Abstract
Objective:
The main objective of this study was to investigate thermosensitive Pluronic® F-127 (PF-127) hydrogel for the modified release of a potent alcohol and opioid antagonist, naltrexone (NTX) hydrochloride, in a subcutaneous injectable dosage form.
Methods:
The NTX hydrogels were prepared by the cold method, and the in vitro release profiles of various formulations were evaluated at 37°C using the Franz diffusion cell system. We examined the different PF-127 concentrations, pH of solution, and inorganic salts on drug release from these gels.
Results:
The data showed an increase in PF-127 content from 20% to 35%, resulting in a decrease in the rate of NTX release. Among the formulations prepared in different pH solutions, pH 7.4 produced the slowest drug release rate. The addition of inorganic salts had no significant effect on drug release. However, these factors appeared to have limited effects on drug release rate. Therefore, to achieve a sustained-release formulation, a NTX and triacetyl β-cyclodextrin (TAβCD) complex was evaluated. The binary systems of NTX/TAβCD in different molar ratios were prepared by the kneading method, and complex formation was demonstrated by differential scanning calorimetry.
Conclusion:
The results of the current in vitro study indicate that PF-127 gel formulations containing drug complexes with hydrophobic cyclodextrin could be useful for the preparation of a controlled delivery system of water-soluble drugs such as NTX, for a period of more than 140 hours.
Keywords: naltrexone, thermosensitive hydrogel, prolonged release, triacetyl β-cyclodextrin, kneading method
Introduction
The usefulness of naltrexone in opioid dependence is limited by low retention during treatment. Naltrexone (NTX) temporarily blocks substance intake and does not affect craving. Sustained-release preparations of NTX have shown rather promising results. The development of new injectable drug delivery systems has received considerable attention over the past few years.1–3 Our research interest has been sparked by the advantages of these delivery systems, which include ease of application, prolonged delivery periods, decreased drug dosage with concurrent reduction in undesirable side effects common to most forms of systemic delivery, and improved patient compliance and comfort.4
PF-127, or poloxamer 407 (Pluronic®) is a commercially available polyoxyethylene-propylene copolymer, of general formula E106 P70 E106, with an average molar mass of 13,000.5,6 It contains approximately 70% ethylene oxide, which accounts for its hydrophilicity.7–9 This thermosensitive amphiphilic polymer has been widely used in the biomedical field due to its low toxicity, excellent compatibility with other chemicals, and high solubilizing capacity for different drugs. PF-127 at concentrations of 20% or higher in aqueous solution exhibits the unique property of reversible thermal gelation. These preparations transform from low-viscosity solutions to semisolid gels upon heating from 4°C to body temperature (37°C) and thus can be localized in the injection site with minimal distribution of the drug.10 In this capacity, PF-127 hydrogels have been used as drug delivery vehicles, alone and in combination with other delivery systems, to provide desirable release profiles.11–14
This kind of depot-like sustained release gel is available for many drugs, but there have been no published reports of its use for delivery of NTX. Oral NTX is safe and effective for the treatment of both alcohol and opioid dependence. However, because of its extensive first-pass metabolism, small treatment effect, plasma level fluctuations, adverse events, and poor patient adherence to the daily dosing schedule, clinical acceptance of this dosage form has been limited.15–17 Injectable sustained-release preparations of NTX can overcome these problems and improve the treatment outcome.18–20
Due to the high solubility of NTX (65 mg/mL) and the amphiphilic nature of PF-127, it appears that drug release from the carrier may be rapid and characterized by a strong burst of drug release.21 Therefore, the effects of formulation variables, such as concentration of polymer, pH, and inorganic salts on in vitro drug release were investigated. For optimizing the sustained-release pattern, cyclodextrin complexes were also evaluated. Bioadaptable and multifunctional cyclodextrin molecules are able to alleviate the undesirable properties of drugs given by various routes of administration via formation of inclusion complexes.
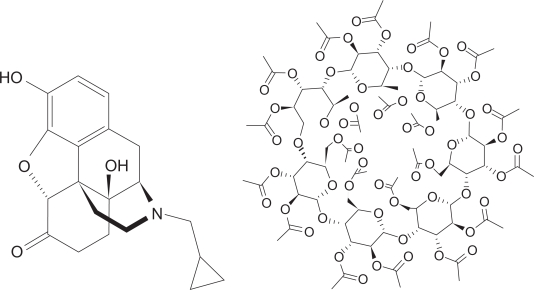
Hydrophobic cyclodextrins, such as triacetyl-β-cyclodextrin (TaβCD, Figure 1), could serve as novel slow-release carriers of water soluble drugs, and a combination of molecular encapsulation and other carrier materials will become an effective and valuable tool in the improvement of drug formulation.22
Figure 1.
Chemical structure of naltrexone and triacetyl-β-cyclodextrin (TAβCD).
The most common preparation method for complexes with hydrophobic cyclodextrins is the kneading technique.23 Thus, the binary systems of NTX/TAβCD in different molar ratios were prepared by the kneading method, and the release behavior of the drug was investigated.
To confirm the formation of a NTX/TAβCD complex, each sample was analyzed by differential scanning calorimetry.
Materials and methods
Materials
NTX, PF-127, and TAβCD were purchased from Sigma-Aldrich (Schrelldorf, Germany). Analytical grade ethanol and inorganic salts were obtained from Merck (Darmstadt, Germany). Cellulose acetate membrane (molecular weight cutoff 12,000 Da) was obtained from Sigma (Germany). All other chemical reagents used were of pharmaceutical grade.
Preparation of NTX PF-127 hydrogel formulation
The PF-127 solutions were prepared by the cold method.20 A weighed amount of PF-127 was slowly added to a cold aqueous solution (5–10°C) with gentle mixing until complete dissolution of the polymer. The solutions used included a phosphate-buffered solution of the polymer at different pH values. When inorganic salts (NaCl, Na2SO4, Na2HPO4) were used, they were dissolved in distilled water. The polymer was then slowly added to the cold water as described.
An appropriate amount of NTX (10 mg/mL) was added to each formulation. All gels were kept overnight at 4°C until a homogenous solution was obtained.
Preparation of NTX/TAβCD inclusion complexes
The binary system of NTX/TAβCD in different molar ratios (1:1, 1:2, and 1:4) was prepared by the kneading method, ie, TAβCD was wetted in a ceramic mortar with ethanol:water 50% (v/v) solution until a paste was obtained. The required amount of NTX was then added slowly whilst grinding, and the slurry was kneaded for about 45 minutes. During this process, an appropriate quantity of solvent was added in order to maintain a suitable consistency. The product was then dried at 40°C over 24 hours and added to the poloxamer solution.
Differential scanning calorimetry
The optimum NTX/TAβCD complex was characterized by thermal analysis, performed using a differential scanning calorimeter (DSC-60, Shimadzu Co., Kyoto, Japan). Thermograms of the different samples (inclusion complex, physical mixture, and pure substances) were obtained from differential scanning calorimetry equipped with a thermal analysis data system. Weighted samples (6–8 mg) were contained in sealed aluminum pans and scanned at a rate of 10°C/min, between 0°C and 300°C, using nitrogen as a purging gas. The thermal analysis was carried out over 300°C during preliminary runs, but only thermal events observed over 240°C involved decomposition of the materials.
In vitro release studies
In this study, diffusion cells were used to evaluate NTX release from the poloxamer hydrogels.21 The diffusion cells are comprised of two compartments separated by a cellulose acetate membrane (cutoff 12,000 Da), which does not constitute a barrier against NTX diffusion. The donor compartment was filled with 1 mL of formulation, and the receiver compartment was filled with pH 7.4 phosphate buffer (30 mL). Temperature was maintained at 37°C for all experiments. At the specified time, aliquots (500 μL) of medium on the receiver side were taken and replaced with an equal volume of fresh phosphate buffer, in order to maintain sink conditions. The collected samples were analyzed by ultraviolet spectroscopy at 281 nm. Each experiment was performed in triplicate.
Effect of formulation factors on in vitro release of NTX from PF-127 gel
Effect of PF-127 concentration on drug release
Phosphate-buffered solutions (pH 7.4) containing different concentrations of PF-127 (20%, 25%, 30%, and 35% [w/v]) without any additive were prepared as described above. The in vitro release of NTX from these gel formulations was studied.
Effect of pH of polymeric solutions on drug release
The effect of pH on drug release was studied using 25% (w/v) PF-127 gels. These formulations were prepared in different pH solutions, ie, 5.5, 7.4, and 8.5, using 0.2 M phosphate buffer.
Effect of inorganic salts on drug release
The effect of salts was studied using 25% Pluronic formulations of NTX in the presence of NaCl, Na2SO4, and Na2HPO4 (1% w/v). The PF-127 gel formulation with no additive was used as a control.
Effect of complexation of NTX with TAβCD on drug release
The effect of TAβCD was studied using a gel containing PF-127 25% (w/v) and binary systems of NTX/TAβCD in different molar ratios which were prepared by the kneading method. The PF-127 gel formulation with no additive was used as a control.
Results and discussion
Effect of PF-127 concentration on drug release
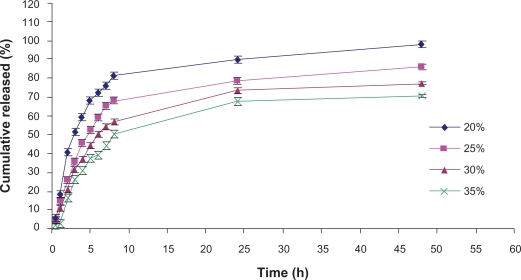
The effect of PF-127 concentration (20%, 25%, 30%, and 35%) on drug release at 37°C is shown in Figure 2. It is apparent that the drug release rate decreases as the PF-127 concentration increases. Increasing the concentration of PF-127 from 20% (w/v) to 35% (w/v) decreased the cumulative percent drug released after eight hours from 80% to 50%. As shown in Figure 2, the highest concentration of PF-127 could sustain NTX release at about 55% in 10 hours and 70% in 48 hours, which is away from our desirable sustained profile release.
Figure 2.
Influence of PF-127 concentration on naltrexone release from hydrogel formulations.
A hydration layer surrounds PF-127 molecules at low temperatures in aqueous solution. However, when the temperature is raised, the hydrophilic chains of the copolymer become desolvated as a result of the breakage of the hydrogen bonds that had been established between the solvent and these chains. This phenomenon favors hydrophobic interactions among the poly oxypropylene domains, leading to increased chain entanglement and gel formation.22 This entanglement is more marked at higher concentrations of PF-127, yielding an increase in gel strength and consequently a decrease of the drug release rate.27 We should mention that 30% and 35% PF-127 hydrogels were very viscous and have very low syringability, so are not suitable vehicles for subcutaneous injection, so 25% PF-127 which has similar release pattern (P > 0.05) was selected for the next experiments.
Effect of polymeric solution pH on drug release
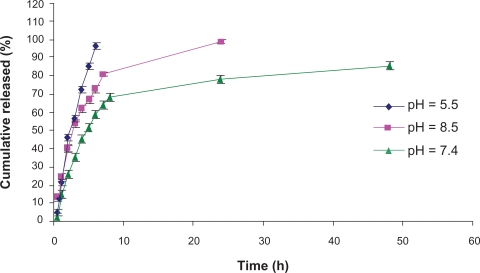
The effect of pH on NTX release was studied at vehicle pH values of 5.5, 7.4, and 8.5 using 25% PF-127 phosphate-buffered solutions (Figure 3). The results showed that pH 5.5 and 8.5 produced faster release of NTX among the studied formulations. This indicates that when the pH of polymeric solution is lower or higher than 7.4, diffusion of NTX is increased. It may be concluded that ionization of hydroxyl groups of polyethylene blocks could increase interchain movement and presence of hydronium or hydroxide ions between these chains, and have effective roles in polymeric chain distance and easier diffusion of drug molecules.
Figure 3.
In vitro release profiles of naltrexone from 25% PF-127 hydrogel formulations prepared in different pH solutions. Each point represents the mean ± standard deviation (n = 3).
Effect of inorganic salts on drug release
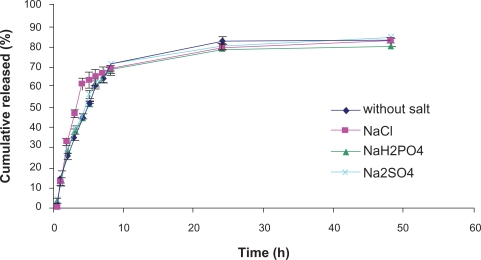
Most pharmaceutical PF-127 gels are formulated with salts, either for buffering or ionic strength adjustment.28 It has been shown that the diffusion of drugs within the gel network is significantly affected by salts. We have examined the effect of NaCl, Na2SO4, and Na2HPO4 (1% w/v) on drug release from PF-127 gels (Figure 4). The results showed no significant effect of any of the salts on drug release rate (Figure 3). This is very different from earlier results where the same salts were shown to decrease diffusion of propranolol across a membrane from F127 gels containing salts.29 We believe that this discrepancy can be explained as follows. The water structure causes salts to reduce the critical micelle concentration and temperature of PF-127, make the poly(ethylene oxide)-poly(ethylene oxide) chain interactions stronger due to a decrease in hydrogen bonding with water, and thus make the poly(ethylene oxide) chain network tighter. Although the inclusion of salts results in a tighter micelle network, it also increases the water uptake rate into the gel due to an osmotic effect.30 The net result is that salts do not have a measurable effect on drug release rate in prepared NTX hydrogel.
Figure 4.
Effect of inorganic salts addition on release of naltrexone from 25% PF-127 hydrogel. Each point represents the mean ± standard deviation (n = 3).
Effect of TAβCD complex on drug release
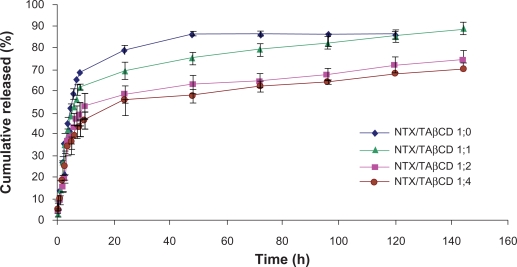
The release profiles of NTX and NTX/TAβCD binary systems in different molar ratios incorporated into 25% w/v PF-127 formulations are presented in Figure 5. The data show that hydrophobic TAβCD slows the release rate, and this effect is dependent on the amount of TAβCD in the matrices. Cumulative release of the drug after 48 hours approached 85% for PF-127 hydrogel alone, compared with less than 60% in a gel containing1:4 NTX/TAβCD complex. This retardation of the drug release rate with TAβCD may be attributed to formation of complexes in the kneaded product.
Figure 5.
Release profiles of naltrexone from 25% PF-127 gel formulations containing binary naltrexone/triacetyl-β-cyclodextrin systems in different molar ratios. Each point represents the mean ± standard deviation (n = 3).
Problems in controlling drug release from polymeric carriers are common, particularly for matrices composed of hydrophilic polymers, eg, PF-127 hydrogels. In such cases, drug release from the carrier may be rapid and characterized by a burst release.21 Narasimhan and Langer31 showed that burst release was controlled by the solubility of drug, the drug diffusion coefficient and the initial drug distributions within the polymeric carrier. Retarded drug release, through a dissolution-limited mechanism, is a common objective for the use of many poorly water-soluble cyclodextrin complexes.32
Poorly water-soluble alkylated cyclodextrin derivatives, such as TaβCD, are useful as slow-release carriers for water soluble drugs.33,34 Furthermore, the incorporation of these agents into polymeric drug delivery systems frequently permits a greater degree of controlled drug release rate and time.35
TAβCD forms noncovalent and poorly water soluble complexes with NTX and reduced drug solubility in aqueous medium and total concentration of diffusible species, resulting in a retarded release. In vitro release experiments showed that the formulations containing drug/TAβCD in molar ratios of 1:2 and 1:4 with no significant difference were able to prolong and control NTX release for more than 144 hours. The optimum formulation showed more than three-week stability at refrigerator temperature and 72 hours at 37°C.
Differential scanning calorimetry
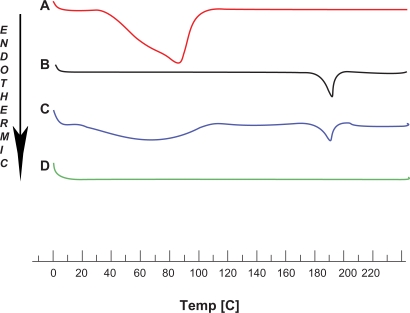
The solid binary systems were analyzed by means of differential scanning calorimetry to detect possible altered thermal properties with regard to the pure substances. When guest molecules are incorporated in the TAβCD cavity, their melting, boiling, and sublimation points generally shift to a different temperature or disappear at the temperature range within which the cyclodextrin lattice is decomposed.36 The differential scanning calorimetry curves for NTX, TaβCD, and respective drug-carrier combinations in a molar ratio of 1:2 are shown in Figure 6. The thermal curve of NTX indicated a mixture of amorphous states and a characteristic broad endothermic peak at 85.19°C. The TAβCD thermogram displayed an endothermic peak at 190.77°C due to the fusion process of this crystalline molecule. The appearance of two endothermic peaks corresponding to the fusion of both components was also evident in the thermogram of the physical mixture, as if this differential scanning calorimetry curve was the superimposition of those for the components analyzed separately. The shape of the NTX melting peak was unaffected by blending with TAβCD, and hence the drug maintained its original amorphous state in the physical mixture. The complete disappearance of the drug endothermic peak was found in the kneaded preparation. The absence of the NTX fusion peak indicated the existence of interactions between drug and TAβCD in the solid state, and could be considered as an indication for the formation of real inclusion complexes. The sustained release of drug from the hydrogel containing NTX/TAβCD binary system supports this theory.
Figure 6.
Differential scanning calorimetry thermograms of A) naltrexone, B) triacetyl-β-cyclodextrin, C) physical mixture, and D) kneaded product.
Conclusion
For a novel drug delivery system, one of the major advantages is extending product life through formulation, and decreasing the number of times that a product is administered. Several approaches have been used to improve the prolonged release dosage forms to increase patient compliance. One of these approaches is the utilization of Pluronic F-127 hydrogels. In this study, we developed a new sustained release and thermoresponsive drug delivery system for a potent opioid receptor antagonist, NTX, to be administrated subcutaneously. Poloxamer hydrogel formulations containing NTX were evaluated by in vitro experiments. The results showed that the release of NTX from PF-127 hydrogel was affected by formulation variables of the gel. Increasing F127 concentration in the gel decreases drug release rate. The pH of the polymeric solution is also an important factor for NTX release. The addition of inorganic salts has no significant effect on drug release. The present results suggested that a combination of TAβCD/drug complexes and Pluronic gels with sustained-release behavior for more than 144 hours is useful for the controlled release of a water-soluble drug, and the release rate may be controlled by adjusting the molar ratio of the components.
Acknowledgments
This work was supported by a grant from the Faculty of Pharmacy, Kermanshah University of Medical Sciences, Iran.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Heller J. Polymers for controlled parenteral delivery of peptides and proteins. Adv Drug Deliv Rev. 1993;10:163–204. [Google Scholar]
- 2.Langer R. New methods of drug delivery. Science. 1990;249:1527–1533. doi: 10.1126/science.2218494. [DOI] [PubMed] [Google Scholar]
- 3.Reddy KR. Controlled release, pegylation, liposomal formulations: New mechanisms in the delivery of injectable drugs. Ann Pharmacother. 2000;34:915–922. doi: 10.1345/aph.10054. [DOI] [PubMed] [Google Scholar]
- 4.Hatefi A, Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Rel. 2002;80:9–28. doi: 10.1016/s0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- 5.Narasimhan B, Peppas NA. The physics of polymer dissolution: Modeling approaches and experimental behavior. Adv Polym Sci. 1997;128:157–207. [Google Scholar]
- 6.Mallapragada SK, Peppas NA. Crystal unfolding and chain disentanglement during semicrystalline polymer dissolution. AlChE J. 1997;43:870–876. [Google Scholar]
- 7.Yang L, Alexandridis P. Mass transport in ordered microstructures formed by block copolymers: Ramifications for controlled release applications. Polym Prep. 1999;40:349–350. [Google Scholar]
- 8.Harland RS, Gazzaniga A, Sangalli ME, Colombo P, Peppas NA. Drug/polymer matrix swelling and dissolution. Pharm Res. 1988;5:488–492. doi: 10.1023/a:1015913207052. [DOI] [PubMed] [Google Scholar]
- 9.Mallapragada SK, Peppas NA. Crystal dissolution-controlled release systems: I. Physical characteristics and modeling analysis. J Control Rel. 1997;45:87–93. [Google Scholar]
- 10.Zhang L, Parsons DL, Navarre C, Kompella UB. Development and in-vitro evaluation of sustained release Poloxamer 407 gel formulations of ceftiofur. J Control Rel. 2002;85:73–81. doi: 10.1016/s0168-3659(02)00273-0. [DOI] [PubMed] [Google Scholar]
- 11.Escobar-Chávez JJ, López-Cervantes M, Naïk A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermoreversible Pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharmaceut Sci. 2006;9:339–358. [PubMed] [Google Scholar]
- 12.Desai SD, Blanchard J. Evaluation of Pluronic F-127 based sustained-release ocular delivery systems for pilocarpine using the albino rabbit eye model. J Pharm Sci. 1998;87:1190–1195. doi: 10.1021/js980222j. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel JG, Balaji KS, Koushik K, et al. Pluronic F127 gel formulations of deslorelin and GnRH reduce drug degradation and sustain drug release and effect in cattle. J Control Rel. 2002;85:51–59. doi: 10.1016/s0168-3659(02)00271-7. [DOI] [PubMed] [Google Scholar]
- 14.Collet JH, Tait C, Attwood D. In vitro evaluation of poloxamer gels as controlled release systems using gamma scintigraphy. Proc Int Symp Contr Rel Bioact Mater. 1985;12:28–30. [Google Scholar]
- 15.Dunbar JL, Turncliff RZ, Dong Q, Silverman BL, Ehrich EW, Lasseter KC. Single and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin Exp Res. 2006;30:480–490. doi: 10.1111/j.1530-0277.2006.00052.x. [DOI] [PubMed] [Google Scholar]
- 16.Garbutt JC, Kranzler HR, O’Malley SS, et al. Efficacy and tolerability of long-acting ingectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- 17.Johnson BA. A synopsis of the pharmacological rationale, properties and therapeutic effects of depot preparations of naltrexone for treating alcohol dependence. Expert Opin Pharmacother. 2006;7:1065–1073. doi: 10.1517/14656566.7.8.1065. [DOI] [PubMed] [Google Scholar]
- 18.Sullivan MA, Vosburg SK, Comer SD. Depot naltrexone: Antagonism of the reinforcing, subjective, and physiological effects of heroin. J Psychopharmacol. 2006;189:37–46. doi: 10.1007/s00213-006-0509-x. [DOI] [PubMed] [Google Scholar]
- 19.Swainston Harrison T, Plosker GL, Keam SJ. Extended-release intramuscular naltrexone. Drugs. 2006;66:1741–1751. doi: 10.2165/00003495-200666130-00006. [DOI] [PubMed] [Google Scholar]
- 20.O’Malley SS, Garbutt JC, Gastfriend DR, Dong Q, Kranzler HR. Efficacy of extended-release naltrexone in alcohol dependent patients who are abstinent before treatment. J Clin Psychopharmacol. 2007;27:507–512. doi: 10.1097/jcp.0b013e31814ce50d. [DOI] [PubMed] [Google Scholar]
- 21.Pongpaibul Y, Maruyama K, Iwatsuru M. Formation and in-vitro evaluation of theophylline-loaded poly (methyl methacrylate) micro-spheres. J Pharm Pharmacol. 1988;40:530–533. doi: 10.1111/j.2042-7158.1988.tb05296.x. [DOI] [PubMed] [Google Scholar]
- 22.Hirayama F, Uekama K. Cyclodextrin-based controlled drug release system. Adv Drug Deliv Rev. 1999;36:125–141. doi: 10.1016/s0169-409x(98)00058-1. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes CM, Veiga FJ. Effect of the hydrophobic nature of triacetyl-β-cyclodextrin on the complexation with nicardipine hydrochloride: Physicochemical and dissolution properties of the kneaded and spray-dried complexes. Chem Pharm Bull. 2002;50:1597–1602. doi: 10.1248/cpb.50.1597. [DOI] [PubMed] [Google Scholar]
- 24.Schmolka IR. Artificial skin I. Preparation and properties of Pluronic F-127 gels for treatment of burns. J Biomed Mater Res. 1972;6:571–582. doi: 10.1002/jbm.820060609. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Lobel E, Trevgada A, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Rel. 1997;44:201–208. [Google Scholar]
- 26.Escobar-Chavez JJ, Lopez-Cervantes M, Naik A, Kalia YN, Quintanar-Guerrero D, Ganem-Quitanar A. applications of thermoreversible Pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci. 2006;9:339–358. [PubMed] [Google Scholar]
- 27.Guzman M, Garsia FF, Molpeceres J, Aberturas MR. Polyoxyethylene-polyoxypropylene block copolymer gels as sustained release vehicles for subcutaneous drug administration. Int J Pharm. 1992;80:119–127. [Google Scholar]
- 28.Gilbert JC, Hadgraft J, Bye A, Brookes L. Drug release from Pluronic F127 gels. Int J Pharm. 1986;32:223–228. [Google Scholar]
- 29.Pandit N, Wang D. Salt effects on the diffusion and release rate of propranolol from poloxamer 407 gels. Int J Pharm. 1998;167:183–189. [Google Scholar]
- 30.Moore T, Croy S, Mallapragada S, Pandit N. Experimental investigation and mathematical modeling of Pluronic F127 gel dissolution: Drug release in stirred systems. J Control Rel. 2000;67:191–202. doi: 10.1016/s0168-3659(00)00215-7. [DOI] [PubMed] [Google Scholar]
- 31.Narasimhan B, Langer R. Zero-order release of micro- and macromolecules from polymeric devices: The rule of the burst effect. J Control Rel. 1997;47:13–20. [Google Scholar]
- 32.Lemesle-Lamache V, Wouessidjewe D, Cheron M, Duchene D. Study of β-cyclodextrin and ethylated β-cyclodextrin salbutamol complexes, in vitro evaluation of sustained-release behavior of salbutamol. Int J Pharm. 1996;141:117–124. [Google Scholar]
- 33.Uekama K, Hirayama F, Irie T. Cyclodextrin drug carrier systems. Chem Rev. 1998;98:2045–2076. doi: 10.1021/cr970025p. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi Y, Hirayama F, Uekama K. Slow-release characteristics of diltiazem from ethylated β-cyclodextrin complexes. J Pharm Sci. 1990;79:128–132. doi: 10.1002/jps.2600790211. [DOI] [PubMed] [Google Scholar]
- 35.Bibby DC, Davies NM, Tucker IG. Mechanisms by which cyclodextrins modify drug release from polymeric drug delivery systems. Int J Pharm. 2000;19:1–11. doi: 10.1016/s0378-5173(00)00335-5. [DOI] [PubMed] [Google Scholar]
- 36.Cabral-Marques HM, Hadgraft J, Kellaway IW. Int J Pharm. 1990;63:259–266. [Google Scholar]