. Vol 13. Oxford, England: Clarendon Press; 1994. Oxford Statistical Science Series. [Google Scholar]
- 32.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York: Wiley and Sons; 1998. [Google Scholar]
- 33.SAS/STAT User’s Guide Version 8.0. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 34.Juniper EF, Bousquet J, Abetz L, Bateman ED GOAL Committee. Identifying 'well- controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med. 2006;100:616–621. doi: 10.1016/j.rmed.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, Pedersen SE GOAL Investigators Group. Can guideline-defined asthma control be achieved? The Gaining Optimal Asthma ControL study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 36.Ciccone DS, Chandler HK, Pate-Carolan L, Janal MN, Lavietes MH. A test of the symptom amplification hypothesis in patients with asthma. J Nerv Ment Dis. 2007;195:119–124. doi: 10.1097/01.nmd.0000254731.68430.a9. [DOI] [PubMed] [Google Scholar]
- 37.Goebel MU, Trebst AE, Steiner J, Xie YF, Exton MS, Frede S, Canbay AE, Michel MC, Heemann U, Schedlowski M. Behavioral conditioning of immunosuppression is possible in humans. FASEB J. 2002;16:1869–1873. doi: 10.1096/fj.02-0389com. [DOI] [PubMed] [Google Scholar]
- 38.Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(8):1084–1091. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.