Abstract
Purpose of Review
Provides an overview of the identified risk factors for chronic kidney disease (CKD) progression emphasizing the pediatric population.
Recent findings
Over the past ten years, there have been significant changes to our understanding and study of pre-terminal kidney failure. Recent refinements in the measurement of glomerular filtration rate (GFR) and GFR estimating equations are important tools for identification and association of risk factors for CKD progression in children. In pediatric CKD, lower level of kidney function at presentation, higher levels of proteinuria, and hypertension are known markers for a more rapid decline in GFR. Anemia and other reported risk factors from the pre-genomic era have need for further study and validation. Genome-wide association studies have identified genetic loci which have provided novel genetic risk factors for CKD progression.
Summary
With cohort studies of children with CKD becoming mature, they have started to yield important refinements to the assessment of CKD progression. While many of the traditional risk factors for renal progression will certainly be assessed, such cohorts will be important for evaluating novel risk factors identified by genome-wide studies.
Keywords: Chronic kidney disease, risk factors, blood pressure, proteinuria, anemia, genome wide association studies
Introduction
The state of kidney damage or reduced kidney function lasting three months or longer, known as chronic kidney disease (CKD), is both progressive and irreversible.[1] In the United States, 16 percent of general population is estimated to have CKD with the rate projected to increase.[2] The worldwide impact of CKD is significant, yet underestimated. According to the World Health Report 2002 and the global burden of disease project, kidney and urinary tract diseases contribute to 850,000 deaths per year and 15,010,167 in disability-adjusted life years.[3] The consequences of CKD in children are devastating, condemning patients to varying levels of chronic, lifelong medical disability.[4] We are clearly facing an urgent public health problem in this country and worldwide.
Operationally, CKD is defined as kidney damage or glomerular filtration rate (GFR) < 60mL/min/1.73m2 for ≥ 3 months or more regardless of diagnosis. Kidney damage is usually identified by abnormalities in the blood, urine, imaging tests; and if needed, by kidney biopsy. Health care providers screen for CKD by either blood testing to estimate glomerular filtration rate (GFR) or urinary screening for the detection of proteinuria. Tests for total urine protein are preferred in children; for children with diabetes, screening for albuminuria should also be performed.[5].
Progression to Kidney Failure
While the dominant causes of CKD in adults are diabetic nephropathy and hypertension, nearly 60%-70% of children affected with CKD have congenital or inherited kidney disorders.[6] Patients with congenital anomalies of the kidney and urinary tract, including those with congenital solitary kidneys, carry an increased risk for end-stage renal disease by young adulthood.[7] Irrespective of the original cause of kidney damage, the onset of CKD initiates a chain of events that describe a common final pathway where pre-terminal kidney damage progresses to kidney failure. The term “renal progression” refers to this progressive decline in kidney function. Although some CKD patients have stable kidney function for years, others decline rapidly. The variability of CKD progression among patients suggests that biologically relevant factors may influence the course of CKD. This review will summarize the historical trends in the study of pre-terminal kidney disease, the recent updates to the determination GFR in children, the identification of clinical risk factors for renal progression, and the genetic risk factors associated with CKD progression. Although findings from the adult CKD population are discussed, there will be an emphasis on pediatric studies.
A Historical Perspective on CKD and Progression
Prior to the 21st century, children with kidney disease were considered to have significant kidney impairment below a GFR of 75ml/min/1.73m2, termed chronic renal insufficiency (CRI). There were active efforts to treat and study pre-terminal kidney disease, yet different kidney diseases were studied as separate entities without enough cases to provide significant statistical power to derive firm conclusions. Although it was understood that patients with CRI progressed to kidney failure, there was little systematic data to define the patterns of decline and clinical measurements of this decline were not standardized. During this era, a significant focus of clinical care and research in children with kidney disease were toward the fundamental challenges of performing dialysis and transplantation in children, growth problems associated with chronic renal failure, treatment of nephrotic syndrome, and detection and treatment of hypertension.[8] By the beginning of the 21st century many of these fundamental challenges were overcome and the concept of CRI had been revised to our current model of CKD.
In 2002, the National Kidney Foundation (NKF) published the first Kidney Dialysis Outcome Quality Initiative (K/DOQI) clinical practice guidelines for CKD: evaluation, classification, and stratification. [9] The NKF committee members who generated the guidelines provided a broad conceptual framework in the identification, management, and the care of all patients with CKD.[9] This work acknowledged that despite multiple causes for kidney damage, all kidney diseases had a characteristic in common—a decline in kidney function overtime. This paradigm shift for the study of pre-terminal kidney failure facilitated epidemiologic study of CKD risk factors and a common nomenclature to define CKD progression. The committee developed the CKD staging system (Table 1) which relies on the level glomerular filtration rate (GFR) as an index of kidney function to categorize patients in a particular stage. Use of this system allows for consistency when describing CKD, accurate assessment of progression risk factors, and ultimately should improve outcomes.
Table 1. NKF-K/DOQI Classification Chronic Kidney Disease.
| Stage | GFR (mL/min/1.73m2) | Description |
|---|---|---|
| - | ≥ 90 (with CKD risk factors) | At increased risk |
| 1 | ≥ 90 | Kidney damage with normal or increased GFR |
| 2 | 60-89 | Kidney damage with mild reduction of GFR |
| 3 | 30-59 | Moderate reduction of GFR |
| 4 | 15-29 | Severe reduction of GFR |
| 5 | <15 (or dialysis) | Kidney failure |
Pediatric Nephrology Collaborative Studies
Multi-center consortia are necessary for clinical research in pediatric CKD due to the relatively small numbers of patients. Compared to the adult CKD population, the incidence of CKD is relatively low among children and is estimated to be 12.1 cases per year per million age-related population (MARP) compared to 74.7 cases per MARP for adults.[10] Several large consortia of multiple pediatric nephrology centers have provided recent data to further our understanding of risk factors and treatment for CKD progression in children, notably groups across Europe and North America. The recently published Effect of Strict Blood Pressure Control and ACE Inhibition on the Progression of CRF in Pediatric Patients (ESCAPE) trial was performed by 33 pediatric nephrology units across Europe. The ESCAPE trial was a randomized intervention evaluating intensified blood pressure control in children 3-18 years of age (GFR between 15-80 ml/min/1.73m2) on a fixed dose of angiotensin-converting-enzyme (ACE) inhibitor and its impact on disease progression.[11] The ongoing Chronic Kidney Disease in Children (CKiD) study is being performed by 43 pediatric nephrology centers across North America.[12] CKiD is gathering detailed epidemiologic information on 567 children with mild to moderate CKD and has provided recent data pertaining to our understanding of GFR assessment and risk factors associated with CKD progression in children.
GFR and renal progression
A reduction in GFR is a marker for CKD[5] and also a risk factor for renal progression. Recent studies in children demonstrate that a higher CKD stage at time of study entry has been shown to be independently associated with an increase rate of progression to renal failure.[4,13] Staples et al., in a retrospective cohort of approximately 4,000 children with CKD demonstrated that moderate (GFR 30-59ml/min/1.73m2) to severe CKD (GFR 15-29ml/min/1.73m2) at study entry had an associated higher risk of disease progression when compared to those with mild (GFR 60-75ml/min/1.73m2) CKD. For moderate CKD the hazard ratio (HR) was 2.02 with a 95% confidence interval (95% CI) 1.67 – 2.46; and for severe CKD the HR was 6.84 with a 95% CI 5.59 – 8.37.[4]
Given the importance of CKD staging and accurate determination of GFR in children, there have been several recent contributions regarding the measurement and estimation of GFR in this population. There is general consensus that measurement of GFR by a timed urine collection for creatinine clearance can be variable and inaccurate.[14] This is accentuated by the high prevalence of urologic problems in the pediatric CKD which may cause inadequate bladder emptying leading to inaccurate measured GFR values. While GFR measurement by inulin clearance is accepted as the gold standard, it is labor intensive; it utilizes a difficult assay to perform; inulin is not readily available; and without bladder catheterization, accurate urine collection in children can be difficult.[15]
Having close agreement to the GFR measured by inulin clearance [16]. GFR measurement by iohexol plasma disappearance method has been validated by the CKiD study as an accurate, reproducible, non-radioactive and safe method for the measurement of GFR in children.[15] It is used as the gold standard to measure GFR in the CKiD study. Since the initial pilot study, the CKiD study has performed over 1000 measurements of GFR by iohexol plasma disappearance in children across 43 pediatric nephrology centers with no adverse events.[17] With the four-point plasma disappearance curve as described in the original pilot study, the rate of unattainable iohexol GFR (iGFR) measurements is approximately six to seven percent due to errors in timing, inadequate documentation, or infiltration of the intravenous line[17], proving that iohexol GFR measurements are relatively successful.
Since the 1970s, the GFR estimating equation by Schwartz (Table 2, formula 1) has been widely accepted as an accurate estimation of GFR in children. It was used in the CKiD study to determine study eligibility. Although convenient and practical, the Schwartz formula has been found to overestimate the true GFR particularly at lower levels of GFR.[9] The overestimation of the GFR by the original Schwartz formula has been attributed to the change in methodology for laboratory creatinine determination.[18] The newer enzymatic determination of creatinine results in a lower value of creatinine compared to the older Jaffe method, on which the original Schwartz formula was based.
Table 2. CKiD GFR Estimating Equations.
The constant k is directly proportional to the muscle component of body, and varies with age. The value for k is 0.33 in premature infants through the first year of life, 0.45 for term infants through the first year of life, 0.55 in children and adolescent girls, and 0.7 in adolescent boys.
Abbreviations: eGFR= estimated GFR, Ht= height, m= meters, cm=centimeters Scr= Serum creatinine, CysC= Cystatin C, BUN= blood urea nitrogen.
Abbreviations: eGFR2 = estimated GFR 1 year after baseline, iGFR1 = iohexol GFR at baseline, Ht(m)/Scr2 = height in meters divided by serum creatinine one year after baseline, Ht(m)/Scr1 = height in meters divided by serum creatinine at baseline.
CKiD has developed an improved GFR estimating formula. Due to the structure of the visits for CKiD, newer GFR estimating equations were needed to accurately determine the GFR when iGFR was not measured (Table 2, formulae 2 and 3). Since the formulae were developed in children with moderate to severe CKD, the formulae have been validated for a range of GFR between 15 to 75ml/min/1.73m2, but need to be evaluated in children with higher levels of GFR for accuracy. Additionally, the CKiD study investigators have assessed longitudinal formulas to determine current or future changes in GFR based on prior measurements of GFR and changes in other clinical parameters (Table 2, formula 4).[19] The limitations for use of the longitudinal equation include the select population of subjects with moderate to severe CKD as well as the minimal decline in GFR over one year within the cohort. With more follow-up time and further progression of the cohort, the longitudinal equation can be refined.
Proteinuria and renal progression
In human studies (both adult and pediatric), elevated urine total protein is shown to be an independent risk factor for more rapid decline in kidney function.[20-22] The increase in urinary protein causes injury to tubular cells, leading to interstitial inflammation and fibrosis.[1] In patients with CKD, blockade of the renin-angiotensin system (RAS) reduces proteinuria: in adults, 1g/day reduction in proteinuria is associated with an abatement of GFR decline by 1-2mL/min per year. The antiproteinuric efficacy of RAS blockade has also been validated in children with CKD.[23-25]
Given the importance of proteinuria as a risk factor for CKD progression, understanding the determinants of proteinuria severity has become an important focus of research. The CKiD study evaluated the patterns of proteinuria severity among children with CKD. From 419 subjects with first morning urine protein-to-creatinine ratios (Upcr) and iGFR, the median Upcr was 0.53 and interquartile range was 0.2 to 1.27; median iGFR was 42 ml/min/1.73m2; and the median duration of CKD was six years. Log-linear regression demonstrated that proteinuria was associated with iGFR, age, race, and glomerular cause of CKD.[26] As seen in Figure 1, it is estimated that for every 10% decrease in iGFR, Upcr increased on average by 14% regardless of cause for CKD.
Figure 1. Level of Proteinuria by level of GFR.
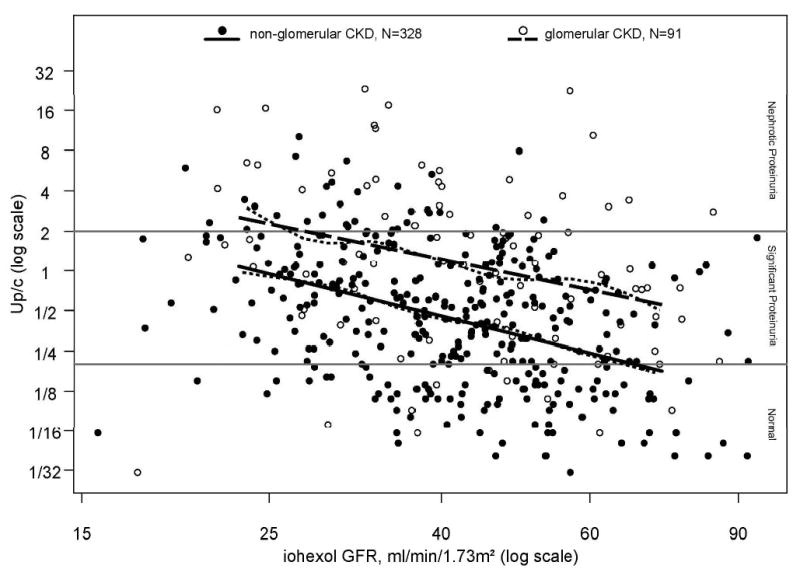
Urine Protein:Creatinine ratios (Up/c) by iohexol-measured glomerular filtration rate (iGFR)) for 328 non-glomerular CKD children and 91 glomerular CKD children, respectively. Non-parametric smoothing splines for the Up/c vs iGFR relationship for each of the CKD diagnosis groups are represented by the small dashed lines. Linear regression models for the non-glomerular and glomerular CKD patients are represented by the solid and large dashed straight lines, respectively. [Reproduced with permission from [26]]
In the multivariate analysis, proteinuria continued to be associated with GFR, age, and cause of CKD. Additionally, independent of these factors, non-Caucasian race was associated with 40% higher level of proteinuria compared to Caucasian race.[26] In summary, while higher levels of proteinuria were associated with lower levels of GFR, the factors accounting for intra-individual variation of proteinuria among children with CKD might be associated with variation in genetic or environmental factors.
Hypertension and renal progression
Systemic hypertension causes intraglomerular hypertension that leads to glomerular hypertrophy and injury. Compared to normotensive CKD patients, hypertension is associated with a more rapid decline in kidney function among adult and pediatric CKD patients.[27-30] Yet, hypertension in pediatric nephropathies, though common, is typically less severe than in adult disorders.[31] Regardless, analysis of CKD registry data in children shows that renal function in children who are hypertensive declines more rapidly than in normotensive children.[29] As discussed by Dr. Wuhl in this issue of Current Opinion in Pediatrics(editor to insert page), CKD decline can be slowed with effective control of hypertension. Studies show that intensified blood pressure control slows renal progression in both adult and pediatric patients with CKD.[11,22,32,33] The results of the ESCAPE trial emphasize the importance of high blood pressure as both a risk factor and a treatment target for pediatric CKD progression.
Despite the known benefits of adequate hypertension control, baseline blood pressure (BP) data from 432 participants in CKiD indicate that hypertension is highly prevalent (54%).[34] In the cohort, risk factors for either elevated systolic BP or elevated diastolic BP included black race, reported antihypertensive use, elevated serum potassium, glomerular cause of CKD, shorter duration of CKD, obesity, younger age, and nephrotic range proteinuria. In the multivariable analysis (adjusting for age, race, GFR, CKD diagnosis, duration of CKD, Upcr, antihypertensive use, obesity, and serum potassium), African American race, elevated serum potassium, longer duration of CKD, and antihypertensive use were independent risk factors for BP elevation.[34]
Among a sub-cohort of 202 CKiD subjects reporting use of antihypertensive medication for BP control, 36% had uncontrolled systolic or diastolic BP. The multivariable analysis demonstrated that male gender and shorter duration of CKD were associated with uncontrolled BP. Furthermore, use of ACE inhibitors or angiotensin receptor blockers was independently associated with BP control compared to other BP medications.[34] Although effective BP control is associated with improved outcomes in patients with CKD, the above data indicate that persistent barriers to achieving these goals exist in clinical practice.
Anemia and renal progression
Anemia is a universal complication of CKD caused by a decrease in renal production of erythropoietin.[35] The prevalence of anemia in the pediatric CKD population depends on CKD stage with a higher prevalence of anemia at higher stages of CKD. Wong et al found 36.6% of the pediatric CKD patients at their single center in Canada were anemic.[36] Compared to an anemia prevalence rate of approximately 30% in the earlier stages of CKD (stages 1 and 2), the rate of anemia was 66% at moderate CKD (stage 3), and 93% with severe CKD and end stage renal disease (ESRD) (stages 4 and 5) at their center. Estimates using the North American Pediatric Renal Trials and Collaborative Studies database indicate that the prevalence of anemia are 19% in CKD stage 2, 31% CKD stage 3, 55% CKD stage 4, and 68% in CKD stage 5.[37] Data from CKiD indicate that below a threshold iGFR of 43 ml/min/1.73m2 there is a statistically significant decrease in hemoglobin by -0.3 grams/dL for each 5ml/min/1.73m2 decrease in iGFR, whereas above the threshold the decline is less: -0.1gram/dL for each 5ml/min/1.73m2 decrease.[38]
Multiple studies have found anemia to be associated with an increased risk of morbidity and mortality in both pediatric ESRD and non-dialysis CKD patients.[37,39,40] More specifically, several studies have found an association between anemia and CKD progression. [41-43] Although there are wide variations in the study methods and definitions of anemia, the associated findings are consistent; anemic patients have a higher risk of CKD progression than patients without anemia.
In children with CKD, there is high interest in determining if anemia correction may slow CKD progression. Development of such trials have been complicated due to recent concerns regarding the increase in risk for morbidity and even mortality associated with normalization of hematocrit levels in adult CKD populations.[44-46] In pediatric CKD patients, increased risk for hospitalization or death were not associated with hematocrit levels above 36% or 39%.[37] Furthermore, correction of anemia with erythropoietin has been associated with a variety of beneficial effects in children, including improvement in quality of life, appetite, exercise tolerance, and Wechsler intelligence score, [47,48] highlighting the importance of attentive anemia management and the need for well-designed clinical trials in the pediatric CKD population.
Other risk factors for renal progression
While level of GFR, proteinuria, and hypertension are strongly associated risk factors for CKD progression, other reported risk factors associated with CKD progression include: low birth weight or prematurity[49,50], uric acid[51-53], lead or heavy metals[54,55], hyperlipidemia[56], metabolic acidosis[57], oxidative stress[58], and disorders of bone and mineral metabolism [4,59-61]. While there is ongoing research to clarify the role of these risk factors on renal progression, the search for genetic susceptibility to CKD and its progression in humans have offered not only new directions for research but also potential targets for intervention.
Genome-Wide Scans and CKD
Recent findings regarding the genetic contribution to renal traits have provided new insights toward the pathogenesis and treatment for CKD. Significant advances in the field of genetics and genomic technology have made scanning the human genome for associations with complex diseases economically feasible. The success of genome-wide association studies (GWAS) is highlighted by the identification of candidate genes and loci for diabetic nephropathy susceptibility.[62-65] Recently, whole genome association studies have identified associations between renal traits and the non-muscle myosin heavy chain type 2 isoform A (MYH9) gene localized to chromosome 22q13[66,67]. This gene is associated with serum creatinine levels in non-CKD healthy Europeans. [67] In African Americans, MYH9 is associated with focal segmental glomerulosclerosis, nondiabetic ESRD, hypertensive ESRD, and among hypertensive patients urinary microalbumin-to-creatinine ratios. [66,68-70] Although the mechanism by which MYH9 participates in CKD susceptibility and progression is unknown, MYH9 is expressed by podocytes and suggested to be a major part of the actin-myosin contractile apparatus of the podocyte foot process.[71] Other genes associated with CKD include uromodulin (UMOD),[72] methenyltetrahydrofolate synthetase (MTHFS),[73,74] eyes absent homolog 1 (EYA1),[74] transcription factor-7-like 2 (TCF7L2),[75] and others[72-74] (Table 3).
Table 3. Genes Associated with CKD (Non-Diabetic).
| Gene | Name | Function | Biological Process |
|---|---|---|---|
| ACTN4[76,77] | Actinin alpha 4 | Alpha actinins belong to the spectrin gene superfamily which represents a diverse group of cytoskeletal proteins. This gene encodes a nonmuscle, alpha actinin isoform which is concentrated in the cytoplasm. | In nonmuscle cells, the cytoskeletal isoform is found along microfilament bundles and adherens-type junctions, where it is involved in binding actin to the membrane. Mutations in this gene have been associated with an inherited form of focal segmental glomerulosclerosis. |
| EYA1[74] | Eyes absent homolog 1 (aka: brachio-oto-renal (BOR)) | EYA1 is a regulator of mammalian organogenesis. EYA protiens do not bind DNA directly but function as coactivators of transcription in the nucleus. | EYA1 is important for pattern formation of early kidney development and works in conjunction with HOX11 and PAX2. The EYA binding domain interacts with transcriptional cofactors SIX and DACH. The binding domain also exibits a tyrosine phosphatase activity that regulates anti-apoptotic signaling with DNA damage. |
| GATM[72] | Glycine amidino-transferase (aka: L-arginine:glycine amidinotransfer-ase) | The gene encodes a mitochondrial enzyme that belongs to the amidinotransferase family. This enzyme is involved in creatine biosynthesis. | GATM is responsible for catalyzing the rate limiting step of creatine biosynthesis. GATM transfers the amidino group from L-arginine to glycine resulting in L-ornithine and guanidinoacetic acid (the immediate precursor to creatine). |
| JAG1[72] | Jagged 1 | JAG1 is a single-pass transmembrane ligand for NOTCH1. Mutations of this gene is associated with Alagille syndrome. | Interactions between the extracellular domain of JAG1 with NOTCH1 triggers proteolytic cleavage of the receptor and activation of the pathway. |
| MTHFS[74] | 5,10-methenylttrahydro-folate synthetase | MTHFS is a protein that catalyzes the transformation of 5-formyltetrahydrofolate to methenyltetrahydrofolate. | MYH9 is associated with serum creatinine level in Europeans. In African Americans it is associated with FSGS, ESRD, and in hypertensive patients albuminuria. The protein is expressed in the kidney, liver, cochlea, and platelets. |
| MYH9[66-70] | Myosin, heavy chain 9, non-muscle | The gene encodes the alpha isoform of non-muscle myosin heavy chain. This class of protein is an important component of the cells motor system. | MYH9 is associated with serum creatinine level in Europeans. In African Americans it is associated with FSGS, ESRD, and in hypertensive patients albuminuria. The protein is expressed in the kidney, liver, cochlea, and platelets. |
| NPHS2[78] | Nephrosis 2, idiopathic steroid resistant (Podicin) | This gene encodes for the glomerular protein podocin which belongs to a family of evolutionarily conserved membrane associated proteins. | NPHS2 can recruit and bind cholesterol, forming multimers. NPHS2 binds and regulates the transient receptor potential channel, TRP6. |
| SHROOM3[72] | Shroom family member 3 | The gene encodes for an actin binding protein and associated with gamma tubulin function. | SHROOM3 associates with gamma tubulin, myosin light chain, and Rho kinase to regulate apical junction organization in epithelial cells. |
| STC1[72] | Stanniocalcin 1 | The gene is a glycoprotein initially characterized in fish with calcium regulating properites. Human STC1 has a wide distribution of tissues and found in mitochondria. The function of STC1 has been difficult to characterize. | STC1 in humans is hypothesized to be involved in the cellular stress response. STC1 gene expression is regulated by tumor suppressors BRCA1 and p53. STC1 is upregulated by oxidative stress and IL6. |
| TCF7L2[75] | Transcription factor 7 like-2 (note: TCF7L2 has an alternative alias, TCF4) | This gene encodes a high mobility group box-containing transcription fctor that plays a role in Wnt signaling pathway. | Genetic variants of this gene has been implicated in blood glucose homeostasis as well as chronic kidney disease in the general population. |
| UMOD[72] | Uromodulin | Uromodulin is the most abundant protein in normal urine. Excretion follows proteolytic cleavage of the ectodomain on the luminal surface of the loop of henle. | Defects of the gene are associated with medullary cystic kidney disease-2 (MCKD2) and familial juvenil hyperuricemic nephropathy. Recently the gene has been associated with CKD by genome wide association in ther general population. |
While GFR and proteinuria are closely associated and are markers for CKD, they are distinct phenotypes.[79] Furthermore, available GWAS evidence from adults with diabetic CKD indicates the genetic loci associated with albuminuria is distinct from the loci associated with GFR.[62,63,80] The gene-disease associations for proteinuria from adult studies thus far do not generalize to children with CKD. This may be due to the fact that the types of diseases causing CKD are different in children versus adults. In fact, the NKF recommendations for proteinuria testing differ between children and adults. Tests for albuminuria are recommended when for screening for and monitoring adults with CKD; while albuminuria screening is recommended for diabetic children, urine total protein is recommended for children with kidney disease.[5] The genetics for proteinuria have yet to be elucidated in children with CKD, and are the focus of ongoing research.
Conclusions
With cohort studies of children with CKD becoming mature, they have started to yield important refinements to the assessment of CKD progression. The measurement of GFR by iohexol disappearance and refinement of GFR estimating equations represent recent improvements for measuring CKD progression in children. These are necessary to define the role of traditional and non-traditional risk factors for CKD progression in children. Traditional risk factors of proteinuria and hypertension are validated targets to slow CKD progression in children while other reported risk factors are still need of confirmation and validation in human studies. Adding to the growing list of putative CKD risk factors, genome-wide investigations in the adult CKD population have provided new genetic markers and pathways associated with CKD. Undoubtedly the ongoing cohort studies will assess the role traditional and novel risk factors for GFR decline. Furthermore, they will be ideal platforms for assessing genetic risk factors for CKD progression in the pediatric population.
Acknowledgments
Dr. Wong is supported by NIH/NIDDK grant # U01DK066143. The authors would like to thank Dr. George Schwartz, Dr. Susan Furth, Dr. Allison Abraham and Dr. Michel Baum for their suggestions and comments in preparing this manuscript.
References
- 1.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis. 2005;12:353–365. doi: 10.1053/j.ackd.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 2.(USRDS) USRDS . In: USRDS 2008 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, editor. Bethesda, MD: 2008. [Google Scholar]
- 3.Schieppati A, Remuzzi G. Chronic renal diseases as a public health problem: Epidemiology, social, and economic implications. Kidney Int. 2005;68:S7–S10. doi: 10.1111/j.1523-1755.2005.09801.x. [DOI] [PubMed] [Google Scholar]
- 4.Staples AO, Smith JC, Gipson DS, Wong CS, Filler G, Warady B, Martz K, Greenbaum LA. Risk factors associated with progression of pediatric chronic kidney disease. 2007 E-PAS2007:61:7919.5. [Google Scholar]
- 5.Vassalotti JA, Stevens LA, Levey AS. Testing for chronic kidney disease: a position statement from the national kidney foundation. Am J Kidney Dis. 2007;50:169–180. doi: 10.1053/j.ajkd.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 6.Wong CS, Mak RHK. Chronic kidney disease. In: Kher KK, Schnapper HW, Makker SP, editors. Clinical Pediatric Nephrology. Informa UK Ltd; 2007. pp. 339–352. [Google Scholar]
- 7.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, et al. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int. 2009;76:528–533. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]; •• Observational study with long-term outcomes demonstrating the increased risk for end-stage renal disease of children with a congenital solitary kidney and of children with hypodysplasia.
- 8.Chesney RW. The future of pediatric nephrology. Pediatr Nephrol. 2005;20:867–871. doi: 10.1007/s00467-005-1902-0. [DOI] [PubMed] [Google Scholar]
- 9.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 10.Ardissino G, Dacco V, Testa S, Bonaudo R, Claris-Appiani A, Taioli E, Marra G, Edefonti A, Sereni F. Epidemiology of chronic renal failure in children: data from the ItalKid project. Pediatrics. 2003;111:e382–387. doi: 10.1542/peds.111.4.e382. [DOI] [PubMed] [Google Scholar]
- 11.Strict Blood-Pressure Control and Progression of Renal Failure in Children. N Engl J Med. 2009;361:1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]; •• This interventional study demonstrates the benefit of intensified blood pressure control to slow CKD progression in children with CKD.
- 12.Furth SL, Cole SR, Moxey-Mims M, Kaskel F, Mak R, Schwartz G, Wong C, Munoz A, Warady BA. Design and Methods of the Chronic Kidney Disease in Children (CKiD) Prospective Cohort Study. Clin J Am Soc Nephrol. 2006;1:1006–1015. doi: 10.2215/CJN.01941205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soares CM, Oliveira EA, Diniz JS, Lima EM, Vasconcelos MM, Oliveira GR. Predictive factors of progression of chronic renal insufficiency: a multivariate analysis. Pediatr Nephrol. 2003;18:371–377. doi: 10.1007/s00467-003-1115-3. [DOI] [PubMed] [Google Scholar]
- 14.Hogg RJ, Furth S, KV L, et al. National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111:1416. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Furth S, Cole SR, Warady B, Munoz A. Glomerular filtration rate via plasma iohexol disappearance: pilot study for chronic kidney disease in children. Kidney Int. 2006;69:2070–2077. doi: 10.1038/sj.ki.5000385. [DOI] [PubMed] [Google Scholar]
- 16.Gaspari F, Perico N, Ruggenenti P, Mosconi L, Amuchastegui CS, Guerini E, Daina E, Remuzzi G. Plasma clearance of nonradioactive iohexol as a measure of glomerular filtration rate. J Am Soc Nephrol. 1995;6:257–263. doi: 10.1681/ASN.V62257. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Furth S. Safety of Plasma Clearance of Iohexol to Measure GFR. Personal communication. 2009 [Google Scholar]
- 18.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is an update of GFR estimating equations based on clinical and laboratory measurements using the GFR by iohexol plasma disappearance as the gold standard.
- 19.Abraham AG, Schwartz GJ, Furth S, Warady BA, Munoz A. Longitudinal Formulas to Estimate GFR in Children with CKD. Clin J Am Soc Nephrol. 2009 doi: 10.2215/CJN.01860309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson JC, Adler S, Burkart JM, Greene T, Hebert LA, Hunsicker LG, King AJ, Klahr S, Massry SG, Seifter JL. Blood pressure control, proteinuria, and the progression of renal disease. The Modification of Diet in Renal Disease Study. Ann Intern Med. 1995;123:754–762. doi: 10.7326/0003-4819-123-10-199511150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) Lancet. 1997;349:1857–1863. [PubMed] [Google Scholar]
- 22.Ardissino G, Testa S, Dacco V, Vigano S, Taioli E, Claris-Appiani A, Procaccio M, Avolio L, Ciofani A, Dello Strologo L, et al. Proteinuria as a predictor of disease progression in children with hypodysplastic nephropathy. Data from the Ital Kid Project Pediatr Nephrol. 2004;19:172–177. doi: 10.1007/s00467-003-1268-0. [DOI] [PubMed] [Google Scholar]
- 23.Wuhl E, Mehls O, Schaefer F. Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int. 2004;66:768–776. doi: 10.1111/j.1523-1755.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 24.Ellis D, Moritz ML, Vats A, Janosky JE. Antihypertensive and renoprotective efficacy and safety of losartan. A long-term study in children with renal disorders. Am J Hypertens. 2004;17:928–935. doi: 10.1016/j.amjhyper.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y, Ohta K, Shimizu M, Nakai A, Kasahara Y, Yachie A, Koizumi S. Treatment with low-dose angiotensin-converting enzyme inhibitor (ACEI) plus angiotensin II receptor blocker (ARB) in pediatric patients with IgA nephropathy. Clin Nephrol. 2005;64:35–40. doi: 10.5414/cnp64035. [DOI] [PubMed] [Google Scholar]
- 26.Wong CS, Pierce CB, Cole SR, Warady BA, Mak RH, Benador NM, Kaskel F, Furth SL, Schwartz GJ. Association of proteinuria with race, cause of chronic kidney disease, and glomerular filtration rate in the chronic kidney disease in children study. Clin J Am Soc Nephrol. 2009;4:812–819. doi: 10.2215/CJN.01780408. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study evaluates the determinants of proteinuria among children with CKD.
- 27.Locatelli F, Marcelli D, Comelli M, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A. Proteinuria and blood pressure as causal components of progression to end-stage renal failure. Northern Italian Cooperative Study Group. Nephrol Dial Transplant. 1996;11:461–467. doi: 10.1093/ndt/11.3.461. [DOI] [PubMed] [Google Scholar]
- 28.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 29.Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephrol. 2003;14:2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 30.Wingen AM, Fabian-Bach C, Schaefer F, Mehls O. Randomised multicentre study of a low-protein diet on the progression of chronic renal failure in children. European Study Group of Nutritional Treatment of Chronic Renal Failure in Childhood. Lancet. 1997;349:1117–1123. doi: 10.1016/s0140-6736(96)09260-4. [DOI] [PubMed] [Google Scholar]
- 31.Wuhl E, Schaefer F. Therapeutic strategies to slow chronic kidney disease progression. Pediatr Nephrol. 2008;23:705–716. doi: 10.1007/s00467-008-0789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 33.Summary of the 2007 European Society of Hypertension (ESH) and European Society of Cardiology (ESC) guidelines for the management of arterial hypertension. Vasc Health Risk Manag. 2007;3:783–795. [PMC free article] [PubMed] [Google Scholar]
- 34.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52:631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study from the CKiD group identifies that there are barriers to achieving controlled blood pressure and assesses the determinants for controlled and uncontrolled blood pressure.
- 35.Chandra M, McVicar M, Clemons GK. Pathogenesis of the anemia of chronic renal failure: the role of erythropoietin. Adv Pediatr. 1988;35:361–389. [PubMed] [Google Scholar]
- 36.Wong H, Mylrea K, Feber J, Drukker A, Filler G. Prevalence of complications in children with chronic kidney disease according to KDOQI. Kidney Int. 2006;70:585–590. doi: 10.1038/sj.ki.5001608. [DOI] [PubMed] [Google Scholar]
- 37.Staples AO, Wong CS, Smith JM, Gipson DS, Filler G, Warady BA, Martz K, Greenbaum LA. Anemia and risk of hospitalization in pediatric chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:48–56. doi: 10.2215/CJN.05301107. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrates the increased risk of hospitalization associated with anemia in children with CKD and shows no association between increased risk of morbidity and normalized hematocrit levels.
- 38.Fadrowski JJ, Pierce CB, Cole SR, Moxey-Mims M, Warady BA, Furth SL. Hemoglobin decline in children with chronic kidney disease: baseline results from the chronic kidney disease in children prospective cohort study. Clin J Am Soc Nephrol. 2008;3:457–462. doi: 10.2215/CJN.03020707. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that there is a threshold of GFR in children with CKD when the rate of anemia becomes more pronounced.
- 39.Amaral S, Hwang W, Fivush B, Neu A, Frankenfield D, Furth S. Association of mortality and hospitalization with achievement of adult hemoglobin targets in adolescents maintained on hemodialysis. J Am Soc Nephrol. 2006;17:2878–2885. doi: 10.1681/ASN.2005111215. [DOI] [PubMed] [Google Scholar]
- 40.Warady BA, Ho M. Morbidity and mortality in children with anemia at initiation of dialysis. Pediatr Nephrol. 2003;18:1055–1062. doi: 10.1007/s00467-003-1214-1. [DOI] [PubMed] [Google Scholar]
- 41.Furth SL, Cole SR, Fadrowski JJ, Gerson A, Pierce CB, Chandra M, Weiss R, Kaskel F. The association of anemia and hypoalbuminemia with accelerated decline in GFR among adolescents with chronic kidney disease. Pediatr Nephrol. 2007;22:265–271. doi: 10.1007/s00467-006-0313-1. [DOI] [PubMed] [Google Scholar]
- 42.Kuriyama S, Tomonari H, Yoshida H, Hashimoto T, Kawaguchi Y, Sakai O. Reversal of anemia by erythropoietin therapy retards the progression of chronic renal failure, especially in nondiabetic patients. Nephron. 1997;77:176–185. doi: 10.1159/000190270. [DOI] [PubMed] [Google Scholar]
- 43.Rossert J, Levin A, Roger SD, Horl WH, Fouqueray B, Gassmann-Mayer C, Frei D, McClellan WM. Effect of early correction of anemia on the progression of CKD. Am J Kidney Dis. 2006;47:738–750. doi: 10.1053/j.ajkd.2006.02.170. [DOI] [PubMed] [Google Scholar]
- 44.Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 45.Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol. 2005;16:2180–2189. doi: 10.1681/ASN.2004121039. [DOI] [PubMed] [Google Scholar]
- 46.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 47.Burke JR. Low-dose subcutaneous recombinant erythropoietin in children with chronic renal failure. Australian and New Zealand Paediatric Nephrology Association. Pediatr Nephrol. 1995;9:558–561. doi: 10.1007/BF00860930. [DOI] [PubMed] [Google Scholar]
- 48.Montini G, Zacchello G, Baraldi E, Zanconato S, Suppiej A, Fabris F, Andreetta B, Pavanello L, Zacchello F. Benefits and risks of anemia correction with recombinant human erythropoietin in children maintained by hemodialysis. J Pediatr. 1990;117:556–560. doi: 10.1016/s0022-3476(05)80688-2. [DOI] [PubMed] [Google Scholar]
- 49.Abitbol CL, Chandar J, Rodriguez MM, Berho M, Seeherunvong W, Freundlich M, Zilleruelo G. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr Nephrol. 2009;24:1363–1370. doi: 10.1007/s00467-009-1120-2. [DOI] [PubMed] [Google Scholar]; •• This retrospective single center study provides compelling data regarding the increased risk for CKD in individuals who are born pre-term. The evaluation of obesity in their study demonstrated that obese patients who were preterm infants had a more rapid decline in kidney function compared to obese patients who were born at term.
- 50.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Cohort study with registry data with long-term follow-up in Norway demonstrates the increased risk of low birth weight and end-stage renal disease.
- 51.Feig DI. Uric acid: a novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens. 2009;18:526–530. doi: 10.1097/MNH.0b013e328330d9d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA. 2008;300:924–932. doi: 10.1001/jama.300.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This interventional study with allopurinol provides a possible mechanism by which elevated serum uric acid levels are associated with CKD progression.
- 53.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169:342–350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This is a population based retrospective cohort study that identified multiple risk factors associated with ESRD. The most important risk factors identified by the authors were proteinuria and excess weight. Other risk factors of interest included: lower hemoglobin level, higher uric acid level, self-reported history of nocturia, and family history of kidney disease.
- 54.Lin JL, Lin-Tan DT, Li YJ, Chen KH, Huang YL. Low-level environmental exposure to lead and progressive chronic kidney diseases. Am J Med. 2006;119:707, e701–709. doi: 10.1016/j.amjmed.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Muntner P, He J, Vupputuri S, Coresh J, Batuman V. Blood lead and chronic kidney disease in the general United States population: results from NHANES III. Kidney Int. 2003;63:1044–1050. doi: 10.1046/j.1523-1755.2003.00812.x. [DOI] [PubMed] [Google Scholar]
- 56.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58:293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 57.de Brito-Ashurst I, Varagunam M, Raftery MJ, Yaqoob MM. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20:2075–2084. doi: 10.1681/ASN.2008111205. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Randomized open-label, prospective parallel-group study of bicarbonate supplementation in adults with CKD. Intervention subjects had a slower decline in creatinine clearance and improved nutritional status compared to control subjects.
- 58.Okamura DM, Himmelfarb J. Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol. 2009 doi: 10.1007/s00467-009-1199-5. [DOI] [PubMed] [Google Scholar]
- 59.Norman LJ, Macdonald IA, Watson AR. Optimising nutrition in chronic renal insufficiency--progression of disease. Pediatr Nephrol. 2004;19:1253–1261. doi: 10.1007/s00467-004-1581-2. [DOI] [PubMed] [Google Scholar]
- 60.Ritz E, Gross ML, Dikow R. Role of calcium-phosphorous disorders in the progression of renal failure. Kidney Int Suppl. 2005:S66–70. doi: 10.1111/j.1523-1755.2005.09912.x. [DOI] [PubMed] [Google Scholar]
- 61.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75:88–95. doi: 10.1038/ki.2008.501. [DOI] [PubMed] [Google Scholar]; •• This prospective observational cohort study among adult patients with CKD demonstrates that low serum vitamin D levels are associated with a higher risk for CKD progression and death.
- 62.Iyengar SK, Abboud HE, Goddard KA, Saad MF, Adler SG, Arar NH, Bowden DW, Duggirala R, Elston RC, Hanson RL, et al. Genome-wide scans for diabetic nephropathy and albuminuria in multiethnic populations: the family investigation of nephropathy and diabetes (FIND) Diabetes. 2007;56:1577–1585. doi: 10.2337/db06-1154. [DOI] [PubMed] [Google Scholar]
- 63.Freedman BI, Bowden DW, Rich SS, Xu J, Wagenknecht LE, Ziegler J, Hicks PJ, Langefeld CD. Genome-wide linkage scans for renal function and albuminuria in Type 2 diabetes mellitus: the Diabetes Heart Study. Diabet Med. 2008;25:268–276. doi: 10.1111/j.1464-5491.2007.02361.x. [DOI] [PubMed] [Google Scholar]
- 64.Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol. 2007;2:1306–1316. doi: 10.2215/CJN.02560607. [DOI] [PubMed] [Google Scholar]
- 65.Bowden DW, Colicigno CJ, Langefeld CD, Sale MM, Williams A, Anderson PJ, Rich SS, Freedman BI. A genome scan for diabetic nephropathy in African Americans. Kidney Int. 2004;66:1517–1526. doi: 10.1111/j.1523-1755.2004.00915.x. [DOI] [PubMed] [Google Scholar]
- 66.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identification of MYH9 as a novel gene associated with primary and secondary forms of focal segmental sclerosis.
- 67.Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS, Polasek O, Kolcic I, Biloglav Z, Campbell S, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009 doi: 10.1038/ki.2009.135. [DOI] [PubMed] [Google Scholar]; • Identification of the MYH9 gene associated with serum creatinine levels in Europeans.
- 68.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports of MYH9 locus associated with ESRD in African Americans accounting for a large proportion of the excess risk for ESRD observed in African Americans compared to European Americans.
- 69.Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, Kopp JB, Winkler CA, Nelson GW, Langefeld CD, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–745. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports of MYH9 locus associated with ESRD in African Americans. Taken together with the other reports, the MYH9 gene is a significant genetic risk factor for CKD.
- 70.Freedman BI, Kopp JB, Winkler CA, Nelson GW, Rao DC, Eckfeldt JH, Leppert MF, Hicks PJ, Divers J, Langefeld CD, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with albuminuria in hypertensive African Americans: the HyperGEN study. Am J Nephrol. 2009;29:626–632. doi: 10.1159/000194791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arrondel C, Vodovar N, Knebelmann B, Grunfeld JP, Gubler MC, Antignac C, Heidet L. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65–74. doi: 10.1681/ASN.V13165. [DOI] [PubMed] [Google Scholar]
- 72.Kottgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, Li M, Yang Q, Gudnason V, Launer LJ, Harris TB, et al. Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet. 2009 doi: 10.1038/ng.377. advanced online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Genome-wide association study demonstrating multiple loci, GATM, JAG1, SHROOM3, STC1, and UMOD are associated with CKD and level of kidney function.
- 73.Hwang SJ, Yang Q, Meigs JB, Pearce EN, Fox CS. A genome-wide association for kidney function and endocrine-related traits in the NHLBI's Framingham Heart Study. BMC Med Genet. 2007;8 Suppl 1:S10. doi: 10.1186/1471-2350-8-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kottgen A, Kao WH, Hwang SJ, Boerwinkle E, Yang Q, Levy D, Benjamin EJ, Larson MG, Astor BC, Coresh J, et al. Genome-wide association study for renal traits in the Framingham Heart and Atherosclerosis Risk in Communities Studies. BMC Med Genet. 2008;9:49. doi: 10.1186/1471-2350-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identified that the EYA1, MTHFS, gene is associated with CKD progression.
- 75.Kottgen A, Hwang SJ, Rampersaud E, Coresh J, North KE, Pankow JS, Meigs JB, Florez JC, Parsa A, Levy D, et al. TCF7L2 variants associate with CKD progression and renal function in population-based cohorts. J Am Soc Nephrol. 2008;19:1989–1999. doi: 10.1681/ASN.2007121291. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Identifies that the TCF7L2 gene is associated with CKD progression.
- 76.Henderson JM, Alexander MP, Pollak MR. Patients with ACTN4 mutations demonstrate distinctive features of glomerular injury. J Am Soc Nephrol. 2009;20:961–968. doi: 10.1681/ASN.2008060613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaplan JM, Kim SH, North KN, Rennke H, Correia LA, Tong HQ, Mathis BJ, Rodriguez-Perez JC, Allen PG, Beggs AH, et al. Mutations in ACTN4, encoding alpha-actinin-4, cause familial focal segmental glomerulosclerosis. Nat Genet. 2000;24:251–256. doi: 10.1038/73456. [DOI] [PubMed] [Google Scholar]
- 78.Dusel JA, Burdon KP, Hicks PJ, Hawkins GA, Bowden DW, Freedman BI. Identification of podocin (NPHS2) gene mutations in African Americans with nondiabetic end-stage renal disease. Kidney Int. 2005;68:256–262. doi: 10.1111/j.1523-1755.2005.00400.x. [DOI] [PubMed] [Google Scholar]
- 79.Garg AX, Kiberd BA, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: Results from the NHANES III. Kidney Int. 2002;61:2165–2175. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 80.Fox CS, Yang Q, Guo CY, Cupples LA, Wilson PWF, Levy D, Meigs JB. Genome-wide linkage analysis to urinary microalbuminuria in a community-based sample: The Framingham Heart Study. Kidney Int. 2005;67:70–74. doi: 10.1111/j.1523-1755.2005.00056.x. [DOI] [PubMed] [Google Scholar]


