Abstract
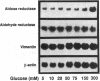
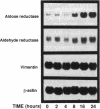
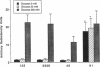
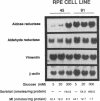
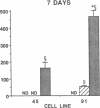
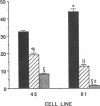
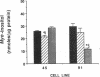
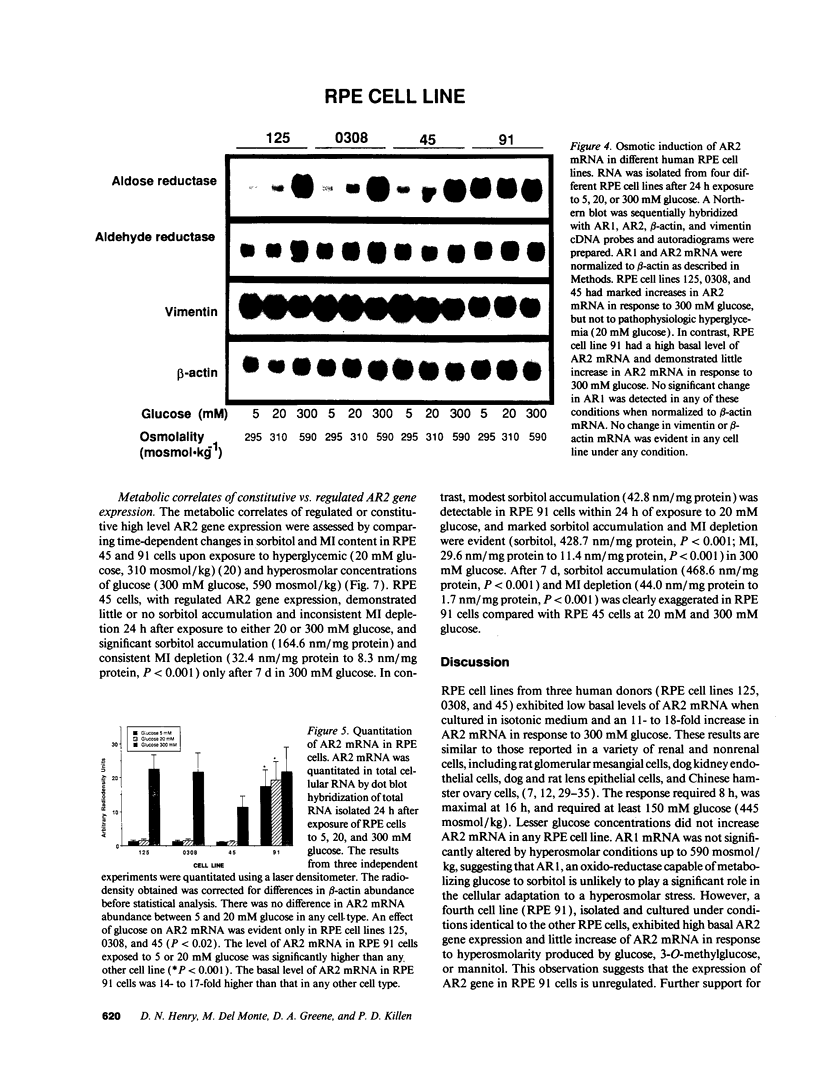
Aldose reductase (AR2), a putative "hypertonicity stress protein" whose gene is induced by hyperosmolarity, protects renal medullary cells against the interstitial hyperosmolarity of antidiuresis by catalyzing the synthesis of millimolar concentrations of intracellular sorbitol from glucose. Although AR2 gene induction has been noted in a variety of renal and nonrenal cells subjected to hypertonic stress in vitro, the functional significance of AR2 gene expression in cells not normally exposed to a hyperosmolar milieu is not fully understood. The physiological impact of basal AR2 expression in such cells may be limited to hyperglycemic states in which AR2 promotes pathological polyol accumulation, a mechanism invoked in the pathogenesis of diabetic complications. Since AR2 overexpression in the retinal pigment epithelium has been associated with diabetic retinopathy, the regulation of AR2 gene expression and associated changes in sorbitol and myo-inositol were studied in human retinal pigment epithelial cells in culture. The relative abundance of aldehyde reductase (AR1) and AR2 mRNA was quantitated by filter hybridization of RNA from several human retinal pigment epithelial cell lines exposed to hyperglycemic and hyperosmolar conditions in vitro. AR2 but not AR1 mRNA was significantly increased some 11- to 18-fold by hyperosmolarity in several retinal pigment epithelial cell lines. A single cell line with a 15-fold higher basal level of AR2 mRNA than other cell lines tested demonstrated no significant increase in AR2 mRNA in response to hypertonic stress. This cell line demonstrated accelerated and exaggerated production of sorbitol and depletion of myo-inositol upon exposure to 20 mM glucose. Therefore, abnormal AR2 expression may enhance the sensitivity of cells to the biochemical consequences of hyperglycemia potentiating the development of diabetic complications.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagnasco S. M., Murphy H. R., Bedford J. J., Burg M. B. Osmoregulation by slow changes in aldose reductase and rapid changes in sorbitol flux. Am J Physiol. 1988 Jun;254(6 Pt 1):C788–C792. doi: 10.1152/ajpcell.1988.254.6.C788. [DOI] [PubMed] [Google Scholar]
- Bekhor I., Shi S., Carper D., Nishimura C., Unakar N. J. Relative abundance of aldose reductase mRNA in rat lens undergoing development of osmotic cataracts. Curr Eye Res. 1989 Dec;8(12):1299–1308. doi: 10.3109/02713688909013910. [DOI] [PubMed] [Google Scholar]
- Beyer T. A., Hutson N. J. Introduction: evidence for the role of the polyol pathway in the pathophysiology of diabetic complications. Metabolism. 1986 Apr;35(4 Suppl 1):1–3. doi: 10.1016/0026-0495(86)90178-2. [DOI] [PubMed] [Google Scholar]
- Bohren K. M., Bullock B., Wermuth B., Gabbay K. H. The aldo-keto reductase superfamily. cDNAs and deduced amino acid sequences of human aldehyde and aldose reductases. J Biol Chem. 1989 Jun 5;264(16):9547–9551. [PubMed] [Google Scholar]
- Bondy C., Cowley B. D., Jr, Lightman S. L., Kador P. F. Feedback inhibition of aldose reductase gene expression in rat renal medulla. Galactitol accumulation reduces enzyme mRNA levels and depletes cellular inositol content. J Clin Invest. 1990 Oct;86(4):1103–1108. doi: 10.1172/JCI114814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Carper D., Kaneko M., Stark H., Hohman T. Increase in aldose reductase mRNA in dog lens epithelial cells under hypertonic conditions. Exp Eye Res. 1990 Jun;50(6):743–749. doi: 10.1016/0014-4835(90)90124-d. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cowley B. D., Jr, Ferraris J. D., Carper D., Burg M. B. In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am J Physiol. 1990 Jan;258(1 Pt 2):F154–F161. doi: 10.1152/ajprenal.1990.258.1.F154. [DOI] [PubMed] [Google Scholar]
- Del Monte M. A., Maumenee I. H. New technique for in vitro culture of human retinal pigment epithelium. Birth Defects Orig Artic Ser. 1980;16(2):327–338. [PubMed] [Google Scholar]
- Del Monte M. A., Rabbani R., Diaz T. C., Lattimer S. A., Nakamura J., Brennan M. C., Greene D. A. Sorbitol, myo-inositol, and rod outer segment phagocytosis in cultured hRPE cells exposed to glucose. In vitro model of myo-inositol depletion hypothesis of diabetic complications. Diabetes. 1991 Oct;40(10):1335–1345. [PubMed] [Google Scholar]
- Dixit V. M., Marks R. M., Sarma V., Prochownik E. V. The antimitogenic action of tumor necrosis factor is associated with increased AP-1/c-jun proto-oncogene transcription. J Biol Chem. 1989 Oct 5;264(28):16905–16909. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garcia-Perez A., Burg M. B. Role of organic osmolytes in adaptation of renal cells to high osmolality. J Membr Biol. 1991 Jan;119(1):1–13. doi: 10.1007/BF01868535. [DOI] [PubMed] [Google Scholar]
- Ghahary A., Luo J. M., Gong Y. W., Chakrabarti S., Sima A. A., Murphy L. J. Increased renal aldose reductase activity, immunoreactivity, and mRNA in streptozocin-induced diabetic rats. Diabetes. 1989 Aug;38(8):1067–1071. doi: 10.2337/diab.38.8.1067. [DOI] [PubMed] [Google Scholar]
- Graham A., Brown L., Hedge P. J., Gammack A. J., Markham A. F. Structure of the human aldose reductase gene. J Biol Chem. 1991 Apr 15;266(11):6872–6877. [PubMed] [Google Scholar]
- Greene D. A., De Jesus P. V., Jr, Winegrad A. I. Effects of insulin and dietary myoinositol on impaired peripheral motor nerve conduction velocity in acute streptozotocin diabetes. J Clin Invest. 1975 Jun;55(6):1326–1336. doi: 10.1172/JCI108052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes. 1988 Jun;37(6):688–693. doi: 10.2337/diab.37.6.688. [DOI] [PubMed] [Google Scholar]
- Greene D. A., Lattimer S. A., Sima A. A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987 Mar 5;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- Hamada Y., Kitoh R., Raskin P. Crucial role of aldose reductase activity and plasma glucose level in sorbitol accumulation in erythrocytes from diabetic patients. Diabetes. 1991 Oct;40(10):1233–1240. doi: 10.2337/diab.40.10.1233. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Carper D., Nishimura C., Millen J., Bock M., Hohman T. C. Induction of aldose reductase expression in rat kidney mesangial cells and Chinese hamster ovary cells under hypertonic conditions. Exp Cell Res. 1990 May;188(1):135–140. doi: 10.1016/0014-4827(90)90288-l. [DOI] [PubMed] [Google Scholar]
- Khatami M. Kinetics of myo-inositol transport in corneal endothelial cells: diverse effects of sugars and implications in corneal deutergensence [corrected]. Membr Biochem. 1990 Apr-Jun;9(2):91–106. doi: 10.3109/09687689009025832. [DOI] [PubMed] [Google Scholar]
- Kinoshita J. H. Mechanisms initiating cataract formation. Proctor Lecture. Invest Ophthalmol. 1974 Oct;13(10):713–724. [PubMed] [Google Scholar]
- Kwon H. M., Yamauchi A., Uchida S., Preston A. S., Garcia-Perez A., Burg M. B., Handler J. S. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992 Mar 25;267(9):6297–6301. [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. Altered retinal metabolism in diabetes. II. Measurement of sodium-potassium ATPase and total sodium and potassium in individual retinal layers. J Biol Chem. 1986 Mar 25;261(9):4052–4058. [PubMed] [Google Scholar]
- MacGregor L. C., Matschinsky F. M. Treatment with aldose reductase inhibitor or with myo-inositol arrests deterioration of the electroretinogram of diabetic rats. J Clin Invest. 1985 Aug;76(2):887–889. doi: 10.1172/JCI112048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor L. C., Rosecan L. R., Laties A. M., Matschinsky F. M. Altered retinal metabolism in diabetes. I. Microanalysis of lipid, glucose, sorbitol, and myo-inositol in the choroid and in the individual layers of the rabbit retina. J Biol Chem. 1986 Mar 25;261(9):4046–4051. [PubMed] [Google Scholar]
- Miceli M. V., Newsome D. A. Cultured retinal pigment epithelium cells from donors with type I diabetes show an altered insulin response. Invest Ophthalmol Vis Sci. 1991 Oct;32(11):2847–2853. [PubMed] [Google Scholar]
- Moriyama T., Garcia-Perez A., Burg M. B. Factors affecting the ratio of different organic osmolytes in renal medullary cells. Am J Physiol. 1990 Nov;259(5 Pt 2):F847–F858. doi: 10.1152/ajprenal.1990.259.5.F847. [DOI] [PubMed] [Google Scholar]
- Morrison A. D., Clements R. S., Jr, Travis S. B., Oski F., Winegrad A. I. Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun. 1970 Jul 13;40(1):199–205. doi: 10.1016/0006-291x(70)91066-1. [DOI] [PubMed] [Google Scholar]
- Nakamura J., Del Monte M. A., Shewach D., Lattimer S. A., Greene D. A. Inhibition of phosphatidylinositol synthase by glucose in human retinal pigment epithelial cells. Am J Physiol. 1992 Apr;262(4 Pt 1):E417–E426. doi: 10.1152/ajpendo.1992.262.4.E417. [DOI] [PubMed] [Google Scholar]
- Nakanishi T., Burg M. B. Osmoregulation of glycerophosphorylcholine content of mammalian renal cells. Am J Physiol. 1989 Oct;257(4 Pt 1):C795–C801. doi: 10.1152/ajpcell.1989.257.4.C795. [DOI] [PubMed] [Google Scholar]
- Rosenstock J., Raskin P. Diabetes and its complications: blood glucose control vs. genetic susceptibility. Diabetes Metab Rev. 1988 Aug;4(5):417–435. doi: 10.1002/dmr.5610040502. [DOI] [PubMed] [Google Scholar]
- Smardo F. L., Jr, Burg M. B., Garcia-Perez A. Kidney aldose reductase gene transcription is osmotically regulated. Am J Physiol. 1992 Mar;262(3 Pt 1):C776–C782. doi: 10.1152/ajpcell.1992.262.3.C776. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Ansari N. H., Hair G. A., Awasthi S., Das B. Activation of human erythrocyte, brain, aorta, muscle, and ocular tissue aldose reductase. Metabolism. 1986 Apr;35(4 Suppl 1):114–118. doi: 10.1016/0026-0495(86)90199-x. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Washington L. D. Region-specific initiation of mouse mammary tumor virus RNA synthesis by endogenous RNA polymerase II in preparations of cell nuclei. J Biol Chem. 1983 Mar 10;258(5):2802–2807. [PubMed] [Google Scholar]
- Strange K., Morrison R., Heilig C. W., DiPietro S., Gullans S. R. Upregulation of inositol transport mediates inositol accumulation in hyperosmolar brain cells. Am J Physiol. 1991 Apr;260(4 Pt 1):C784–C790. doi: 10.1152/ajpcell.1991.260.4.C784. [DOI] [PubMed] [Google Scholar]
- Uchida S., Garcia-Perez A., Murphy H., Burg M. Signal for induction of aldose reductase in renal medullary cells by high external NaCl. Am J Physiol. 1989 Mar;256(3 Pt 1):C614–C620. doi: 10.1152/ajpcell.1989.256.3.C614. [DOI] [PubMed] [Google Scholar]
- Vinores S. A., Campochiaro P. A., Williams E. H., May E. E., Green W. R., Sorenson R. L. Aldose reductase expression in human diabetic retina and retinal pigment epithelium. Diabetes. 1988 Dec;37(12):1658–1664. doi: 10.2337/diab.37.12.1658. [DOI] [PubMed] [Google Scholar]
- Williamson J. R., Ostrow E., Eades D., Chang K., Allison W., Kilo C., Sherman W. R. Glucose-induced microvascular functional changes in nondiabetic rats are stereospecific and are prevented by an aldose reductase inhibitor. J Clin Invest. 1990 Apr;85(4):1167–1172. doi: 10.1172/JCI114549. [DOI] [PMC free article] [PubMed] [Google Scholar]