Abstract
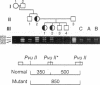
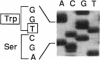
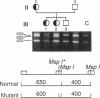
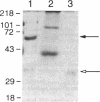
We analyzed the DNA sequence of the cDNA encoding the NH2 terminal region of beta spectrin from members of a kindred with autosomal dominant hereditary spherocytosis associated with defective protein 4.1 binding. We found a point mutation at codon 202 within the 272 amino acid NH2-terminal region of beta spectrin. TGG was changed to CGG, resulting in the replacement of tryptophan by arginine. The base change eliminates a normally occurring PvuII restriction site and creates a new MspI site. This finding enabled rapid detection or exclusion of the mutation at the DNA level among the family members, including one member for whom this analysis was performed prenatally. The mutation was found only in the affected family members and occurred as a de novo mutation in the proband. It has not been found in 20 other kindreds. The recombinant peptide derived from the normal cDNA retains the capacity to sediment with protein 4.1 and F-actin. The mutant peptide spontaneously degrades. This variant represents both the first point mutation and the first beta spectrin mutation demonstrated in autosomal dominant hereditary spherocytosis. Furthermore, the mutation is located within a conserved sequence among spectrinlike proteins and may define an amino acid critical for protein 4.1 binding activity.
Full text
PDF


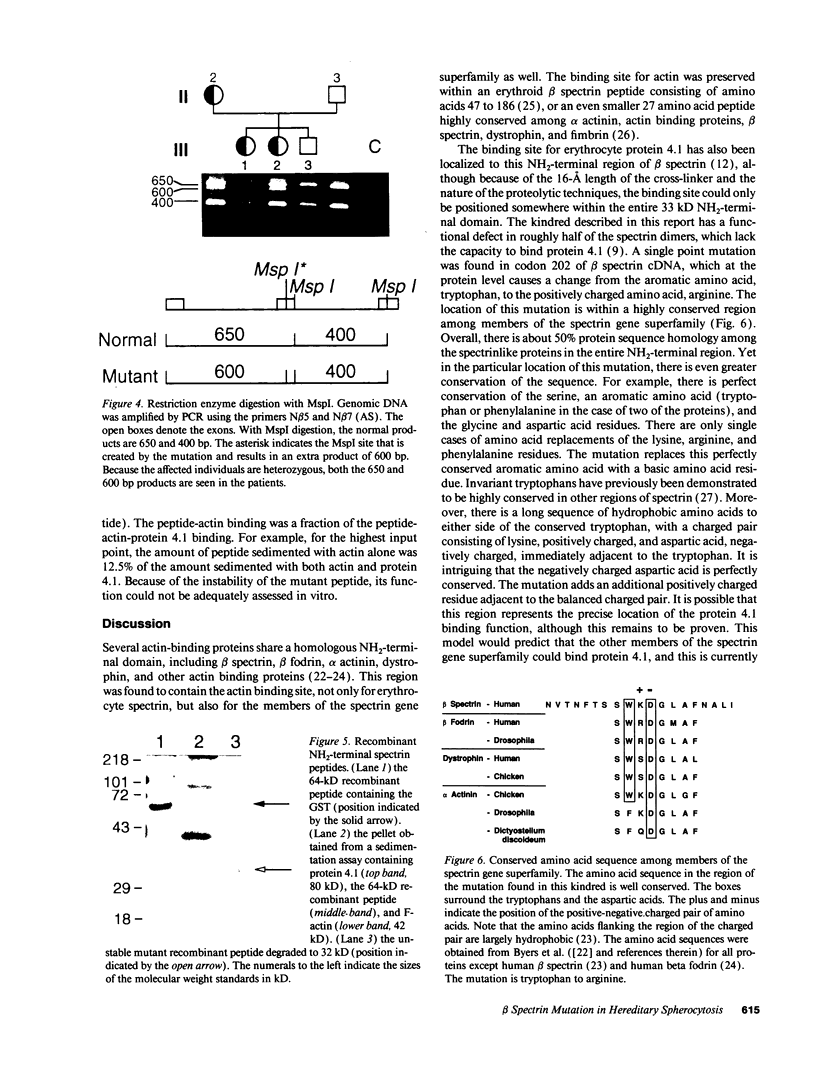

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Asimos A., Casella J. F., McMillan C. Inheritance pattern and clinical response to splenectomy as a reflection of erythrocyte spectrin deficiency in hereditary spherocytosis. N Engl J Med. 1986 Dec 18;315(25):1579–1583. doi: 10.1056/NEJM198612183152504. [DOI] [PubMed] [Google Scholar]
- Agre P., Casella J. F., Zinkham W. H., McMillan C., Bennett V. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. 1985 Mar 28-Apr 3Nature. 314(6009):380–383. doi: 10.1038/314380a0. [DOI] [PubMed] [Google Scholar]
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Becker P. S., Morrow J. S., Lux S. E. Abnormal oxidant sensitivity and beta-chain structure of spectrin in hereditary spherocytosis associated with defective spectrin-protein 4.1 binding. J Clin Invest. 1987 Aug;80(2):557–565. doi: 10.1172/JCI113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker P. S., Schwartz M. A., Morrow J. S., Lux S. E. Radiolabel-transfer cross-linking demonstrates that protein 4.1 binds to the N-terminal region of beta spectrin and to actin in binary interactions. Eur J Biochem. 1990 Nov 13;193(3):827–836. doi: 10.1111/j.1432-1033.1990.tb19406.x. [DOI] [PubMed] [Google Scholar]
- Bresnick A. R., Janmey P. A., Condeelis J. Evidence that a 27-residue sequence is the actin-binding site of ABP-120. J Biol Chem. 1991 Jul 15;266(20):12989–12993. [PubMed] [Google Scholar]
- Byers T. J., Husain-Chishti A., Dubreuil R. R., Branton D., Goldstein L. S. Sequence similarity of the amino-terminal domain of Drosophila beta spectrin to alpha actinin and dystrophin. J Cell Biol. 1989 Oct;109(4 Pt 1):1633–1641. doi: 10.1083/jcb.109.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget B. G., Chang J. G., Coupal E., Stanislovitis P., Costa F. F., Winkelmann J. C., Agre P. C., Marchesi V. T., Watkins P. C. Molecular genetics of the human beta-spectrin gene. Trans Assoc Am Physicians. 1988;101:149–154. [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Goossens M., Kan Y. Y. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 1981;76:805–817. doi: 10.1016/0076-6879(81)76159-7. [DOI] [PubMed] [Google Scholar]
- Hanspal M., Hanspal J. S., Kalraiya R., Liu S. C., Sahr K. E., Howard D., Palek J. Asynchronous synthesis of membrane skeletal proteins during terminal maturation of murine erythroblasts. Blood. 1992 Jul 15;80(2):530–539. [PubMed] [Google Scholar]
- Hanspal M., Palek J. Synthesis and assembly of membrane skeletal proteins in mammalian red cell precursors. J Cell Biol. 1987 Sep;105(3):1417–1424. doi: 10.1083/jcb.105.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanspal M., Yoon S. H., Yu H., Hanspal J. S., Lambert S., Palek J., Prchal J. T. Molecular basis of spectrin and ankyrin deficiencies in severe hereditary spherocytosis: evidence implicating a primary defect of ankyrin. Blood. 1991 Jan 1;77(1):165–173. [PubMed] [Google Scholar]
- Hu R. J., Watanabe M., Bennett V. Characterization of human brain cDNA encoding the general isoform of beta-spectrin. J Biol Chem. 1992 Sep 15;267(26):18715–18722. [PubMed] [Google Scholar]
- Karinch A. M., Zimmer W. E., Goodman S. R. The identification and sequence of the actin-binding domain of human red blood cell beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11833–11840. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagai K., Thøgersen H. C. Generation of beta-globin by sequence-specific proteolysis of a hybrid protein produced in Escherichia coli. 1984 Jun 28-Jul 4Nature. 309(5971):810–812. doi: 10.1038/309810a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Tse W. T., Gallagher P. G., Pothier B., Costa F. F., Scarpa A., Delaunay J., Forget B. G. An insertional frameshift mutation of the beta-spectrin gene associated with elliptocytosis in spectrin nice (beta 220/216). Blood. 1991 Jul 15;78(2):517–523. [PubMed] [Google Scholar]
- Tse W. T., Lecomte M. C., Costa F. F., Garbarz M., Feo C., Boivin P., Dhermy D., Forget B. G. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J Clin Invest. 1990 Sep;86(3):909–916. doi: 10.1172/JCI114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]