Abstract
A distributed limbic-corticostriatal circuitry is implicated in cue-induced drug craving and relapse. Exposure to drug-paired cues not only precipitates relapse, but also triggers the reactivation and reconsolidation of the cue-drug memory. However, the limbic cortical-striatal circuitry underlying drug memory reconsolidation is unclear. The aim of this study was to investigate the involvement of the nucleus accumbens core and the basolateral amygdala in the reconsolidation of a cocaine-conditioned stimulus-evoked memory. Antisense oligodeoxynucleotides (ASO) were infused into each structure to knock down the expression of the immediate-early gene zif268, which is known to be required for memory reconsolidation. Control infusions used missense oligodeoxynucleotides (MSO). The effects of zif268 knockdown were measured in two complementary paradigms widely used to assess the impact of drug-paired CSs upon drug seeking: the acquisition of a new instrumental response with conditioned reinforcement and conditioned place preference. The results show that both intranucleus accumbens core and intrabasolateral amygdala zif268 ASO infusions at memory reactivation impaired the reconsolidation of the memory underlying a cocaine-conditioned place preference. However, knockdown of zif268 in the nucleus accumbens at memory reactivation had no effect on the memory underlying the conditioned reinforcing properties of the cocaine-paired CS measured subsequently, and this is in contrast to the marked impairment observed previously following intrabasolateral amygdala zif268 ASO infusions. These results suggest that both the basolateral amygdala and nucleus accumbens core are key structures within limbic cortical-striatal circuitry where reconsolidation of a cue-drug memory occurs. However reconsolidation of memory representations formed during Pavlovian conditioning are differentially localized in each site.
Through Pavlovian association with the effects of addictive drugs, a conditioned stimulus (CS) acquires both general motivational and sensory-specific conditioned reinforcing properties (Everitt et al. 2000). These associations contribute to the high likelihood of relapse in addicted individuals, yet the extinction of drug CSs by nonreinforced exposure has proved to be of limited therapeutic utility (Conklin and Tiffany 2002). In abstinent humans, drug CSs evoke salient and persistent memories of drug-taking experiences, inducing craving and relapse (Childress et al. 1988; O'Brien et al. 1992), while in animals they also precipitate relapse to, or reinstatement of, drug-seeking behavior (de Wit and Stewart 1981; Meil and See 1996; Fuchs et al. 1998; Weiss 2000). Thus, disrupting drug-related memories might significantly diminish relapse propensity on subsequent exposure to drug-paired CSs, and thereby promote abstinence.
Exposure to a drug-associated CS also triggers a process of memory reconsolidation, which restabilizes the reactivated and labile memory (Nader 2003). While reconsolidation may adaptively update memories (Dudai 2006; Hupbach et al. 2007; Rossato et al. 2007; Lee 2009), its disruption may reduce the impact of intrusive or aberrant memories on behavior subsequently (Lee et al. 2005, 2006; Brunet et al. 2008; Kindt et al. 2009; Taubenfeld et al. 2009). The reconsolidation of CS–cocaine memories has been shown to depend upon protein synthesis and expression of the plasticity-associated immediate-early gene, zif268, in the basolateral amygdala (BLA), since zif268 knockdown at memory reactivation disrupted the acquired conditioned reinforcing properties of the CS measured in drug-seeking tasks days or weeks later (Lee et al. 2005, 2006).
Although the BLA has an established role in CS-drug memory reconsolidation, it remains unclear whether other sites within limbic cortical-ventral striatal circuitry participate in this process. The nucleus accumbens core (AcbC) is a primary candidate, as zif268 is up-regulated in the AcbC as well as in the BLA following exposure to cocaine CSs (Thomas et al. 2003). Furthermore, the AcbC, which is strongly implicated in Pavlovian influences on drug seeking and relapse (Cardinal et al. 2002; Kalivas and McFarland 2003), has been shown to be a site where the reconsolidation of a drug conditioned place preference (CPP) memory can be disrupted (Miller and Marshall 2005).
Given the evidence of increased zif268 expression in the AcbC following CS-drug memory reactivation, we investigated its requirement in the reconsolidation of cocaine-associated memories. To address this issue, we employed two different but complementary paradigms widely used to measure the conditioned effects of CSs associated with drugs of abuse: the acquisition of a new instrumental response with conditioned reinforcement (ANR) and CPP. These procedures have been used successfully to investigate the mechanisms underlying the reconsolidation of appetitive Pavlovian memories, but it is likely that they depend upon different associative mechanisms (Everitt et al. 1991; White and McDonald 1993) that in turn depend upon different neural loci within limbic cortical-striatal circuitry (Cardinal et al. 2002). Therefore, to enable a full comparison with the functional involvement of the BLA, we investigated the necessity for BLA zif268 expression in drug memory reconsolidation as assessed in the CPP paradigm.
Results
Experiment 1: zif268 knockdown in the nucleus accumbens core impaired the reconsolidation of a cocaine-induced conditioned place preference memory
Histological assessment
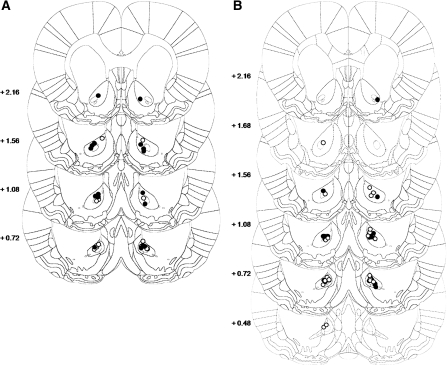
Rats included in the behavioral analysis had cannulae placed bilaterally either within, or adjacent to the boundaries of, the nucleus accumbens core (Fig. 1). For the reactivated group, after conditioning, 22 of 40 rats had acquired a preference for the cocaine-paired chamber, a necessary prerequisite for observing reconsolidation deficits. After histological analysis, five animals were excluded due to cannulae misplacement (Fig. 1A). In total, 17 animals were included in the statistical analysis, seven zif268 ASO-infused rats and 10 zif268 MSO-infused rats.
Figure 1.
Location of the injector tips within the AcbC for both reactivated (A) and nonreactivated (B) groups. (Black circles) zif268 ASO-infused rats; (white circles) zif268 MSO-infused rats.
For the nonreactivated group, one of the 24 rats was excluded from the analysis due to cannulae misplacement (Fig. 1B). Hence, the overall statistical analysis was performed on 23 animals of which 11 were ASO treated and 12 were MSO treated.
Behavioral results
Intra-AcbC zif268 ASO infusions at memory reactivation disrupted the performance of a previously acquired cocaine-conditioned place preference, in a reactivation-dependent manner
Rats underwent a single preconditioning session prior to the 8 d of training to assess any intrinsic preference for either of the chambers (see Fig. 2A,B for an overview of training, memory reactivation, and testing). Prior to conditioning, there was no difference in the time spent on each side of the conditioning apparatus between the experimental groups (Side: F(1,15) = 2.787, P = 0.157; Treatment: F(1,15) = 0.683, P = 0.422; Side × Treatment: F(1,15) = 0.536, P = 0.476) (Fig. 2C). During the memory-reactivation session, in which rats were reexposed to the CPP apparatus in the absence of drug, there was no difference between the two groups, with both groups spending more time on the cocaine-paired side than on the saline-paired side (Side: F(1,15) = 26.979, P < 0.001; Treatment: F(1,15) = 0.033, P = 0.859; Treatment × Side: F(1,15) = 0.079, P = 0.783). Moreover, both experimental groups had acquired a place preference as evidenced by a greater preference for the cocaine-paired side during reactivation compared with the preconditioning session (Fig. 2D; Test × Side: F(1,15) = 46.577, P < 0.001, Test × Side × Treatment: F(1,15) = 0.918, P = 0.353). Forty-eight hours after the last conditioning session and 24 hours after memory reactivation, all animals were reexposed again to the CPP apparatus. During this post-reactivation test, while zif268 MSO-infused rats still showed a preference for the cocaine-paired side, the zif268 ASO-infused rats did not (Fig. 2E). Compared with the previous robust preference showed during memory reactivation, the knockdown of zif268 in the AcbC prior to memory reactivation impaired the subsequent expression of the cocaine-induced CPP (Test × Side × Treatment: F(1,15) = 7.476, P < 0.05).
Figure 2.
Cocaine-induced CPP is disrupted in a reactivation-dependent manner following intra-AcbC zif268 ASO prior to memory reactivation. Time spent on the cocaine- (black bars) and saline-paired (white bars) sides are shown for the reactivated (C–E; MSO = 10, ASO = 7) and nonreactivated (F,G; MSO = 12, ASO = 11) groups. The timeline of the experimental procedures for the reactivated and nonreactivated memory conditions are shown in A and B, respectively. (C) There was no difference between the groups during the preconditioning test. (D) Both groups acquired a significant preference for the cocaine-paired side as assessed in the memory-reactivation test. (E) The ASO group had a significantly impaired preference for the cocaine-paired side during the post-reactivation test. (F) The nonreactivated groups also did not differ during the preconditioning test. (G) Both groups showed a significant preference for the cocaine-paired side during the post-treatment preference test. Data are presented as mean ± SEM.
The deficit induced by zif268 ASO was reactivation dependent because intra-AcbC zif268 ASO in the absence of reexposure to the conditioning apparatus did not affect the subsequent expression of a previously learned cocaine-CPP (Fig. 2G). Twenty-four hours following intra-AcbC zif268 ASO in the absence of memory reactivation, both the zif268 MSO- and ASO-infused groups spent more time in the cocaine-paired side than in the saline-paired side during the post-conditioning test (Side: F(1,21) = 12.379, P < 0.01; Treatment: F(1,21) = 1.859, P = 0.187; Treatment × Side: F(1,21) = 0.106, P = 0.748). The significant preference observed was not attributable to an initial preference for one of the conditioning chambers as no bias was observed during the preconditioning test (Fig. 2F; Side × Treatment: F(1,21) = 0.157, P = 0.696; Side: F(1,21) = 0.067, P = 0.799; Treatment: F(1,21) = 0.154, P = 0.699).
Experiment 2: zif268 knockdown in the basolateral amygdala impaired the reconsolidation of a cocaine-induced conditioned place preference memory
Histological assessment
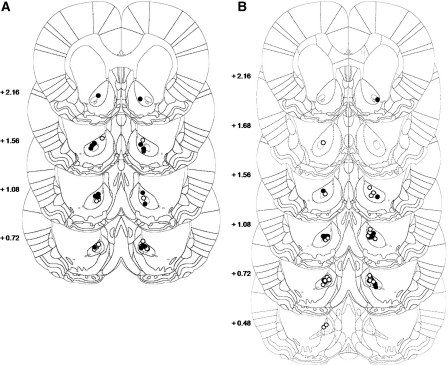
Animals included in the behavioral analysis had cannulae placed bilaterally either within, or adjacent to the boundaries of, the basolateral amygdala (Fig. 3). In the reactivated group 24 of 39 rats acquired a cocaine CPP. After histological assessments, nine animals were excluded from the study due to cannulae misplacement (Fig. 3, left panel). A further animal was excluded as an outlier following the behavioral analysis (data point > 2 SDs from the group mean) (Cardinal and Aitken 2006). In total, 15 animals were included in the statistical analysis, four zif268 MSO-treated rats and 11 zif268 ASO-infused animals.
Figure 3.
Location of the injector tips within the BLA for both reactivated (A) and nonreactivated (B) groups. (Black circles) zif268 ASO-infused rats; (white circles) zif268 MSO-infused rats.
In the nonreactivated group, 19 of 31 rats were included in the statistical analysis while 11 were excluded due either to cannulae misplacement or bilateral damage in the BLA and surrounding areas (Fig. 3, right panel). An additional animal was considered as an outlier. Among the 19 animals included in the analysis, eight were treated with zif268 MSO, while 11 were infused with zif268 ASO.
Behavioral results
Intra-BLA zif268 ASO infusions at memory reactivation disrupted the performance for a previously acquired cocaine-conditioned place preference, in a reactivation-dependent manner
To assess the impact of intra-BLA zif268 knockdown, rats were trained and tested exactly as in the previous experiment (see Fig. 4A,B for an overview of training, memory reactivation, and testing). Prior to behavioral conditioning, all reactivated animals showed no bias toward either side of the CPP apparatus (Fig. 4C; Side: F(1,13) = 1.397, P = 0.258; Treatment: F(1,13) = 0.004, P = 0.953; Side × Treatment: F(1,13) = 0.657, P = 0.432). Twenty-four hours after the last conditioning session, rats received intra-BLA zif268 ASO infusions and were reexposed to the conditioning apparatus in the absence of any drug infusion in order to reactivate the memory. Both zif268 MSO- and ASO-infused animals expressed a preference for the cocaine-paired side as for both the amount of time spent in this chamber was higher than in the saline-paired side (Side: F(1,13) = 17.453, P = 0.001; Treatment: F(1,13) = 0.494, P = 0.495; Treatment × Side: F(1,13) = 0.698, P = 0.419) (Fig. 4D). Furthermore, for both experimental groups, the time spent in the cocaine-paired side was greater during the reactivation session than during the preconditioning session (Test × Side: F(1,13) = 27.479, P < 0.001; Test × Side × Treatment: F(1,13) = 0.190, P = 0.67), indicating that both groups acquired a significant cocaine-CPP. Forty-eight hours after the last conditioning session, animals experienced a second reexposure to the CPP apparatus, but this time without receiving ASO or MSO infusions. During this post-reactivation test, while zif268 MSO-infused rats still showed a preference for the cocaine-paired side, the zif268 ASO-infused rats did not (Fig. 4E), thereby demonstrating that the knockdown of zif268 in the BLA prior to memory reactivation impaired the expression of a previously acquired cocaine-induced CPP (Test × Side × Treatment: F(1,13) = 5.975, P < 0.05).
Figure 4.
Intra-BLA zif268 ASO, prior to memory reactivation, impaired cocaine-induced CPP in a reactivation-dependent manner. Time spent on the cocaine- (black bars) and saline-paired (white bars) sides are shown for the reactivated (C–E; MSO = 4, ASO = 11) and nonreactivated (F,G; MSO = 8, ASO = 11) groups. The timeline of the experimental procedures for the reactivated and nonreactivated memory conditions are shown in A and B, respectively. (C) There was no difference between the groups during the preconditioning test. (D) Both groups acquired a significant preference for the cocaine-paired side as assessed in the memory-reactivation test. (E) The ASO group had a significantly impaired preference for the cocaine-paired side during the post-reactivation test. (F) The nonreactivated groups also did not differ during the preconditioning test. (G) Both groups showed a significant preference for the cocaine-paired side during the post-treatment preference test. Data are presented as mean ± SEM.
The deficit induced by zif268 ASO was reactivation dependent since intra-BLA zif268 ASO infusions in the absence of reexposure to the conditioning apparatus did not affect the expression of the previously acquired cocaine-CPP (Fig. 4G). Twenty-four hours following intra-BLA zif268 ASO infusions in the absence of memory reactivation, both the zif268 MSO- and ASO-infused groups spent more time in the cocaine-paired side than in the saline-paired side during the post-conditioning test (Side: F(1,17) = 12.08, P < 0.01; Treatment: F(1,17) = 0.282, P = 0.068; Treatment × Side: F(1,17) = 0.274, P = 0.608). This deficit was not due to an initial preference for one of the conditioning chambers by one of the experimental groups. Although the ASO-treated rats spent more time in the center compartment than the MSO-treated animals during the preconditioning test (Fig. 4F; Treatment: F(1,17) = 6.052, P = 0.025), all animals spent on average the same amount of time on each conditioning side (Fig. 4F; Side: F(1,17) = 0.368, P = 0.552; Side × Treatment: F(1,17) = 0.001, P = 0.979).
Experiment 3: zif268 knockdown in the nucleus accumbens core did not impair the reconsolidation of the representation underlying the conditioned reinforcing properties of a cocaine-paired CS
Histological assessment
Rats were implanted with chronic indwelling cannulae aimed at the nucleus accumbens core, and animals included in the analysis had injectors placed bilaterally either within, or adjacent to the boundaries of, the nucleus accumbens core (Fig. 5). After histological analysis, 12 of the 50 animals used in this experiment were excluded due to cannulae misplacement. The final numbers of animals per group were 16 for the contingent reactivation experiment (ASO = 9, MSO = 7), and 22 for the noncontingent reactivation experiment (ASO = 11, MSO = 11).
Figure 5.
Location of the injector tips within the AcbC for both contingent (A) and noncontingent (B) reactivated groups. (Black circles) zif268 ASO-infused rats; (white circles) zif268 MSO-infused rats.
Behavioral results
Self-administration training
The rats were first trained to nosepoke for cocaine and a contingently presented CS light (see Fig. 6A for an overview of training, memory reactivation, and testing). In both the prospective designated response contingent and noncontingent memory-reactivation groups, all animals showed similar levels of nosepoke responding (data not shown; contingent: MSO = 47.3 ± 12.2, ASO = 54.5 ± 14.4, Treatment: F(1,14) = 0.209, P = 0.654; noncontingent: MSO = 53.4 ± 8.8, ASO = 50.6 ± 7.6, Treatment: F(1,20) = 0.059, P = 0.811) and thus received similar numbers of CS–cocaine pairings (data not shown; contingent: MSO = 22.6 ± 2.6, ASO = 23.8 ± 2.5, Treatment: F(1,14) = 0.218, P = 0.648; noncontingent: MSO = 23.5 ± 1.6, ASO = 23.3 ± 1.8, Treatment: F(1,20) = 0.008, P = 0.932). Therefore, the level of conditioning of the CS–cocaine association was equivalent across experimental groups.
Figure 6.
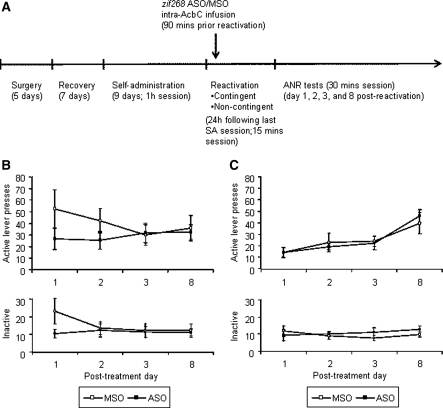
Prereactivation intra-AcbC zif268 ASO had no effect on subsequent acquisition of a new instrumental response with conditioned reinforcement. (A) Timeline of the experimental procedures. (B) The number of active (reinforced by CS presentations) and inactive (nonreinforced) lever presses was compared over four testing sessions after treatment given to rats submitted to a response contingent memory reactivation (n = 7–9 per group). (C) The number of active (reinforced by CS presentations) and inactive (nonreinforced) lever presses was compared over four testing sessions after treatment given to rats submitted to a response noncontingent memory reactivation (n = 11 per group). Data are presented as mean ± SEM.
Memory reactivation
The cue–cocaine association was reactivated either response contingently, or response noncontingently. For response contingent memory reactivation, nosepokes were reinforced by the CS, but not cocaine. During this 15-min session, both MSO and ASO groups performed similar numbers of nosepoke responses (Supplemental Fig. S1A; MSO = 29.1 ± 4.3, ASO = 26.6 ± 3.4; t(14) = −0.482, P = 0.638) and thereby received on average the same number of nonreinforced CS presentations (Supplemental Fig. S1B; MSO = 14.1 ± 1.4, ASO = 14.4 ± 1.6, t(14) = 0.137, P = 0.893).
The rats which received response noncontingent memory reactivation were exposed to 15 passive CS presentations across the 15 min of reactivation session (nosepoke responses had no programmed consequence). In this condition, both groups, MSO and ASO, performed a similar number of nosepoke responses (Fig. S2; MSO = 26.4 ± 5.2, ASO = 23.4 ± 4.2, t(20): −0.449, P = 0.658) and therefore underwent the same level of extinction of the primary nosepoke response that previously delivered cocaine infusions.
Acquisition of a new response with conditioned reinforcement
The integrity of the cue–cocaine association was assessed by measuring the drug cue's ability to reinforce the discriminated acquisition of a new active, rather than inactive, lever press response over four sessions of testing. In both response contingent and noncontingent memory-reactivation conditions, intra-AcbC infusions of zif268 ASO 90 min prior to memory reactivation did not affect the subsequent acquisition of a new instrumental response (Fig. 6B,C). ANOVA did not reveal any significant Treatment × Lever × Session interaction (contingent: F(3,42) = 0.832, P = 0.484; noncontingent: F(2.206,44.118) = 0.600, P = 0.569). Moreover, while there was a main effect of Lever (contingent: F(1,14) = 29.617, P < 0.001; noncontingent: F(1,20) = 27.079, P < 0.001), there were no Treatment × Lever interactions (contingent: F(1,14) = 0.807, P = 0.384; noncontingent: F(1,20) = 0.032, P = 0.859) demonstrating that in both memory-reactivation conditions, MSO- and ASO-treated animals distinguished between the two levers to an equivalent extent during ANR testing. Further pairwise comparisons revealed that all groups showed a preference for the active lever (CS producing) over the inactive lever during the last two sessions of ANR testing indicating that intra-AcbC infusions of zif268 ASO prior to memory reactivation (contingent and noncontingent) did not disrupt the previously acquired conditioned reinforcing properties of the CS (contingent group: ASO day 3, P = 0.014; ASO day 8; P = 0.008; MSO day 3, P = 0.049; MSO day 8, P = 0.01; noncontingent group: ASO day 3, P = 0.015; ASO day 8, P = 0.000; MSO day 3, P = 0.001; MSO day 8, P = 0.001).
Primary instrumental responding during ANR tests
In both, contingent and noncontingent, reactivation conditions the knockdown of zif268 in the AcbC prior to memory reactivation did not affect the subsequent performance of the previously acquired instrumental nosepoke response for drug (Fig. 7A,B) (Treatment: contingent: F(1,14) = 0.336, P = 0.555; noncontingent: F(1,20) = 2.169, P = 0.156; Treatment × Session: contingent: F(1.9,27.2) = 0.965, P = 0.391; noncontingent: F(3,60) = 1.921, P = 0.136).
Figure 7.
The knockdown of zif268 in the AcbC prior to both response contingent (A) and noncontingent (B) memory reactivation did not affect the performance of the previously drug-reinforced instrumental behavior, nosepoke responding (n = 7–9 per group in contingent reactivation; 11 per group in noncontingent reactivation). Data are presented as mean ± SEM.
Discussion
The present study demonstrates that knockdown of zif268 by intra-AcbC or intra-BLA zif268 ASO infusion at reactivation impairs the reconsolidation of a cocaine-CPP memory. The memory impairment was reactivation-dependent because animals infused with zif268 ASO in the absence of memory reactivation continued to express a cocaine-CPP when tested 24 h later. These findings are consistent with previous studies which showed, using different manipulations of intracellular signaling pathways, that both the BLA and AcbC are involved in CPP memory reconsolidation (Miller and Marshall 2005; Milekic et al. 2006). In contrast, the administration of zif268 ASO in the AcbC at memory reactivation did not affect the reconsolidation of the memory representations underlying the conditioned reinforcing properties of a cocaine-paired CS. Following response contingent or noncontingent memory reactivation, zif268 ASO-treated rats readily acquired a new lever pressing response solely reinforced by presentation of the cocaine-paired CS. Given that under identical experimental conditions, zif268 knockdown in the BLA at memory reactivation disrupted subsequent acquisition of a new instrumental response with conditioned reinforcement (Lee et al. 2005), the lack of effect following zif268 knockdown in the AcbC clearly does not reflect a failure to reactivate the drug-associated memory. Instead these data together indicate that the increased expression of zif268 in the AcbC following presentation of a cocaine-associated CS (Thomas et al. 2003) is related to the reconsolidation of the memory trace arising from Pavlovian associations that underlie conditioned place preference, but not conditioned reinforcement.
The observed behavioral impairments are most likely due to the effects of zif268 knockdown in the targeted neural loci. Our previous studies using the same dose and volume of zif268 ASO resulted in a specific decrease in zif268 expression in the brain loci targeted by the infusions (Lee et al. 2004, 2005; Hellemans et al. 2006). The infusion parameters used may have resulted in diffusion from the BLA or AcbC into neighboring areas such as the central nucleus of the amygdala (CeN) or the nucleus accumbens shell (AcbS), respectively. However, there is no evidence to suggest that the CeN or AcbS are functionally engaged in the present tasks. In the procedure we and others have used, CPP is cue-dependent and not dependent on spatial (“place”) or context processing (Everitt et al. 1991; McDonald and White 1993; Ito et al. 2006), hence requiring the integrity of the AcbC and not the AcbS (Ito et al. 2008). Second, the reactivation of a cocaine-CPP has been shown to induce the phosphorylation of the extracellular-regulated kinase (ERK) signaling pathway (a kinase upstream to zif268 activation) specifically in the AcbC (Miller and Marshall 2005). Moreover, the cocaine CS-dependent increase in zif268 expression was observed selectively in the BLA (Thomas et al. 2003), and not the CeN. Finally, we have previously demonstrated that even though intra-BLA zif268 ASO infusions do spread into the CeN, they selectively knock down zif268 protein levels only in the BLA and not in the CeN (Hellemans et al. 2006).
The lack of effect of intra-AcbC zif268 knockdown on the reconsolidation of the conditioned reinforcing properties of a cocaine-paired CS requires further consideration. First, the increased zif268 expression in the AcbC following presentation of a cocaine CS (Thomas et al. 2003) may reflect neuronal activation rather than engagement of a zif268-dependent plasticity process. Indeed, activity-dependent plasticity in the visual cortex is associated with increased zif268 expression, yet occurs in the absence of zif268 in mutant mice (Mataga et al. 2001). Therefore, even if up-regulated, zif268 does not appear to be required for all types of synaptic plasticity in all brain regions (Davis et al. 2003), and this leaves open the possibility that alternative or parallel molecular pathways in the AcbC are engaged in the reconsolidation of associations underlying conditioned reinforcement. Second, zif268 expression at memory reactivation in the AcbC may be implicated in the reconsolidation of some, but not all, of the different representations formed during Pavlovian conditioning (Cardinal et al. 2002) indicating, therefore, that the CPP and conditioned reinforcement procedures depend upon dissociable representations and neural substrates. Thus, through association with the effects of a reinforcer such as cocaine, a CS acquires both general motivational and sensory-specific properties (Killcross and Balleine 1996; Cardinal et al. 2002) that are activated by CS presentation. The associations mediating both types of representation undergo reconsolidation and can be disrupted by the administration of an amnestic agent at memory reactivation (Lee et al. 2005; Blaiss and Janak 2006; Lee and Everitt 2008). Conditioned Pavlovian approach and Pavlovian-to-instrumental transfer (PIT) tasks, which measure the general motivational properties of a CS, depend upon structures within limbic cortical-ventral striatal circuitry that can be dissociated from those that mediate conditioned reinforcement (Cador et al. 1989; Everitt et al. 1999; Hall et al. 2001; Balleine 2005). For example, the BLA, but not the CeN is required for conditioned reinforcement, but not Pavlovian approach or general PIT, whereas the AcbC, but not the AcbS is required for the latter (Everitt et al. 1999; Cardinal et al. 2002; Balleine 2005). Thus, the present results clearly indicate that although zif268 knockdown in the AcbC does not impair reconsolidation of the memory for the sensory-specific conditioned reinforcing properties of a cocaine-paired CS, it may impair the reconsolidation of the representation in memory of its general motivational attributes.
These results strongly suggest that the reconsolidation of Pavlovian memory representations underlying conditioned reinforcement and conditioned place preference behavior depend upon different neural sites within limbic cortical-ventral striatal circuitry. The psychological basis of conditioned place preference is complex; choice of the preferred compartment may reflect any, or a combination of, an automatic Pavlovian approach response (Mead et al. 2005), conditioned reinforcing properties of the CSs in the environment supporting instrumental choice (Everitt et al. 1991), or reward expectancy (Everitt et al. 1991; White and McDonald 1993). These various Pavlovian mechanisms differentially depend upon subcompartments of the amygdala and nucleus accumbens (for review, see Cardinal et al. 2002). Here we show that both the BLA and AcbC are engaged during the reconsolidation of a cocaine-induced CPP memory. One explanation for the involvement of both structures is that CPP is mediated by both conditioned approach and conditioned reinforcement mechanisms. Thus, the conditioned reinforcement element is encoded by the BLA, while the AcbC is involved in the storage of the conditioned approach response. This hypothesis is consistent with Miller and Sweatt's (2006) suggestion that different brain structures within a neural circuit may interact in the storage of different components of the CPP memory trace. However, it is not clear from this hypothesis why, when zif268 is knocked down in only one of these structures, animals are unable subsequently to express a preference for the cocaine-paired compartment. Instead, the reconsolidation of the memory or memories underlying CPP requires concurrent processing in both the BLA and AcbC, indicating that the memory trace at retrieval is distributed across both nodes of this amygdalo-striatal system.
The differential involvement of the BLA and AcbC in conditioned reinforcement and CPP memories may stem from the different representations formed in these structures during Pavlovian conditioning. Thus, while the BLA is critical for the reconsolidation of the specific CS–US association (Lee et al. 2005, 2006; Milton et al. 2008), the AcbC is required for the impact of a CS upon instrumental responding and may therefore be critical for encoding the selection of the behavioral response to the CS (i.e., [CS–US]-R; see Cardinal et al. [2002]). This view resonates with the notion of a limbic–motor interface as a site for the “translation” of motivation or emotion into action (Mogenson et al. 1980). This hypothesis suggests that knockdown of zif268 in the BLA at memory reactivation impairs reconsolidation of the CS–US representation stored in this area. Accordingly, the cocaine-induced CPP and the acquisition of a new response with conditioned reinforcement, which both rely on the CS–US association, will be disrupted in subsequent performance tests. Knockdown of zif268 at memory reactivation in the AcbC may, in contrast, disrupt the reconsolidation of the (CS–US)-R association. Therefore, during CPP memory reactivation, the influence of the CS–US association upon the behavioral approach to the drug-paired compartment is reactivated and rendered susceptible to disruption by intra-AcbC zif268 ASO. In contrast, while CS exposure would be expected to reactivate any (CS–US)-R representation within the AcbC, as observed through the up-regulation of zif268 expression (Thomas et al. 2003), importantly that (CS–US)-R association is not critical for the subsequent acquisition of a new response with conditioned reinforcement. A key difference between the CPP and conditioned reinforcement procedures is that, during self-administration training, the CS–US association is itself associated only with the nosepoke cocaine self-administration response, and not the lever press in the ANR task that assesses the conditioned reinforcing properties of the CS. Hence, the putative AcbC encoded (CS–US)–lever press association is only acquired during the ANR test and, since it is not present during the reactivation session, it cannot be disrupted following intra-AcbC zif268 ASO infusion. The hypothesis of a functional role for the AcbC in encoding selection of the behavioral response to the CS is supported by the demonstration that reversible inactivation of the AcbC impaired the expression of the appropriate behavioral response (Di Ciano et al. 2008).
While the BLA appears to be critical for the reconsolidation of CS–US associations, we show here that the AcbC instead appears to be required for the reconsolidation of the representations linking a CS–US association with its appropriate behavioral response. A clear test of the hypothesized functional role of the AcbC in the reconsolidation of Pavlovian influences upon instrumental responding would be to use a “relapse/reinstatement” task. If valid, this hypothesis would predict that the knockdown of zif268 in the AcbC at cocaine–CS memory reactivation may disrupt the ability of the CS subsequently to reinstate cocaine-seeking behavior, a process known to depend upon glutamatergic afferents to the AcbC (Cornish et al. 1999; Kalivas and McFarland 2003; McFarland et al. 2003). Taken together, the results of the present study demonstrate that the BLA and the AcbC are two structures importantly involved in the reconsolidation of a cocaine–CS memory. The results extend those from other studies investigating the functional role of different brain loci in memory reconsolidation. The originality of the present work lies in the demonstration that, depending on the psychological processes involved, different neural substrates within limbic cortical-ventral striatal circuitry are required for the reconsolidation of a Pavlovian memory.
Materials and Methods
Subjects
The animals used were Lister Hooded rats (N = 184) (Charles River, UK) weighing 280–300 g at the time of surgery. Subjects were housed in pairs from their arrival until surgical procedures were carried out. Thereafter, rats were singly housed and kept in a room maintained at constant temperature (21°C) and humidity levels under a reversed dark/light cycle (12-h-dark, 12-h-light with lights on at 19:00). One week after arrival, animals were placed on a restricted diet of 20 g of Purina lab chow a day, sufficient to maintain body weight and growth. Water was available ad libitum except during behavioral testing. All procedures were conducted in accordance with the United Kingdom 1986 Animals (Scientific Procedures) Act (Project License PPL 80/1767 Personal License PIL 80/9268).
Surgery
For experiments 1 and 2, subjects were implanted bilaterally with cannulae targeting the AcbC or the BLA. For experiment 3, animals were implanted with chronic indwelling bilateral cannulae targeting the AcbC and with an indwelling, intravenous catheter in the right jugular vein. The coordinates for cannula implantation were (mm, from Bregma): AcbC: AP +1.2 ML ± 1.8 DV −4.6 and BLA: AP −2.6 ML ± 4.5 DV −5.6 (from dura). Details of the intravenous catheterization and intracerebral cannulation procedures have been described previously (Di Ciano and Everitt 2004; Lee et al. 2005). A minimum of 7 d was allowed between surgery and behavioral testing.
Infusions
The infusions of zif268 antisense (ASO)/missense (MSO) oligodeoxynucleotides were performed as previously described (Lee et al. 2005) using a syringe pump and 5-µL Hamilton syringes linked to the injectors (28 gauge stainless steel) by Silastic tubing. The injectors for the AcbC infusions extended 2.5 mm below the guide cannulae (AP +1.2 ML ± 1.8 DV –7.2). For the BLA infusions, injectors extended 2 mm below the guide cannulae (AP –2.6 ML ± 4.5 DV –7.6). The oligodeoxynucleotides (ODN) were PAGE-purified phosphorothioate end-capped 18 mer sequences and were resuspended in sterile PBS to a concentration of 2 nmol/µL (1.0 µL/side; 0.125 µL/min): zif268 ASO: 5′-GGTAGTTGTCCATGGTGG-3′, zif268 MSO: 5′-GTGTTCGGTAGGGTGTCA-3′ (see Lee et al. 2005 for full details). The dose and volume of ODN infused has previously been shown to knock down zif268 expression, to be restricted to the amygdala under the employed infusion parameters, and to disrupt the reconsolidation of both aversive and appetitive memories when infused into the amygdala (Lee et al. 2005; Hellemans et al. 2006) or hippocampus (Lee et al. 2004). Rats were habituated to the infusion procedure with prior training infusions of the vehicle solution (0.125 µL/min) twice during the conditioning phase.
Injections
For CPP conditioning, cocaine and saline were administered via intraperitoneal injection on alternate days. No previous habituation sessions were given. A dose of 10 mg/kg (10 mg/mL, 1 mL/kg) cocaine hydrochloride was used for the conditioning sessions. This dose has been previously shown to induce a significant place preference in rats (Sellings et al. 2006). Rats were weighed every 2 d in order to maintain appropriate doses according to body weight. The volume of saline (0.9%) injected was identical to the volume of cocaine for each subject.
Behavioral procedures
Acquisition of a new instrumental response with conditioned reinforcement
Rats were tested in operant chambers (Med Associates) described previously (Di Ciano and Everitt 2004) and were based on previous experiments (Lee et al. 2005). Animals underwent 9 d of cocaine self-administration training under a fixed-ratio schedule of reinforcement. No levers were present during training. Each head-entry (nosepoke) was recorded and resulted in extinction of the houselight and contingent presentation of the CS light above one of the retracted levers (20 sec; counterbalanced left and right) with a single intravenous cocaine infusion (0.25 mg/0.1 mL infused over 5.46 sec) delivered under a fixed-ratio 1 schedule. During this 20-sec “time-out,” further nosepokes were recorded but had no programmed consequences. Following this 20-sec period, the houselight was illuminated again and the CS light was switched off. No “priming” infusions of cocaine were given at any time. The animals were allowed 30 infusions in total per session or a maximum session length of 60 min, whichever occurred first.
Reactivation of the CS-drug memory occurred 24 h after the final training session and consisted of a single 15-min session. Animals were randomly assigned to two experimental groups: ASO and MSO. zif268 ODN infusions were given 90 min prior to the start of the reactivation session, since this has been shown in our earlier work to result in significant attenuation of zif268 up-regulation (Lee et al. 2005; Hellemans et al. 2006). Two conditions of memory reactivation were used: (1) response-contingent and (2) response noncontingent. In the response-contingent reactivation condition, the parameters of the reactivation session were identical to the training phase except that the syringe pump contained heparinized saline instead of cocaine. Hence, animals received a presentation of the 20-sec light stimulus associated with an infusion of heparinized saline following each nosepoke. As in the training session, the houselight was switched off during the 20-sec CS presentation and switched back on at the end of the time out period. For the response noncontingent reactivation, CS light presentations were delivered independently of the animal's behavior. Each nosepoke still resulted in a single saline infusion during which the houselight was switched off. A total of 15 CS light presentations were made during the 15-min reactivation session (CS duration = 20 sec; interstimulus interval = 40 sec).
The acquisition of a new instrumental response (ANR) test occurred 24 h after the memory-reactivation session. Animals were placed back in the same operant chambers used for training and reactivation with the exception that two levers were now extended into the chamber. A response on the active lever resulted in a 1-sec presentation of the previously drug-associated CS under a variable ratio (VR) 1–3 schedule during which the houselight was extinguished. Depression of the inactive lever, located on the same side as the drug-paired cue to avoid confounding effects with Pavlovian approach (Di Ciano and Everitt 2004), had no programmed consequences. Nosepoke responses were also recorded but had no programmed consequences. Although the animals were connected to the intravenous line, the pumps were never activated during the 30-min session. Test sessions were conducted on days 1, 2, 3, and 8 following reactivation.
Cocaine-conditioned place preference
All behavioral training and testing took place in a Y-maze apparatus (Med Associates) described elsewhere (Ito et al. 2006). The CPP apparatus comprised two main chambers which were distinguished by specific physical features and separated by a smaller central compartment by manually operated guillotine doors.
The CPP protocol consisted of three different phases: preconditioning (day 1), conditioning (days 2–9), and post-conditioning tests (reactivation on day 10, post-reactivation long-term memory on day 11). During the preconditioning test, baseline preferences were assessed by placing rats in the central compartment of the place preference apparatus and allowing free access to all compartments for 15 min. The time spent in each compartment was recorded. An animal was considered to have acquired CPP if the time spent in the cocaine-paired side at post-conditioning/reactivation test minus the time spent in the cocaine-unpaired side at post-conditioning/reactivation test was greater than zero and the difference between post-conditioning (paired-unpaired) minus preconditioning (paired-unpaired) was also greater than zero.
During the subsequent 8 d, the conditioning phase was conducted using a procedure in which the side and the order of injections were counterbalanced across rats. Hence, in each experimental group (ASO and MSO), half of the animals received cocaine injections in their less preferred compartment while the other half received cocaine injections in their preferred compartment, and, in each subgroup, half were given cocaine injections (10 mg/kg) prior to placement in the drug-paired compartment on the first day, and half received saline (same volume as the cocaine injection) prior to placement in the unpaired compartment. This counterbalanced procedure resulted in the mean time of each experimental group being approximately equal both in the paired and unpaired compartments during preconditioning. At the beginning of the conditioning session, animals were placed in the center compartment. The animal only had access to one of the main compartments, to which it was confined for 15 min following entry.
Twenty-four hours after the last conditioning session, a post-conditioning memory-reactivation test was performed. Infusions of zif268 ODN were given 90 min prior to the start of the post-conditioning test. Between infusions and the memory-reactivation test, animals were returned to their home cage. The post-conditioning test was similar to the preconditioning phase as animals were placed in the center compartment and were given free access to all compartments for 15 min. The time spent on each side was recorded. Because one of the aims of this study was to determine whether the reactivation of a CPP memory causes this memory again to become labile and susceptible to disruption by amnestic agents, a nonreactivated control group was included in the study for each experimental group (AcbC and BLA cannulated rats). For the nonreactivated group, the CPP conditioning procedure was similar to that described above. However, the nonreactivated animals were never reexposed to the conditioning apparatus on the day of the infusions. These rats received the infusion treatment in a control environment which was never experienced during the training period and were returned to their home cage upon completion of the treatment.
Twenty-four hours after the memory-reactivation test, another post-conditioning test (post-reactivation long-term memory, PR-LTM) was performed and the time spent in each compartment was recorded.
Histological assessment of cannula placements
At the end of behavioral testing, animals were perfused and their brains sectioned coronally at 60 µm and stained with Cresyl Violet. The cannulae placements were analyzed using light microscopy and recorded on standardized sections of the rat brain (Paxinos and Watson 2004). Animals were only included in the statistical analysis if the guide cannula tracks were just dorsal to the brain area targeted and there was evidence of the tip of the injector being placed within the brain area studied. Any animal with bilateral damage or extensive lesions was excluded from the analysis.
Statistical analysis
All data are presented as means ± SEM and were analyzed using SPSS 11.5 for Windows.
Three-way repeated-measures ANOVA were performed on the data with between-subject factor Treatment (two levels, ASO versus MSO), and within-subject factors Session, Levers, Test, and Side as appropriate. Main effects and significant interactions were followed by pairwise comparisons (multiple t-test) with Bonferroni adjustments. Violations of the sphericity assumption underwent the Huynh-Feldt correction. A significance level of P < 0.05 was selected for all analyses.
Acknowledgments
This work was supported by United Kingdom Medical Research Council (MRC) Grant 9536855 and was conducted within the MRC/Wellcome Trust Behavioural and Clinical Neuroscience Institute. F.R.M.T. was financially supported by an Isaac Newton and Cambridge European scholarships, and the Oon Khye Beng Ch'hia Tsio studentship from Downing College, Cambridge.
Footnotes
[Supplemental material is available online at http://www.learnmem.org.]
References
- Balleine BW 2005. Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiol Behav 86: 717–730 [DOI] [PubMed] [Google Scholar]
- Blaiss CA, Janak PH 2006. Post-training and post-reactivation administration of amphetamine enhances morphine conditioned place preference. Behav Brain Res 171: 329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Orr SP, Tremblay J, Robertson K, Nader K, Pitman RK 2008. Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatr Res 42: 503–506 [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ 1989. Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience 30: 77–86 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Aitken MRF 2006. ANOVA for the behavioural sciences researcher. Lawrence Erlbaum Associates, Mahwah, NJ [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ 2002. Emotion and motivation: The role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26: 321–352 [DOI] [PubMed] [Google Scholar]
- Childress AR, McLellan AT, Ehrman R, O'Brien CP 1988. Classically conditioned responses in opioid and cocaine dependence: A role in relapse? NIDA Res Monogr 84: 25–43 [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST 2002. Applying extinction research and theory to cue-exposure addiction treatments. Addiction 97: 155–167 [DOI] [PubMed] [Google Scholar]
- Cornish JL, Duffy P, Kalivas PW 1999. A role for nucleus accumbens glutamate transmission in the relapse to cocaine-seeking behavior. Neuroscience 93: 1359–1367 [DOI] [PubMed] [Google Scholar]
- Davis S, Bozon B, Laroche S 2003. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning?. Behav Brain Res 142: 17–30 [DOI] [PubMed] [Google Scholar]
- de Wit H, Stewart J 1981. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75: 134–143 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ 2004. Conditioned reinforcing properties of stimuli paired with self-administered cocaine, heroin or sucrose: Implications for the persistence of addictive behaviour. Neuropharmacology 47: 202–213 [DOI] [PubMed] [Google Scholar]
- Di Ciano P, Robbins TW, Everitt BJ 2008. Differential effects of nucleus accumbens core, shell, or dorsal striatal inactivations on the persistence, reacquisition, or reinstatement of responding for a drug-paired conditioned reinforcer. Neuropsychopharmacology 33: 1413–1425 [DOI] [PubMed] [Google Scholar]
- Dudai Y 2006. Reconsolidation: The advantage of being refocused. Curr Opin Neurol Neurobiol 16: 1–5 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O'Brien A, Robbins TW 1991. The basolateral amygdala-ventral striatal system and conditioned place preference: Further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience 42: 1–18 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Parkinson JA, Olmstead MC, Arroyo M, Robledo P, Robbins TW 1999. Associative processes in addiction and reward: The role of amygdala-ventral striatal subsystems. Ann N Y Acad Sci 877: 412–438 [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Hall J, Parkinson JA, Robbins TW 2000. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In The amygdala: A functional analysis (ed. Aggleton JP), pp. 353–390 Oxford University Press, New York [Google Scholar]
- Fuchs RA, Tran-Nguyen LTL, Specio SE, Groff RS, Neisewander JL 1998. Predictive validity of the extinction/reinstatement model of drug craving. Psychopharmacology 135: 151–160 [DOI] [PubMed] [Google Scholar]
- Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ 2001. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci 13: 1984–1992 [DOI] [PubMed] [Google Scholar]
- Hellemans KG, Everitt BJ, Lee JL 2006. Disrupting reconsolidation of conditioned withdrawal memories in the basolateral amygdala reduces suppression of heroin seeking in rats. J Neurosci 26: 12694–12699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupbach A, Gomez R, Hardt O, Nadel L 2007. Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learn Mem 14: 47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, McNaughton BL, Everitt BJ 2006. Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci 23: 3071–3080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ 2008. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci 28: 6950–6959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, McFarland K 2003. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168: 44–56 [DOI] [PubMed] [Google Scholar]
- Killcross S, Balleine B 1996. Role of primary motivation in stimulus preexposure effects. J Exp Psychol Anim Behav Process 22: 32–42 [DOI] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B 2009. Beyond extinction: Erasing human fear responses and preventing the return of fear. Nat Neurosci 12: 256–258 [DOI] [PubMed] [Google Scholar]
- Lee JL 2009. Reconsolidation: Maintaining memory relevance. Trends Neurosci 32: 413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ 2008. Reactivation-dependent amnesia in Pavlovian approach and instrumental transfer. Learn Mem 15: 597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JL, Everitt BJ, Thomas KL 2004. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 304: 839–843 [DOI] [PubMed] [Google Scholar]
- Lee JL, Di Ciano P, Thomas KL, Everitt BJ 2005. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47: 795–801 [DOI] [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ 2006. Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26: 5881–5887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mataga N, Fujishima S, Condie BG, Hensch TK 2001. Experience-dependent plasticity of mouse visual cortex in the absence of the neuronal activity-dependent marker egr1/zif268. J Neurosci 21: 9724–9732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald RJ, White NM 1993. A triple dissociation of memory systems: Hippocampus, amygdala, and dorsal striatum. Behav Neurosci 107: 3–22 [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW 2003. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci 23: 3531–3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AN, Brown G, Le Merrer J, Stephens DN 2005. Effects of deletion of gria1 or gria2 genes encoding glutamatergic AMPA-receptor subunits on place preference conditioning in mice. Psychopharmacology 179: 164–171 [DOI] [PubMed] [Google Scholar]
- Meil WM, See RE 1996. Conditioned cued recovery of responding following prolonged withdrawal from self-administered cocaine in rats: An animal model of relapse. Behav Pharmacol 7: 754–763 [PubMed] [Google Scholar]
- Milekic MH, Brown SD, Castellini C, Alberini CM 2006. Persistent disruption of an established morphine conditioned place preference. J Neurosci 26: 3010–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF 2005. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47: 873–884 [DOI] [PubMed] [Google Scholar]
- Miller CA, Sweatt JD 2006. Amnesia or retrieval deficit? Implications of a molecular approach to the question of reconsolidation. Learn Mem 13: 498–505 [DOI] [PubMed] [Google Scholar]
- Milton AL, Lee JL, Everitt BJ 2008. Reconsolidation of appetitive memories both natural and drug reinforcement is dependent on beta-adrenergic receptors. Learn Mem 15: 88–92 [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY 1980. From motivation to action: Functional interface between the limbic system and the motor system. Prog Neurobiol 14: 69–97 [DOI] [PubMed] [Google Scholar]
- Nader K 2003. Memory traces unbound. Trends Neurosci 26: 65–72 [DOI] [PubMed] [Google Scholar]
- O'Brien C, Childress AR, Ehrman R, Robbins S, McLellan AT 1992. Conditioning mechanisms in drug dependence. Clin Neuropharmacol 15: 66A–67A [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 2004. The rat brain in stereotaxic coordinates. Elsevier Academic Press, San Diego, CA [Google Scholar]
- Rossato JI, Bevilaqua LR, Myskiw JC, Medina JH, Izquierdo I, Cammarota M 2007. On the role of the hipppocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learn Mem 14: 36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellings LHL, McQuade LE, Clarke PBS 2006. Evidence for multiple sites within rat ventral striatum mediating cocaine-conditioned place preference and locomotor activation. J Pharmacol Exp Ther 317: 1178–1187 [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Riceberg JS, New AS, Alberini CM 2009. Preclinical assessment for selectively disrupting a traumatic memory via postretrieval inhibition of glucocorticoid receptors. Biol Psychiatry 65: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KL, Arroyo M, Everitt BJ 2003. Induction of the learning and platicity-associated gene zif268 following exposure to a discrete cocaine-associated stimulus. Eur J Neurosci 17: 1964–1972 [DOI] [PubMed] [Google Scholar]
- Weiss F 2000. Control of cocaine-seeking behavior by drug-associated stimuli in rats: Effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Neurobiology 97: 4321–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White NM, McDonald RJ 1993. Acquisition of a spatial conditioned place preference is impaired by amygdala lesions and improved by fornix lesions. Behav Brain Res 55: 269–281 [DOI] [PubMed] [Google Scholar]