This Journal feature begins with a case vignette highlighting a rare clinical problem. The clinical problem and the underlying pathophysiology and molecular mechanisms are then presented, followed by the evaluation and management of the condition. The article ends with the authors’ thoughts and clinical recommendations.
Clinical Case
A 29 yr-old woman presented with a long-standing history of fatigue, profound anxiety, acne, hirsutism, menstrual irregularities and hypertension. The family history revealed anxiety and hypertension in her father and a paternal aunt. On clinical examination, she was noted to have acne, hirsutism and elevated blood pressure despite adherence to antihypertensive treatment, but no clinical signs suggestive of Cushing syndrome. Her weight was 79 kg, her height 170 cm, and her body mass index 27 kg/m2. Biochemical and endocrinological evaluation at presentation revealed elevated 0800h serum cortisol concentrations [56.2 ug/dL; normal range (nr), 8–19 ug/dL], increased 24-hour urinary free cortisol excretion (187.6 ug/day; nr, 10–34 ug/day), and elevated 0800h plasma ACTH (80 pg/mL; nr, 10–60 pg/mL) and serum testosterone (93 ng/dL; nr, 10–55 ng/dL), androstenedione (209 ng/dL; nr, 85–275 ng/dL), and dehydroepiandrosterone sulfate (458 ng/dL; nr, 60–255 ng/dL) concentrations. A low dose dexamethasone suppression test (0.5 mg dexamethasone every 6 hours for 48 hours) revealed resistance of the hypothalamic-pituitary-adrenal axis to dexamethasone suppression (0800h serum cortisol, 13.9 ug/dL; 0800h plasma ACTH, 53 pg/mL). Additional investigations excluded Cushing syndrome, primary hyperaldosteronism and other causes of hypertension. How should her condition be further evaluated and managed?
The Clinical Problem
The patient suffers from “Primary Generalized Glucocorticoid Resistance”, a syndrome first described and elucidated by Chrousos et al. as a rare, familial or sporadic, genetic condition characterized by generalized, partial, end-organ insensitivity to glucocorticoids (1–3). In this syndrome, the resultant compensatory activation of the hypothalamic-pituitary-adrenal (HPA) axis leads to inferred hypersecretion of hypothalamic corticotropin-releasing hormone (CRH) and arginine-vasopressin (AVP) in the hypophysial portal system and, hence, to elevated pituitary adrenocorticotropic hormone (ACTH) in the systemic circulation (1–3). In turn, the excess ACTH secretion results in adrenal cortical hyperplasia and increased cortisol secretion as a compensation for the reduced action of glucocorticoids at target tissues regulating glucocorticoid negative feedback, while it increases production of adrenal steroids with salt-retaining (mineralocorticoid) [cortisol, deoxycorticosterone (DOC) and corticosterone] and/or androgenic activity [androstenedione, dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEAS)] (1–3). In recognition of Professor George P. Chrousos' extensive and ground-breaking research work in this field, and for the sake of brevity, we propose that the term “Chrousos Syndrome” is heretofore used in place of “Primary Generalized Familial or Sporadic Glucocorticoid Resistance”.
The clinical presentation of Chrousos syndrome reflects the pathophysiologic alterations described above and is, therefore, mainly associated with, respectively, hypertension and/or hypokalemic alkalosis and hyperandrogenism (1–3) (Table 1). Clinical manifestations of glucocorticoid deficiency might occur, but are rare and were only reported in a young child with hypoglycemic generalized tonic-clonic seizures during the course of a febrile illness (4), in a newborn baby with severe hypoglycemia, excessive fatigability with feeding, increased susceptibility to infections and concurrent growth hormone deficiency (5), and in several adult patients with chronic fatigue. The latter might indicate inadequate glucocorticoid target tissue compensation at the central nervous system (CNS) and/or the skeletal muscles by the increased circulating cortisol concentrations (1–3). Clinical manifestations of androgen excess include ambiguous genitalia in a karyotypic female at birth and gonadotropin-independent precocious puberty in children of either gender; acne, hirsutism and hypofertility in both sexes; male-pattern hair loss, menstrual irregularities and oligo-anovulation in females; and oligospermia in males (1–3) (Table 1). The impaired fertility in both sexes has been attributed in part to the feedback inhibition of gonadotropin secretion by the elevated androgen concentrations, while the profound anxiety observed in some subjects is probably due to compensatory increases in hypothalamic CRH and AVP secretion. The latter might also predispose the patients to the development of an ACTH-secreting pituitary adenoma. Finally, the elevated circulating ACTH concentrations may be responsible for the observed growth of intratesticular adrenal rests and oligospermia (3).
TABLE 1.
| TABLE 1A: Clinical Manifestations and Diagnostic Evaluation of Chrousos Syndrome * | |
|---|---|
| Clinical Presentation | |
| Apparently normal glucocorticoid function in most cases | |
| Asymptomatic | |
| Hypoglycemia, chronic fatigue (glucocorticoid deficiency?) | |
| Mineralocorticoid excess | |
| Hypertension | |
| Hypokalemic alkalosis | |
| Androgen excess | |
|
Children: Ambiguous genitalia at birth**, clitoromegaly, premature adrenarche, gonadotropin- independent precocious puberty |
|
|
Females: Acne, hirsutism, male-pattern hair loss, menstrual irregularities, oligo-anovulation, hypofertility |
|
| Males: Acne, hirsutism, oligospermia, adrenal rests in the testes, hypofertility | |
| Increased HPA axis activity (CRH/AVP and ACTH hypersecretion) | |
| Anxiety | |
| Adrenal rests (oligospermia) | |
| Pituitary corticotropinoma | |
| Diagnostic Evaluation | |
| Absence of clinical features of Cushing syndrome | |
| Normal or elevated plasma ACTH concentrations | |
| Elevated serum or plasma cortisol concentrations | |
| Increased 24-hour urinary free cortisol excretion | |
| Normal circadian and stress-induced pattern of cortisol and ACTH secretion | |
| Resistance of the HPA axis to dexamethasone suppression | |
| Thymidine incorporation assays: Increased resistance to dexamethasone-induced suppression of phytohemaglutinin-stimulated thymidine incorporation compared to control subjects | |
| Dexamethasone-binding assays: Decreased concentration or affinity of the glucocorticoid receptor for the ligand compared to control subjects | |
| Molecular studies: Mutations/deletions of the glucocorticoid receptor; functional studies of mutant receptors | |
| TABLE 1B: Mutations of the Human Glucocorticoid Receptor Gene Causing Chrousos Syndrome * | |||||
|---|---|---|---|---|---|
| Mutation Position | |||||
| Author (Reference) | cDNA | Amino acid | Molecular Mechanisms | Genotype | Phenotype |
| Chrousos et al. (1) Hurley et al. (11) |
1922 (A→T) | 641 (D→V) | Transactivation ↓ Affinity for ligand ↓ (× 3) Nuclear translocation: 22 min Abnormal interaction with GRIP1 |
Homozygous | Hypertension Hypokalemic alkalosis |
| Karl et al. (12) | 4 bp deletion in exon-intron 6 | hGRα number: 50% of control Inactivation of the affected allele |
Heterozygous | Hirsutism Male-pattern hair-loss Menstrual irregularities |
|
| Malchoff et al. (13) | 2185 (G→A) | 729 (V→I) | Transactivation ↓ Affinity for ligand ↓ (× 2) Nuclear translocation: 120 min Abnormal interaction with GRIP1 |
Homozygous | Precocious puberty Hyperandrogenism |
| Karl et al. (10) Kino et al. (14) |
1676 (T→A) | 559 (I→N) | Transactivation ↓ Decrease in hGR binding sites Transdominance (+) Nuclear translocation: 180 Abnormal interaction with GRIP1 |
Heterozygous | Hypertension Oligospermia Infertility |
| Ruiz et al. (15) Charmandari et al. (20) |
1430 (G→A) | 477 (R→H) | Transactivation ↓ No DNA binding Nuclear translocation: 20 min |
Heterozygous | Hirsutism Fatigue Hypertension |
| Ruiz et al. (15) Charmandari et al. (20) |
2035 (G→A) | 679 (G→S) | Transactivation ↓ Affinity for ligand ↓ (× 2) Nuclear translocation: 30 min Abnormal interaction with GRIP1 |
Heterozygous | Hirsutism Fatigue Hypertension |
| Mendonca et al. (16) | 1712 (T→C) | 571 (V→A) | Transactivation ↓ Affinity for ligand ↓ (× 6) Nuclear translocation: 25 min Abnormal interaction with GRIP1 |
Homozygous | Ambiguous genitalia Hypertension Hypokalemia Hyperandrogenism |
| Vottero et al. (17) | 2241 (T→G) | 747 (I→M) | Transactivation ↓ Transdominance (+) Affinity for ligand ↓ (× 2) Nuclear translocation ↓ Abnormal interaction with GRIP1 |
Heterozygous | Cystic acne Hirsutism Oligo-amenorrhea |
| Charmandari et al. (19) | 2318 (T→C) | 773 (L→P) | Transactivation ↓ Transdominance (+) Affinity for ligand ↓ (× 2.6) Nuclear translocation: 30 min Abnormal interaction with GRIP1 |
Heterozygous | Fatigue Anxiety Acne Hirsutism Hypertension |
| Charmandari et al. (21) | 2209 (T→C) | 737 (F→L) | Transactivation ↓ Transdominance (time-dependent) (+) Affinity for ligand ↓ (× 1.5) Nuclear translocation: 180 min |
Heterozygous | Hypertension Hypokalemia |
| McMahon et al. (5) | 2 bp deletion at nt 2318-9 |
773 | Transactivation ↓ Affinity for ligand: absent No suppression of IL-6 |
Homozygous | Hypoglycemia Fatigability with feeding Hypertension |
| Nader et al. (4) | 2141 (G→A) | 714 (R→Q) | Transactivation ↓ Transdominance (+) Affinity for ligand ↓ (× 2) Nuclear translocation ↓ Abnormal interaction with GRIP1 |
Heterozygous | Hypoglycemia Hypokalemia Hypertension Mild clitoromegaly Advanced bone age Precocious pubarche |
Modified from Reference 3.
This is the only case of ambiguous genitalia documented in a child with 46,XX karyotype who also harbored a heterozygous mutation of the 21-hydroxylase gene.
The clinical spectrum of the Chrousos syndrome is broad, ranging from most severe to mild forms, and a number of patients may be asymptomatic, displaying biochemical alterations only (1–3) (Table 1). This variable clinical phenotype is owing to variations in the tissue sensitivity of the glucocorticoid, mineralocorticoid and/or androgen receptor signaling pathways; variations in the activity of key hormone-inactivating or -activating enzymes, such as the 11β-hydroxysteroid dehydrogenase (6) and 5α-reductase (7); and other genetic or epigenetic factors, such as the presence of insulin resistance and visceral obesity (2).
The Molecular Mechanisms
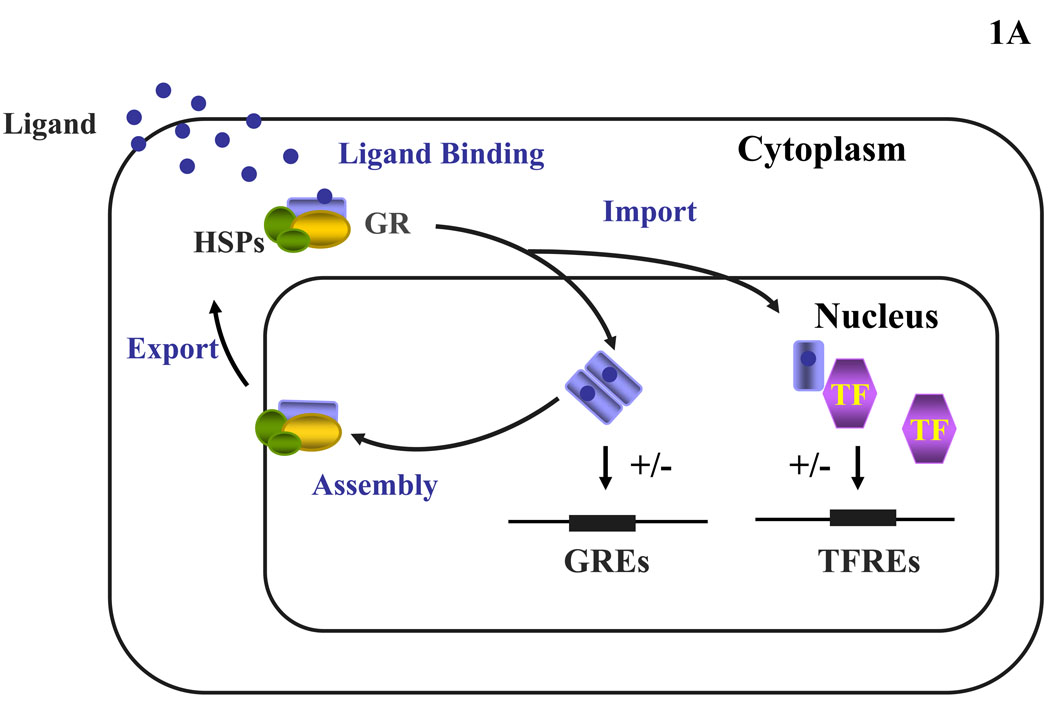
Glucocorticoids regulate a wide spectrum of physiologic functions essential for life and contribute to the maintenance of basal and stress-related homeostasis (8, 9). At the cellular level, glucocorticoids interact with their ubiquitously expressed, classic cytoplasmic/nuclear receptors, the glucocorticoid receptors (GRs), through which they exert genomic and possibly non-genomic actions. The genomic actions of glucocorticoids are mediated by the cytoplasmic/nuclear receptors, which are activated by ligand-binding, liberated from protein oligomers containing heat shock proteins and translocate into the nucleus, where they either bind as dimers to glucocorticoid-response elements (GREs) or they interact – possibly as monomers – with other transcription factors (Figure 1A). Glucocorticoids, thus, regulate the expression of their own GRE-containing target genes and/or the target genes of interacting transcription factors positively or negatively. Glucocorticoid receptors mediating the non-genomic actions of glucocorticoids have not been elucidated as yet, however, they are under intensive investigation.
Figure 1.
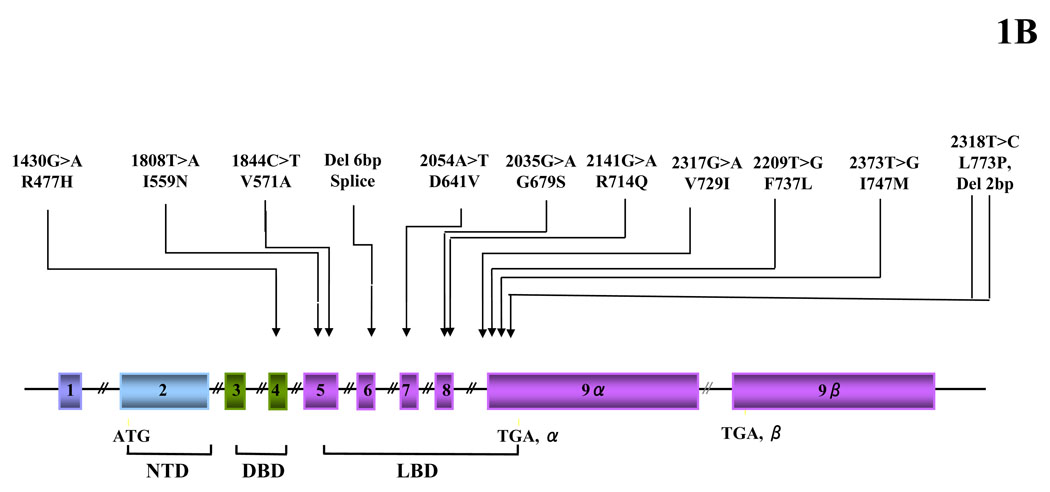
Heuristic, simplified representation of the glucocorticoid signaling system and gene and pathologic mutations of the GR gene causing Chrousos syndrome. (A) Nucleocytoplasmic shuttling of the glucocorticoid receptor. Upon binding to the ligand, the activated hGRα dissociates from heat shock proteins (HSPs) and translocates into the nucleus, where it homodimerizes and binds to glucocorticoid response elements (GREs) in the promoter region of target genes or interacts with other transcription factors (TFs), such as activator protein-1 (AP-1), nuclear factor-κB (NF-κB) and signal transducer and activator of transcription-5 (STAT5), ultimately modulating the transcriptional activity of respectively GRE- or TFRE-containing genes. (B) Location of the known mutations of the glucocorticoid receptor (hGR) gene causing Chrousos syndrome. DBD: DNA-binding domain; GR: glucocorticoid receptor; GREs: glucocorticoid response element; HSP: heat shock protein; LBD: ligand-binding domain; NTD: amino terminal domain; TF: transcription factor; TFRE: transcription factor response element.
The molecular basis of Chrousos syndrome has been ascribed primarily to mutations in the human (h) glucocorticoid receptor (hGR) gene, which impair the molecular mechanisms of hGR action and decrease tissue sensitivity to glucocorticoids. The pathologic hGR gene mutations causing Chrousos syndrome that have been reported to date are shown in Table 1B and Figure 1B (4, 5, 10–21). Eight out of 12 of these mutations are heterozygous (4 are homozygous), while 11 out of 12 partially inactivate GR function. Although studies of GR knock-out mice suggested that complete loss-of-function of the GR is incompatible with extrauterine life (22), one out of 12 of the mutations completely inactivated GR function (5).
The pathophysiologic mechanisms leading to the manifestations of Primary Generalized Glucocorticoid Resistance, the first therapeutic use of a mineralocorticoid-sparing glucocorticoid compound (dexamethasone) and the majority of the hGR mutations associated with the syndrome were respectively elucidated, employed and identified by Professor George P. Chrousos, his team at the National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA, and various collaborators (10–21). The pathologic hGR mutants reported were functionally characterized by various members of his team and collaborators throughout the years. As a result, the molecular mechanisms through which these various natural hGR mutants affected glucocorticoid signal transduction were systematically investigated in all reported cases with the condition. These mechanisms included: i) the transcriptional activity of the mutant receptors; ii) the ability of the heterozygous mutant receptors to exert a dominant negative effect upon the wild-type receptor; iii) the concentrations and affinity of the mutant receptors for the ligand; iv) the subcellular localization of the mutant receptors and their nuclear translocation following exposure to the ligand; v) the ability of the mutant receptors to bind to GREs; vi) the interaction of the mutant receptors with the glucocorticoid receptor-interacting protein 1 (GRIP1) coactivator, which belongs to the p160 family of nuclear receptor coactivators and plays an important role in hGRα-mediated transactivation of glucocorticoid-responsive genes; and vii) the motility of the mutant receptors inside the nucleus (10–21).
The molecular defects that have been elucidated in cases with Chrousos syndrome and have been reported to date are summarized in Table 1B. Compared with the wild-type receptor, all mutant receptors demonstrated variable reduction in their ability to transactivate glucocorticoid-responsive genes following exposure to dexamethasone, with the most severe impairment observed in the cases of R477H, I559N, V571A and D641V mutations (10–21). Furthermore, the mutant receptors hGRαI559N, hGRαF737L, hGRαI747M and hGRαL773P exerted a dominant negative effect upon the wild-type receptor, which might have contributed to the manifestations of the disease at the heterozygote state (10, 14, 17, 19, 21). All mutant receptors in which the mutations were located in the ligand-binding domain (LBD) of the receptor showed a variable reduction in their affinity for the ligand, with the most severe reduction observed in the cases of I559N, I747M and V571A mutations (10–21). The only mutant receptor that demonstrated normal affinity for the ligand was the hGRαR477H, in which the mutation was located at the DNA-binding domain (DBD) (20).
In subcellular localization and nuclear translocation studies, the pathologic mutant receptors were observed primarily in the cytoplasm of cells in the absence of ligand, except for the hGRαV729I and hGRαF737L receptors, which were localized both in the cytoplasm and the nucleus of cells. Exposure to dexamethasone induced a slow translocation of the mutant receptors into the nucleus, which ranged from 20 min (R477H) to 180 min (I559N and F737L) compared with the wild-type hGRα, which required only 12 min for complete translocation (10–21). These findings suggest that all hGR mutations affect the nucleocytoplasmic shuttling of the receptor, probably through impairment of the nuclear localization signal (NL)-1 and/or NL2 functions (23).
All mutant receptors in which the mutations were located in the LBD preserved their ability to bind to DNA (10–21). The only mutant receptor that failed to bind to DNA was the hGRαR477H, in which the mutation was located at the C-terminal zinc finger of the DBD (20). A major function of the C-terminal zinc finger of the DBD of hGRα is to contribute to receptor homodimerization, a prerequisite for potent receptor binding to GREs and efficient transactivation of glucocorticoid-responsive genes (24). All mutant receptors except hGRαR477H displayed an abnormal interaction with the GRIP1 (SRC-2) coactivator in vitro (10–21). Finally, all mutant receptors had dynamic motility defects inside the nucleus of living cells, possibly caused by their inability to properly interact with key partner nuclear molecules of the transcription initiation complex necessary for full activation of glucocorticoid-responsive genes (25).
Evolutionary and Phylogenetic Aspects of Glucocorticoid Resistance: An as yet Unresolved Enigma
The glucocorticoid receptor (GR) is a member of the subfamily of steroid hormone receptors of the superfamily of nuclear receptors. Vertebrate genomes contain six evolutionarily related nuclear receptors for steroid hormones: two for estrogens (ERα and ERβ) and one each for androgens (AR), progestins (PR), glucocorticoids (GR) and mineralocorticoids (MR). Steroid receptors evolved in the chordate lineage after the separation of deuterostomes and protostomes, prior to or at the base of the Cambrian explosion about 540 million years ago (26). The genome of ‘higher’ vertebrates is thought to be the result of two genome duplication events that occurred early in chordate evolution (26, 27). The first gene duplication created an estrogen receptor (ER) and a 3-ketosteroid receptor, whereas the second duplicated the latter gene to produce a corticoid receptor and a receptor for 3-ketogonadal steroids. Therefore, the ancestral vertebrates had three steroid receptors: an estrogen receptor (ER), a corticosteroid receptor (CR) and a receptor that bound androgens, progestins or both. At some later stage within the gnathostome lineage, each of these three receptors duplicated yet again to yield the six steroid receptors currently found in jawed vertebrates: the ER to create ERα and ERβ, the CR to yield the GR and the MR, and the 3-ketogonadal steroid receptor to create the PR and the AR. The GR and MR are thought to have evolved approximately 450 million years ago (28).
These phylogenetic data suggest many commonalities between the steroid hormone and possibly other nuclear receptors. In the early 80’s, Chrousos et al. showed that many or, most likely, all New World primates, which evolved from a common ancestor that separated from Old World primates approximately 60 million years ago, exhibited a “physiologic” form of Chrousos syndrome (29). These species have markedly elevated plasma total and free cortisol concentrations, increased urinary free cortisol excretion, marked increases in plasma ACTH concentrations and resistance of their HPA axis to dexamethasone suppression, without any clinical evidence of hypercortisolism. Studies of the glucocorticoid receptors in circulating mononuclear leukocytes and cultured skin fibroblasts from both New and Old World primate species showed that the receptor content was the same in all species, but the New World monkeys had a markedly decreased binding affinity for dexamethasone. These alterations in the affinity of the glucocorticoid receptors for the ligand must have occurred after the bifurcation of the New World from the Old World primates and before the diversion of the New World monkeys from each other (29–31).
More recent studies demonstrated that the generalized glucocorticoid resistance in squirrel monkeys arose as a result of both naturally occurring mutations in the GR gene that led to inefficient transactivation and overexpression of FKBP51 that inhibits ligand binding (32). These hypotheses indicate that the glucocorticoid resistance seen in New World monkeys may be caused in part through the introduction of de novo harmless and well tolerated over a long time mutations, different from those that underlie the Chrousos syndrome observed in human patients that were recent and apparently not well tolerated.
In the early 80’s, Chrousos et al. and other investigators demonstrated that New World primates had what could be called “pansteroid/sterol” resistance to steroid and sterol hormones, including all steroid hormones and the sterol hormone 1, 25-dihydroxy Vitamin D (29–31). Indeed, the circulating levels of all these hormones are markedly elevated compared to those of Old World Primates and, accordingly, the target tissues are resistant to them. Chrousos proposed that these findings could only be explained by changes of a molecule common and crucial in the signal transduction of all steroid/sterol hormones, possibly a nuclear receptor coactivator or a corepressor, predicting the presence of such molecules many years before their discovery by O’Malley and co-workers (29, 33). This molecule(s) has(ve) eluded attempts to identify it(them) and the pansteroid/sterol resistance of New World primates remains an interesting enigma to be resolved.
Clinical Implications of Glucocorticoid Signaling Changes beyond the Syndrome
Chrousos et al. early on formulated two hypotheses related to potential human glucocorticoid signaling system-associated pathophysiology: First, that tissue-specific changes of glucocorticoid action could lead to a variety of human diseases and, second, that tissue-specific glucocorticoid hypersensitivity would be equally or more important than tissue-specific glucocorticoid resistance (Table 2) (2, 8, 9, 34). Chrousos and co-workers identified two mutations associated with glucocorticoid hypersensitivity, one as early as 1993 and the other recently (12, 35). This condition represents the mirror image of Chrousos syndrome and is not discussed further in this review.
TABLE 2.
Expected Clinical Manifestations in Target Tissue Hypersensitivity or Resistance to Glucocorticoids *
| Target area | Glucocorticoid excess = Glucocorticoid hypersensitivity | Glucocorticoid deficiency = Glucocorticoid resistance |
|---|---|---|
| Central nervous system | Insomnia, anxiety, depression, defective cognition | Fatigue, somnolence, malaise, defective cognition |
| Liver | + Gluconeogenesis, + lipogenesis | Hypoglycemia, resistance to diabetes mellitus |
| Fat | Accumulation of visceral fat (metabolic syndrome) | Loss of weight, resistance to weight gain |
| Blood vessels | Hypertension | Hypotension |
| Bone | Stunted growth, osteoporosis | |
| Inflammation/immunity | Immune suppression, anti-inflammation, vulnerability to certain infections and tumors |
+ Inflammation, + autoimmunity, + allergy |
Clinical Evaluation of the Patients
The first step in evaluating a patient with suspected Chrousos syndrome is to obtain a complete personal and family history, with particular attention to evidence suggesting hyperactivity of the HPA axis and ACTH hypersecretion-related pathology. In addition, any evidence suggesting possible CNS dysfunction, such as headaches, visual impairment or seizures, should be noted. In female subjects, the regularity of menstrual cycles should be documented. In children and adolescents, growth and sexual maturation should be evaluated carefully, given that progressive hyperandrogenism is almost invariably associated with an increased growth velocity, an advanced bone age and changes in pubertal development.
The physical examination should include an assessment for signs of hyperandrogenism and/or virilization, such as acne, hirsutism, pubic and axillary hair development, male-pattern hair loss and clitoromegaly. Hirsutism should be assessed using the Ferriman-Gallwey score (36), while pubic hair development should be classified according to Tanner (37, 38). Arterial blood pressure should be recorded and preferably monitored over a 24-hour period. All subjects should be screened for signs suggestive of Cushing syndrome and undergo a complete neurologic examination.
Endocrinologic Evaluation of the Patients
The concentrations of plasma ACTH, plasma renin activity (recumbent and upright) and aldosterone, as well as those of serum cortisol, testosterone, androstenedione, DHEA and DHEAS should be recorded in the morning. Determination of the 24-hour urinary free cortisol (UFC) excretion on 2 or 3 consecutive days is central to the diagnosis, given that patients with Chrousos syndrome demonstrate increased 24h UFC excretion in the absence of clinical manifestations suggestive of hypercortisolism. Plasma ACTH concentrations may be normal or high. However, the circadian pattern of ACTH and cortisol secretion and their responsiveness to stressors are preserved, albeit at higher concentrations.
The responsiveness of the HPA axis to exogenous glucocorticoids should also be tested with dexamethasone. Increasing doses of dexamethasone should be given orally at midnight every other day, and a serum sample should be drawn at 0800h the following morning for determination of serum cortisol and dexamethasone concentrations. Affected subjects demonstrate resistance of the HPA axis to dexamethasone suppression, which may vary depending on the severity of the condition. The concurrent measurement of serum dexamethasone concentrations is suggested in order to exclude the possibility of increased metabolic clearance or decreased absorption of this medication.
Molecular Studies in the Patients
Thymidine incorporation assays and dexamethasone-binding assays on peripheral blood mononuclear cells in association with sequencing of the hGR gene are necessary to confirm the diagnosis in patients suspected to have Chrousos syndrome and to be able to provide genetic counseling (10–21) (Table 1A). In affected subjects, the thymidine incorporation assays reveal resistance to dexamethasone-induced suppression of phytohemaglutinin-stimulated thymidine incorporation, while the dexamethasone-binding assays often show decreased affinity of the hGR receptor for the ligand compared to control subjects (3). Sequencing of the coding region of the hGR gene, including the intron/exon junctions, will reveal mutations or deletions in most (10–21) but not all (39) cases with Primary Generalized Glucocorticoid Resistance. Finally, once the structural defect is determined, its adverse effects on receptor function should be confirmed using in vitro mutagenesis and standardized assays that examine the ability of the mutant receptor to transactivate glucocorticoid-responsive genes.
Management of the Patients
The aim of treatment in Chrousos syndrome is to suppress the excess secretion of ACTH, thereby suppressing the increased production of adrenal steroids with mineralocorticoid and androgenic activity. Treatment involves administration of high doses of mineralocorticoid-sparing synthetic glucocorticoids, which activate the mutant and/or wild-type hGRα, and suppress the endogenous secretion of ACTH in affected subjects (1–3). Adequate suppression of the HPA axis is of particular importance in cases of severe impairment of hGRα action, given that long-standing corticotroph hyperstimulation in association with decreased glucocorticoid negative feedback inhibition at the hypothalamic and pituitary levels may lead to the development of an ACTH-secreting adenoma (10). Long-term dexamethasone treatment should be carefully titrated according to the clinical manifestations and biochemical profile of the affected subjects (1–3).
Conclusions and Recommendations
In the case described in the vignette, the profound anxiety, acne, hirsutism, menstrual irregularities and hypertension, along with the increased 24h UFC excretion and resistance of HPA axis to low-dose dexamethasone suppression in the absence of Cushing’s manifestations, suggested the diagnosis of Chrousos syndrome or Primary Generalized Glucocorticoid Resistance. Written informed consent was obtained from the patient and additional molecular studies were undertaken. The thymidine incorporation assays revealed resistance to dexamethasone-induced suppression of phytohemagglutinin-stimulated thymidine incorporation in the patient white cells compared with cells from a matched control subject. The dexamethasone-binding assays showed that the affinity of the hGR for the ligand was 2.7-fold lower in the patient than in the control subject. No difference was noted in the number of binding sites between the two subjects. Sequencing of the hGR gene in the patient revealed a single heterozygous thymine to cytosine (T → C) substitution at nucleotide position 2318 (exon 9α), resulting in leucine (L) to proline (P) substitution (CTG → CCG) at amino acid 773 in the ligand-binding domain of the receptor (19).
Molecular studies were undertaken to systematically investigate the molecular mechanisms through which the natural hGRαL773P mutant impaired glucocorticoid signal transduction. Compared with the wild-type hGRα, the mutant receptor hGRαL773P demonstrated a 2-fold reduction in its ability to transactivate glucocorticoid-responsive genes, exerted a dominant negative effect on the wild-type receptor, had a 2.6-fold reduction in the affinity for ligand, showed delayed nuclear translocation and, although it preserved its ability to bind to DNA, displayed an abnormal interaction with the GRIP1 coactivator in vitro (19).
Following treatment with high dose of dexamethasone (2 mg at night), the clinical manifestations of the condition subsided, the serum cortisol concentration was suppressed, and the concentrations of plasma ACTH and serum testosterone, androstenedione, and dehydroepiandrosterone sulfate were normalized. The patient’s father did not provide consent or tissues for any additional endocrinologic or genetic testing despite our advice to the contrary (19).
The variable clinical phenotype of Chrousos syndrome, including chronic fatigue, mild hypertension and hyperandrogenism, in association with the difficulties encountered in establishing the correct diagnosis may account for the low reported prevalence of the condition, given that many cases may be unrecognized and misclassified. We recommend screening with 24h UFC excretion and sequencing of the hGR gene in patients with manifestations of mineralocorticoid and androgen excess (hypertension, hirsutism, menstrual irregularities, oligo-anovulation, impaired fertility), in whom detailed investigations fail to reveal an underlying etiology.
Acknowledgements
Literary work of this article was in part funded by the Biomedical Research Foundation of the Academy of Athens, Athens, Greece, and the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, 20892, USA.
Footnotes
DISCLOSURE STATEMENT:
The authors E.C. and T.K. have nothing to disclose
References
- 1.Chrousos GP, Vingerhoeds A, Brandon D, Eil C, Pugeat M, DeVroede M, Loriaux DL, Lipsett MB. Primary cortisol resistance in man. A glucocorticoid receptor-mediated disease. J Clin Invest. 1982;69(6):1261–1269. doi: 10.1172/JCI110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chrousos GP, Detera-Wadleigh SD, Karl M. Syndromes of glucocorticoid resistance. Ann Intern Med. 1993;119(11):1113–1124. doi: 10.7326/0003-4819-119-11-199312010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Charmandari E, Kino T, Ichijo T, Chrousos GP. Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder. J Clin Endocrinol Metab. 2008;93(5):1563–1572. doi: 10.1210/jc.2008-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nader N, Bachrach BE, Hurt DE, Gajula S, Pittman A, Lescher R, Kino T. A novel point mutation in the helix 10 of the human glucocorticoid receptor causes Generalized Glucocorticoid Resistance by disrupting the structure of the ligand-binding domain. J Clin Endocrinol Metab. 2010;95(5):2281–2285. doi: 10.1210/jc.2009-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McMahon SK, Pretorius CJ, Ungerer JP, Salmon NJ, Conwell LS, Pearen MA, Batch JA. Neonatal complete generalized glucocorticoid resistance and growth hormone deficiency caused by a novel homozygous mutation in Helix 12 of the ligand binding domain of the glucocorticoid receptor gene (NR3C1) J Clin Endocrinol Metab. 2010;95(1):297–302. doi: 10.1210/jc.2009-1003. [DOI] [PubMed] [Google Scholar]
- 6.Tomlinson JW, Walker EA, Bujalska IJ, Draper N, Lavery GG, Cooper MS, Hewison M, Stewart PM. 11β-hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr Rev. 2004;25(5):831–866. doi: 10.1210/er.2003-0031. [DOI] [PubMed] [Google Scholar]
- 7.Wilson JD, Griffin JE, Russell DW. Steroid 5α-reductase 2 deficiency. Endocr Rev. 1993;14(5):577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 8.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity. Endo. Rev. 1996;17:221–244. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 9.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;304:pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 10.Karl M, Lamberts SW, Koper JW, Katz DA, Huizenga NE, Kino T, Haddad BR, Hughes MR, Chrousos GP. Cushing's disease preceded by generalized glucocorticoid resistance: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians. 1996;108(4):296–307. [PubMed] [Google Scholar]
- 11.Hurley DM, Accili D, Stratakis CA, Karl M, Vamvakopoulos N, Rorer E, Constantine K, Taylor SI, Chrousos GP. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest. 1991;87(2):680–686. doi: 10.1172/JCI115046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karl M, Lamberts SW, Detera-Wadleigh SD, Encio IJ, Stratakis CA, Hurley DM, Accili D, Chrousos GP. Familial glucocorticoid resistance caused by a splice site deletion in the human glucocorticoid receptor gene. J Clin Endocrinol Metab. 1993;76(3):683–689. doi: 10.1210/jcem.76.3.8445027. [DOI] [PubMed] [Google Scholar]
- 13.Malchoff DM, Brufsky A, Reardon G, McDermott P, Javier EC, Bergh CH, Rowe D, Malchoff CD. A mutation of the glucocorticoid receptor in primary cortisol resistance. J Clin Invest. 1993;91(5):1918–1925. doi: 10.1172/JCI116410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kino T, Stauber RH, Resau JH, Pavlakis GN, Chrousos GP. Pathologic human GR mutant has a transdominant negative effect on the wild-type GR by inhibiting its translocation into the nucleus: importance of the ligand-binding domain for intracellular GR trafficking. J Clin Endocrinol Metab. 2001;86(11):5600–5608. doi: 10.1210/jcem.86.11.8017. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz M, Lind U, Gafvels M, Eggertsen G, Carlstedt-Duke J, Nilsson L, Holtmann M, Stierna P, Wikstrom AC, Werner S. Characterization of two novel mutations in the glucocorticoid receptor gene in patients with primary cortisol resistance. Clin Endocrinol (Oxf) 2001;55(3):363–371. doi: 10.1046/j.1365-2265.2001.01323.x. [DOI] [PubMed] [Google Scholar]
- 16.Mendonca BB, Leite MV, de Castro M, Kino T, Elias LL, Bachega TA, Arnhold IJ, Chrousos GP, Latronico AC. Female pseudohermaphroditism caused by a novel homozygous missense mutation of the GR gene. J Clin Endocrinol Metab. 2002;87(4):1805–1809. doi: 10.1210/jcem.87.4.8379. [DOI] [PubMed] [Google Scholar]
- 17.Vottero A, Kino T, Combe H, Lecomte P, Chrousos GP. A novel, C-terminal dominant negative mutation of the GR causes familial glucocorticoid resistance through abnormal interactions with p160 steroid receptor coactivators. J Clin Endocrinol Metab. 2002;87(6):2658–2667. doi: 10.1210/jcem.87.6.8520. [DOI] [PubMed] [Google Scholar]
- 18.Charmandari E, Kino T, Vottero A, Souvatzoglou E, Bhattacharyya N, Chrousos GP. Natural glucocorticoid receptor mutants causing generalized glucocorticoid resistance: Molecular genotype, genetic transmission and clinical phenotype. J Clin Endocrinol Metab. 2004;89(4):1939–1949. doi: 10.1210/jc.2003-030450. [DOI] [PubMed] [Google Scholar]
- 19.Charmandari E, Raji A, Kino T, Ichijo T, Tiulpakov A, Zachman K, Chrousos GP. A novel point mutation in the ligand-binding domain (LBD) of the human glucocorticoid receptor (hGR) causing generalized glucocorticoid resistance: the importance of the C terminus of hGR LBD in conferring transactivational activity. J Clin Endocrinol Metab. 2005;90(6):3696–3705. doi: 10.1210/jc.2004-1920. [DOI] [PubMed] [Google Scholar]
- 20.Charmandari E, Kino T, Ichijo T, Zachman K, Alatsatianos A, Chrousos GP. Functional characterization of the natural human glucocorticoid receptor (hGR) mutants hGRαR477H and hGRαG679S associated with generalized glucocorticoid resistance. J Clin Endocrinol Metab. 2006;91(4):1535–1543. doi: 10.1210/jc.2005-1893. [DOI] [PubMed] [Google Scholar]
- 21.Charmandari E, Kino T, Ichijo T, Jubiz W, Mejia L, Zachman K, Chrousos GP. A novel point mutation in helix 11 of the ligand-binding domain of the human glucocorticoid receptor gene causing generalized glucocorticoid resistance. J Clin Endocrinol Metab. 2007;92(10):3986–3990. doi: 10.1210/jc.2006-2830. [DOI] [PubMed] [Google Scholar]
- 22.Cole TJ, Blendy JA, Monaghan AP, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9(13):1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 23.Savory JG, Hsu B, Laquian IR, Giffin W, Reich T, Hache RJ, Lefebvre YA. Discrimination between NL1- and NL2-mediated nuclear localization of the glucocorticoid receptor. Mol Cell Biol. 1999;19(2):1025–1037. doi: 10.1128/mcb.19.2.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahlman-Wright K, Wright A, Gustafsson JA, Carlstedt-Duke J. Interaction of the glucocorticoid receptor DNA-binding domain with DNA as a dimer is mediated by a short segment of five amino acids. J Biol Chem. 1991;266(5):3107–3112. [PubMed] [Google Scholar]
- 25.Kino T, Liou SH, Charmandari E, Chrousos GP. Glucocorticoid receptor mutants demonstrate increased motility inside the nucleus of living cells: time of fluorescence recovery after photobleaching (FRAP) is an integrated measure of receptor function. Mol Med. 2004;10(7–12):80–88. doi: 10.2119/2005-00026.Kino. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci U S A. 2001;98(10):5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301(5640):1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- 28.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312(5770):97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- 29.Chrousos GP, Renquist D, Brandon D, Eil C, Pugeat M, Vigersky R, Cutler GB, Jr, Loriaux DL, Lipsett MB. Glucocorticoid hormone resistance during primate evolution: receptor-mediated mechanisms. Proc Natl Acad Sci U S A. 1982;79(6):2036–2040. doi: 10.1073/pnas.79.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chrousos GP. The defective glucocorticoid receptor in man and nonhuman primates. In: Lipsett MB, Chrousos GP, Brandon DD, Tomita M, Loriaux DL, editors. Rec. Prog. Horm. Res. Vol. 41. 1985. pp. 199–247. (discussion) [DOI] [PubMed] [Google Scholar]
- 31.Chrousos GP, Loriaux DL, Lipsett MB, editors. Advances in Experimental Medicine and Biology. Vol. 196. New York: Plenum Press; 1986. Steroid Hormone Resistance: Mechanisms and Clinical Aspects. [PubMed] [Google Scholar]
- 32.Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG. Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol. 2006;100(1–3):34–41. doi: 10.1016/j.jsbmb.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Chrousos GP. A new “New” syndrome in the New World: Is multiple postreceptor steroid hormone resistance due to a coregulator defect? J. Clin. Endocrinol. Metab. 1999;84:4450–4453. doi: 10.1210/jcem.84.12.6073. [DOI] [PubMed] [Google Scholar]
- 34.Chrousos GP, Kino T. Glucocorticoid Signaling in the Cell: Expanding Clinical Implications to Complex Human Behavioral and Somatic Disorders. Glucocorticoids and Mood: Clinical Manifestations, Risk Factors, and Molecular Mechanisms. Proc. NY Acad Sci. 2009;1179:153–166. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Charmandari E, Ichijo T, Jubiz W, Baid S, Zachman K, Chrousos GP, Kino T. A novel point mutation in the amino terminal domain of the human glucocorticoid receptor (hGR) gene enhancing hGR-mediated gene expression. J Clin Endocrinol Metab. 2008;93:4963–4968. doi: 10.1210/jc.2008-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferriman D, Gallwey J. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;83:2694–2698. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 37.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. 1969;44(235):291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13–24. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huizenga NA, de Lange P, Koper JW, de Herder WW, Abs R, Kasteren JH, de Jong FH, Lamberts SW. Five patients with biochemical and/or clinical generalized glucocorticoid resistance without alterations in the glucocorticoid receptor gene. J Clin Endocrinol Metab. 2000;85(5):2076–2081. doi: 10.1210/jcem.85.5.6542. [DOI] [PubMed] [Google Scholar]