Abstract
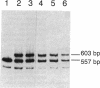
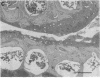
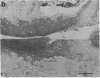
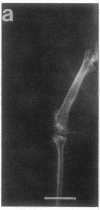
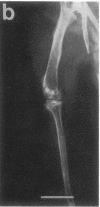
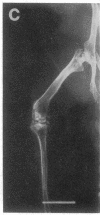
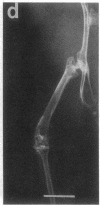
Studies were carried out on a line of transgenic mice that expressed an internally deleted COL2A1 gene and developed a phenotype resembling human chondrodysplasias (Vandenberg et al. 1991. Proc. Natl. Acad. Sci. USA. 88:7640-7644. Marked differences in phenotype were observed with propagation of the mutated gene in an inbred strain of mice in that approximately 15% of the transgenic mice had a cleft palate and a lethal phenotype, whereas the remaining mice were difficult to distinguish from normal littermates. 1-d- and 3-mo-old transgenic mice that were viable showed microscopic signs of chondrodysplasia with reduced amounts of collagen fibrils in the cartilage matrix, dilatation of the rough surfaced endoplasmic reticulum in the chondrocytes, and decrease of optical path difference in polarized light microscopy. The transgenic mice also showed signs of disturbed growth as evidenced by lower body weight, lower length and weight of the femur, decreased bone collagen, decreased bone mineral, and decreased resistance of bone to breakage. Comparisons of mice ranging in age from 1 d to 15 mo demonstrated that there was decreasing evidence of a chondrodysplasia as the mice grew older. Instead, the most striking feature in the 15-mo-old mice were degenerative changes of articular cartilage similar to osteoarthritis.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad N. N., Ala-Kokko L., Knowlton R. G., Jimenez S. A., Weaver E. J., Maguire J. I., Tasman W., Prockop D. J. Stop codon in the procollagen II gene (COL2A1) in a family with the Stickler syndrome (arthro-ophthalmopathy). Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6624–6627. doi: 10.1073/pnas.88.15.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N. N., McDonald-McGinn D. M., Zackai E. H., Knowlton R. G., LaRossa D., DiMascio J., Prockop D. J. A second mutation in the type II procollagen gene (COL2AI) causing stickler syndrome (arthro-ophthalmopathy) is also a premature termination codon. Am J Hum Genet. 1993 Jan;52(1):39–45. [PMC free article] [PubMed] [Google Scholar]
- Ala-Kokko L., Baldwin C. T., Moskowitz R. W., Prockop D. J. Single base mutation in the type II procollagen gene (COL2A1) as a cause of primary osteoarthritis associated with a mild chondrodysplasia. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6565–6568. doi: 10.1073/pnas.87.17.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadio J., Saunders T. L., Tsai E., Goldstein S. A., Morris-Wiman J., Brinkley L., Dolan D. F., Altschuler R. A., Hawkins J. E., Jr, Bateman J. F. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. M., Nichols B. E., Weingeist T. A., Sheffield V. C., Kimura A. E., Stone E. M. Procollagen II gene mutation in Stickler syndrome. Arch Ophthalmol. 1992 Nov;110(11):1589–1593. doi: 10.1001/archopht.1992.01080230089027. [DOI] [PubMed] [Google Scholar]
- Byers P. H., Steiner R. D. Osteogenesis imperfecta. Annu Rev Med. 1992;43:269–282. doi: 10.1146/annurev.me.43.020192.001413. [DOI] [PubMed] [Google Scholar]
- Cheah K. S., Lau E. T., Au P. K., Tam P. P. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991 Apr;111(4):945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Liberfarb R. M., Hirose T., Maumenee I. H., Streeten E. A., Meyers D. A., Pyeritz R. E. The Stickler syndrome: evidence for close linkage to the structural gene for type II collagen. Genomics. 1987 Dec;1(4):293–296. doi: 10.1016/0888-7543(87)90027-9. [DOI] [PubMed] [Google Scholar]
- Fryer A. E., Upadhyaya M., Littler M., Bacon P., Watkins D., Tsipouras P., Harper P. S. Exclusion of COL2A1 as a candidate gene in a family with Wagner-Stickler syndrome. J Med Genet. 1990 Feb;27(2):91–93. doi: 10.1136/jmg.27.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo S., Vuorio E., Metsaranta M., Rosati R., Toman D., Vaughan J., Lozano G., Mayne R., Ellard J., Horton W. Reduced amounts of cartilage collagen fibrils and growth plate anomalies in transgenic mice harboring a glycine-to-cysteine mutation in the mouse type II procollagen alpha 1-chain gene. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9648–9652. doi: 10.1073/pnas.88.21.9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khillan J. S., Olsen A. S., Kontusaari S., Sokolov B., Prockop D. J. Transgenic mice that express a mini-gene version of the human gene for type I procollagen (COL1A1) develop a phenotype resembling a lethal form of osteogenesis imperfecta. J Biol Chem. 1991 Dec 5;266(34):23373–23379. [PubMed] [Google Scholar]
- Kiebzak G. M., Smith R., Gundberg C. C., Howe J. C., Sacktor B. Bone status of senescent male rats: chemical, morphometric, and mechanical analysis. J Bone Miner Res. 1988 Feb;3(1):37–45. doi: 10.1002/jbmr.5650030107. [DOI] [PubMed] [Google Scholar]
- Knowlton R. G., Katzenstein P. L., Moskowitz R. W., Weaver E. J., Malemud C. J., Pathria M. N., Jimenez S. A., Prockop D. J. Genetic linkage of a polymorphism in the type II procollagen gene (COL2A1) to primary osteoarthritis associated with mild chondrodysplasia. N Engl J Med. 1990 Feb 22;322(8):526–530. doi: 10.1056/NEJM199002223220807. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Genetic causes of aortic aneurysms. Unlearning at least part of what the textbooks say. J Clin Invest. 1991 Nov;88(5):1441–1444. doi: 10.1172/JCI115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Vissing H., Ramirez F., Rogers D., Rimoin D. Identification of the molecular defect in a family with spondyloepiphyseal dysplasia. Science. 1989 May 26;244(4907):978–980. doi: 10.1126/science.2543071. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Decker G., Rintala M., de Crombrugghe B., Vuorio E. Chondrodysplasia in transgenic mice harboring a 15-amino acid deletion in the triple helical domain of pro alpha 1(II) collagen chain. J Cell Biol. 1992 Jul;118(1):203–212. doi: 10.1083/jcb.118.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palotie A., Väisänen P., Ott J., Ryhänen L., Elima K., Vikkula M., Cheah K., Vuorio E., Peltonen L. Predisposition to familial osteoarthrosis linked to type II collagen gene. Lancet. 1989 Apr 29;1(8644):924–927. doi: 10.1016/s0140-6736(89)92507-5. [DOI] [PubMed] [Google Scholar]
- Pereira R., Khillan J. S., Helminen H. J., Hume E. L., Prockop D. J. Transgenic mice expressing a partially deleted gene for type I procollagen (COL1A1). A breeding line with a phenotype of spontaneous fractures and decreased bone collagen and mineral. J Clin Invest. 1993 Feb;91(2):709–716. doi: 10.1172/JCI116252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Prockop D. J. Mutations that alter the primary structure of type I collagen. The perils of a system for generating large structures by the principle of nucleated growth. J Biol Chem. 1990 Sep 15;265(26):15349–15352. [PubMed] [Google Scholar]
- Prockop D. J. Seminars in medicine of the Beth Israel Hospital, Boston. Mutations in collagen genes as a cause of connective-tissue diseases. N Engl J Med. 1992 Feb 20;326(8):540–546. doi: 10.1056/NEJM199202203260807. [DOI] [PubMed] [Google Scholar]
- Sangiorgi F. O., Benson-Chanda V., de Wet W. J., Sobel M. E., Tsipouras P., Ramirez F. Isolation and partial characterization of the entire human pro alpha 1(II) collagen gene. Nucleic Acids Res. 1985 Apr 11;13(7):2207–2225. doi: 10.1093/nar/13.7.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey A., Bateman J., Choi T., Mascara T., Cole W., Jaenisch R. Perinatal lethal osteogenesis imperfecta in transgenic mice bearing an engineered mutant pro-alpha 1(I) collagen gene. Nature. 1988 Mar 10;332(6160):131–136. doi: 10.1038/332131a0. [DOI] [PubMed] [Google Scholar]
- Thorogood P., Bee J., von der Mark K. Transient expression of collagen type II at epitheliomesenchymal interfaces during morphogenesis of the cartilaginous neurocranium. Dev Biol. 1986 Aug;116(2):497–509. doi: 10.1016/0012-1606(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Tiller G. E., Rimoin D. L., Murray L. W., Cohn D. H. Tandem duplication within a type II collagen gene (COL2A1) exon in an individual with spondyloepiphyseal dysplasia. Proc Natl Acad Sci U S A. 1990 May;87(10):3889–3893. doi: 10.1073/pnas.87.10.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg P., Khillan J. S., Prockop D. J., Helminen H., Kontusaari S., Ala-Kokko L. Expression of a partially deleted gene of human type II procollagen (COL2A1) in transgenic mice produces a chondrodysplasia. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7640–7644. doi: 10.1073/pnas.88.17.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961 May;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- Wilhelmi G., Faust R. Suitability of the C57 black mouse as an experimental animal for the study of skeletal changes due to ageing, with special reference to osteo-arthrosis and its response to tribenoside. Pharmacology. 1976;14(4):289–296. doi: 10.1159/000136607. [DOI] [PubMed] [Google Scholar]
- Wood A., Ashhurst D. E., Corbett A., Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991 Apr;111(4):955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]