Abstract
Drug delivery systems such as nanoparticles can provide enhanced efficacy for anticancer agents. Noscapine, a widely used cough suppressant for decades has recently been shown to cause significant inhibition and regression of tumor volumes without any detectable toxicity in cells or tissues. Nanoparticles made of human serum albumin (HSA) represent promising strategy for targeted drug delivery to tumor cells by enhancing the drug’s bioavailability and distribution, and reducing the body’s response towards drug resistance. In the present study, we report for the first time the incorporation and delivery of noscapine-loaded HSA nanoparticles to tumor cells. The nanoparticles were designed and optimized to achieve a particle size in the range of 150–300 nm with a drug-loading efficiency of 85%–96%. The nanoparticles were evaluated in vitro for their anticancer activity and efficacy on breast cancer cells.
Keywords: HSA, encapsulation, microcapsule, nanomedicine, nanotechnology, tumor volumes
Introduction
Most commonly used chemotherapeutic drugs such as paclitaxel interact with tubulin, the major protein of mitotic spindles, causing growth arrest in metaphase. These agents induce the polymerization of tubulin and stop mitosis of rapidly dividing cells; thereby leading to apoptosis. Despite impressive results, available chemotherapeutic drugs have severe symptoms associated with them including myelosuppresion.1 Also as a result of repeated and prolonged administration of chemotherapeutic agents, drug resistance can occur.1,2 In addition, these drugs are toxic to healthy tissues, and have poor bioavailability that results in the need of extended intravenous infusions and the use of toxic solubility agents.1,3
Noscapine, a phthalide isoquinoline alkaloid derived from opium, has been used as an oral antitussive agent and has shown very few toxic effects in animals and humans. It is a naturally occurring tubulin-binding agent currently undergoing clinical trials for anticancer therapy. Noscapine, which lacks sedative, analgesic, and euphoric properties, has been used for decades as a cough suppressant. Noscapine showed little or no toxicity on kidney, liver, heart, bone marrow, spleen, and small intestine at tumor suppressive doses.4 Noscapine was also shown to cause growth arrest of tumor cells during mitosis and induces apoptosis5–7 and can therefore be used as an alternative to current chemotherapeutic drugs.8–10 It was recently reported that noscapine was effective in reducing the growth of lymphoma and increasing the survival of tumor-bearing mice when administered in their drinking water.7,11–15 However, current drug delivery methods of high concentration of noscapine are inadequate.
To increase the concentration of noscapine at the tumor site, we have encapsulated noscapine into colloidal drug nanocarriers made of human serum albumin (HSA). Nanoparticles of minimal size can enhance the efficacy of drug metabolism with minimal side effects since they allow for the possibility of site-specific targeted delivery.16–18 Nanoparticles also offer benefits to carry functional groups (amino and carboxylic groups) that can be used for surface modifications. The emergence of nanoscaled devices as drug delivery systems has been remarkable in such a short span of time and has surpassed conventional methods of drug delivery. Nanoparticles help to increase the stability of drugs and possess useful controlled release properties. HSA-based nanoparticles can be well tolerated without any serious side effects, which is supported by clinical studies with registered HSA-based particle formulations such as Albunex.18–21 Albumin has been shown to be biodegradable, nontoxic, easy to purify, and soluble in water, allowing ease of delivery by injection and thus an ideal candidate for nanoparticle preparation. Incorporation of suitable drugs in nanoparticles has been shown to protect the pharmacological active substances from degradation during storage as well as from early degradation/inactivation after injection.22–24
Many different possibilities have been discussed and tested to reduce the toxicity and increase antitumor efficacy of anticancer drugs using nanodevices.25–27 Encapsulating noscapine in nanoparticles will help to increase its efficacy and lowers any side effects. In this paper we report for the first time encapsulation of noscapine into HSA nanoparticles with a described protocol on optimization of drug loading and preparation of nanoparticles and their effectiveness on breast cancer cell line (SK-BR-3).
Materials and methods
Materials
Human serum albumin (HSA fraction V, purity 96%–99%), noscapine hydrochloride and 8% glutaraldehyde, were purchased from Sigma Aldrich (Oakville, Ontario, Canada). For cell culture, fetal bovine serum (FBS), trypsin, McCoy’s 5a medium, penicillin/streptomycin, and the SK-BR-3 cell line were purchased from American Type Culture Collection (Ontario, Canada). Cell-line was cultured according to supplier’s instructions. All other reagents were purchased from Fisher (Ontario, Canada).
Preparation of noscapine nanoparticles
Drug-free HSA nanoparticles and noscapine-loaded nanoparticles crosslinked with glutaraldehyde were prepared using a pH-coacervation method.28,29 100 mg of HSA was dissolved in 2 mL of water or NaCl. Noscapine at a concentration of 5–30 mg/mL was incubated with solution for 4–8 h at room temperature.30 The pH was adjusted to 8 by the addition of 1 M NaOH. Nanoparticles were formed by adding 8 mL of ethanol drop-wise at a constant rate of 1 mL/min under constant magnetic stirring. The particles were stabilized by crosslinking with 100 μL of 8% glutaraldehyde solution. The crosslinking was performed for at least 24 h under constant magnetic stirring at room temperature.30,31 Drug loading was evaluated following an indirect method of collecting the supernatant of purified particles. The quantity of unloaded free drug present in the supernatant was measured by spectrophotometer that lead to the quantification of the percentage of drug loaded into the nanoparticles.
Purification of HSA nanoparticles
The resulting albumin nanoparticles were purified by three cycles of ultra-centrifugation (20,000 g, 30 min) followed by redispersion of pellet in water to original volume. Each redispersion step was performed in an ultrasonication bath (Branson 2510; Bransonic, Danbury, CT) for 15 min.
Particle size and zeta potential of nanoparticles
The size of noscapine albumin nanoparticles was determined by photon correlation spectroscopy (PCS) using a high performance particle size analyzer (Malvern Instruments, Westborough, MA). The samples were diluted with distilled water and measured at 25°C at a scattering angle of 90°. Size distribution was characterized by a polydispersity index (PI) and the zeta potential was measured with the technique of electrophoretic laser Doppler anemometry, using a Zeta Potential Analyzer (Brookhaven Instruments, Holtsville, NY). Morphological characteristics were examined using scanning electron microscope (Hitachi S-4700 FE-SEM; Hitachi, Tokyo, Japan). In order to determine the stability of the nanocarriers when dispersed in aqueous solution, they were stored at room temperature for 5 days while being regularly monitored for size and zeta potential each day. The size and shape of the nanoparticles were also examined by scanning electron microscopy (SEM).
Drug release profile of noscapine-loaded HSA nanoparticles
Determination of drug release of noscapine was performed by dispersing 10 mg of noscapine-loaded nanoparticles in 10 mL phosphate-buffered saline (PBS; pH 7.4) under constant shaking at 200 rpm/min at 37°C. The samples were centrifuged and the amount of free noscapine in the supernatant was determined at predefined time intervals using a UV spectrophotometer Victor3 V 1420 Multilabel Counter (Perkin Elmer, Boston, MA) at 310 nm. This analysis was performed three times for each sample.
Cell culture
SK-BR-3 cells were grown in McCoy’s 5a medium with 10% FBS and 1% penicillin/streptomycin. Cells were cultured in a humidified incubator containing 5% CO2 at 37°C.
In vitro cell viability of noscapine-loaded HSA nanoparticles
The determination of cell viability is a common assay to evaluate the in vitro cytotoxicity of biomaterials. The MTS assay is a quantitative and rapid colorimetric method for measuring the viability of cells. The cytotoxicity study of the nanoparticles was examined in vitro on the breast cancer cell-line SK-BR-3; the cell lines were cultured in 96-well plates at an initial concentrations of 5000 cells/well in fresh medium supplemented with 10% FBS. The cell proliferation was determined by cell counting after trypsinization and trypan blue staining. After 24 h of culture, cells were adherent and the medium was replaced by noscapine-loaded nanoparticles at a final concentration of 50 μg/mL. The media was removed after 24 h and replaced with fresh medium supplemented with 10% FBS. The cell viability and cytotoxicity were determined using MTS cell proliferation kit. At predetermined time intervals, 20 μl of MTT was added in each well and incubated for 4 h in a humidified incubator containing 5% CO2 at 37°C and absorbance was measured at 490 nm using a Victor3V 1420 Multilabel Counter spectrophotometer (Perkin Elmer).32–34
Results and discussion
Preparation and visualization of noscapine nanoparticles
The objective of the present study was to design HSA nanoparticles for noscapine delivery and establish a standard protocol for their preparation. HSA nanoparticles containing noscapine were prepared by coacervation method, in which ethanol was used as a dissolving agent, followed by crosslinking using glutaraldehyde.29 The pH-coacervation method has widely been used in the encapsulation of both water-soluble and insoluble drugs. Initially we investigated the effect of the process conditions such as the aqueous HSA concentration, the rotation speed of the magnetic stirrer, the pH of the solution prior to ethanol addition, and the rate of ethanol addition. After experimentation and optimization a final preparation method was prepared. Uniform particles with narrower size distribution were achieved at higher rotation speed of the magnetic stirrer with ethanol, dropped at a constant flow rate using a peristaltic pump. With the modified pH-coacervation technique reported in this study, we achieved noscapine-loaded HSA nanoparticles of diameters between 150 and 300 nm and narrow size distribution determined by the PI of < 0.4. The SEM micrographs reveal morphological aspects of nanoparticles with a spherical shape and uniform size distribution in the desired range of nanoparticle sizes (Figure 1).
Figure 1.
Scanning electron micrograph of glutaraldehyde crosslinked nanoparticles prepared at pH 8, 60k resolution. The nanoparticles were found to be of uniform size and narrow size distribution.
Degree of crosslinking of noscapine-loaded nanoparticles
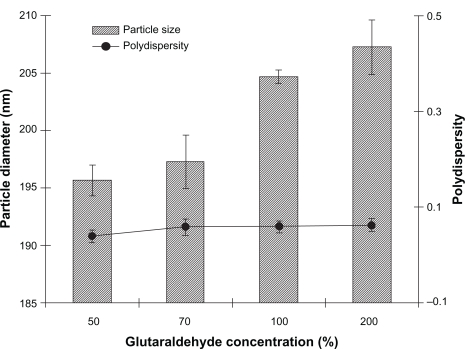
Crosslinking of nanoparticles with a crosslinking agent can limit their degradation rate and hydration potential, thereby attaining slow-release kinetics.35,36 The influence of the cross-linking process (glutaraldehyde concentration, 50%–200%) on the size of albumin nanoparticles was investigated. This process plays a major role in the stability and drug release of albumin nanoparticles. Figure 2 shows the effect of different glutaraldehyde concentrations on particle diameter and PI of noscapine-loaded nanoparticles. The results reveal that the concentration of glutaraldehyde had little or no effect on particle size or polydispersity.
Figure 2.
Effect of glutaraldehyde concentration on diameter and polydispersity index of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein at pH 8.
Influence of pH and crosslinking on size and zeta potential of HSA nanoparticles
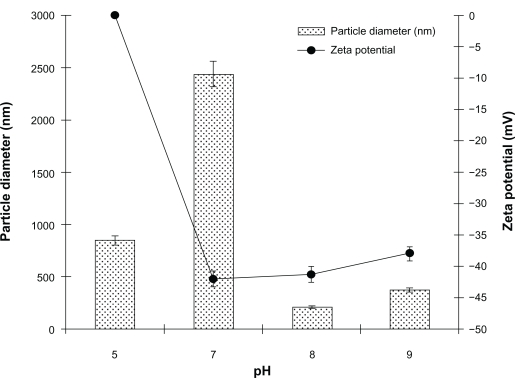
It was found that the pH of the noscapine-HSA solution prepared prior to ethanol addition was a crucial factor in affecting the particle size of the resulting nanoparticles (Figure 3). At pH 5–7, particles were of larger diameter with high polydispersity indices. The particle size was greatest at pH 7 at approximately 2500 nm. At pH greater than 7 the particle diameter was reduced and at pH 8 the particles had optimal size and narrow size distribution at approximately 175–200 nm. The nanoparticles prepared at higher pH of 8–8.2, when viewed under SEM, revealed uniform and spherical particles.
Figure 3.
Effect of pH on diameter and polydispersity index of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein.
The electrical behavior of the noscapine-loaded HSA nanoparticles was also evaluated at different pH (Figure 3). Higher surface charge was attained on HSA nanoparticles by increasing the pH of the HSA solution. It was observed that with increasing pH > 7, the surface charge of prepared nanoparticles was reduced; particles prepared at pH 8 had their surface charge reduced to −47mV. It was noted that preparation of particles at higher pH also leads to repulsion among the HSA molecules due to steric effect and lead to aggregation during particle formation.
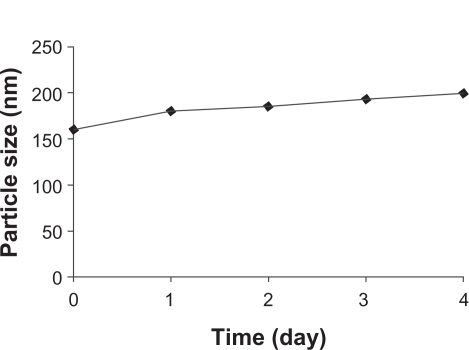
The stability of the nanoparticles was also investigated. Noscapine-loaded nanoparticles prepared at pH 8.2 were stored for 5 days in water at 4°C and at predefined times the samples were analyzed with regard to size (Figure 4). The particle size increased slightly to 190 nm, but particles were found to be stable after 5 days.
Figure 4.
Stability of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles over 5 days. Particle size was monitored. Noscapine-loaded HSA nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein at pH 8.
Investigation of noscapine delivery by nanoparticles
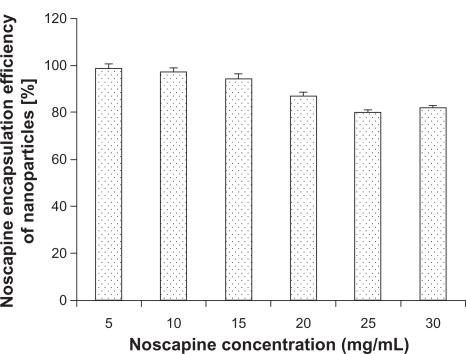
Noscapine was adsorbed to HSA in solution prior to nanoparticle preparation. The pH was adjusted to 8.2 at different noscapine concentrations ranging from 5 mg/mL to 30 mg/mL of HSA. All preparations were stabilized with glutaraldehyde. Figure 5 illustrates the encapsulation efficiency of HSA nanoparticles at different drug concentrations. Maximum encapsulation was attained at noscapine concentrations of 5 mg/mL with an efficiency of 97%. Encapsulation efficiency was shown to gradually decrease with increasing drug concentration. The lowest encapsulation efficiency was seen at noscapine concentrations of 25 mg/mL and 30 mg/mL suggesting that at higher concentrations of noscapine more incubation time is probably required as well as greater concentration of HSA is needed for efficient encapsulation.
Figure 5.
Noscapine encapsulation of human serum albumin nanoparticles (50 mg/mL) in dependence on noscapine concentration.
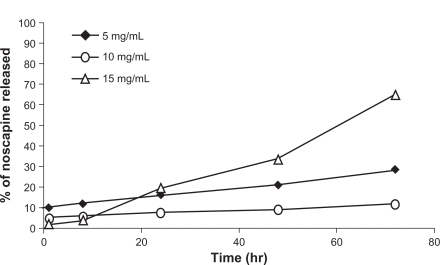
The drug release profiles of noscapine-loaded HSA nanoparticles were also investigated. Noscapine albumin nanoparticles prepared under experimental conditions described previously were tested in vitro and released at 37°C in PBS at pH 7.4. It was found that with increasing drug concentrations the particle size and polydispersity increased dramatically. Figure 6 shows the in vitro release of cumulative amounts of noscapine from albumin nanoparticles as a function of time. For noscapine concentrations of 5 mg/mL, the initial drug released was around 10% which was considered a biphasic way of release; it is characterized by an initial rapid release period followed by a step of slower release. For noscapine-loaded nanoparticles with noscapine concentrations of 5 mg/mL and 10 mg/mL, the release profile was slow after 72 h, with less than 30% of the drug being released. While at concentrations of 15 mg/mL of noscapine more than 60% of the drug had been released after 72 h. The release profile seemed moderately slow.
Figure 6.
Drug release profile of noscapine-loaded human serum albumin (HSA) nanoparticles (noscapine concentration 5–15 mg/mL) over predetermined time intervals. Noscapine-loaded HSA nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein at pH 8.
Viability of HSA nanoparticles loaded with noscapine in a SK-BR-3 cell line
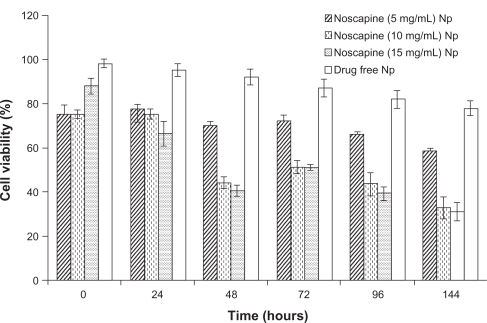
HER-2 positive SK-BR-3 breast cancer cells were used to examine the efficacy of noscapine prepared nanoparticles. The cell lines were cultured in 96-well plates at an initial concentration of 5000 cells/well in fresh medium. After 24 h of culture, cells were adherent, and medium was replaced with prepared noscapine nanoparticles and drug-free HSA nanoparticles. We analyzed the cell viability and cytotoxicity of noscapine-loaded nanoparticles prepared at noscapine concentrations of 5, 10, and 15 mg/mL, and compared them with drug-free nanoparticles. Both drug-loaded and drug-free nanoparticles were added in SK-BR-3 breast cancer cell lines over time periods of 24, 48, 72, 96, and 144 h (Figure 7). As expected the efficacy of the particles depended on the concentration of drug-loaded. Noscapine nanoparticles with concentrations of 5 mg/mL had the lowest efficacy with cell viability of 60% after 144 hrs. The cell viability was less than 35% for noscapine nanoparticles prepared with noscapine concentrations of 10 and 15 mg/mL after 144 h. The drug-free nanoparticles had little effect on the cell viability of the cells, and after 48 h the cell viability of cells with drug-free nanoparticles was approximately 93%. Gradually, the viability of cells treated with drug-free nanoparticles slightly decreased to about 80% after 144 h. Hence noscapine-loaded nanoparticles could potentially be used in the treatment of tumor cells.
Figure 7.
Breast cancer efficiency of human serum albumin (HSA) nanoparticles-loaded with noscapine (5–15 mg/mL concentration) and controls (drug-free nanoparticles). Nanoparticle concentrations of 50 μg/mL were seeded in SK-BR-3 breast cancer cell line incubated at 24, 48, 72, 96, and 144 h.
Conclusion
HSA nanoparticles can be successfully used to encapsulate noscapine and be prepared by the coacervation method. Preparation pH appeared to have an influence on size and particle yield; however it did not induce any effect in the drug-loading capacity. In addition, the in vitro drug delivery studies indicated controlled slow release profiles. In conclusion noscapine-loaded HSA nanoparticles have the potential to be used to deliver a maximum amount of noscapine to target sites at a rate and concentration that permits optimal therapeutic efficacy, while reducing any undesirable side effects to a minimum. Further experiments using surface modification of noscapine nanoparticles are being conducted for tumor cell-specific targeting applications, and for further evaluation of cell cytotoxicity and efficacy in cancer cells.
Acknowledgments
This work was supported by the Canadian Institute of Health Research (CHIR) MOP-86722 Research Grants to Dr Prakash. We also acknowledge Dr Maryam Tabrizian and Dr David Juncker for the use of their facilities. S Sebak acknowledges financial support from McGill University and a CGSM Scholarship from Natural Sciences and Engineering Research Council (NSERC), Canada. M Malhotra acknowledges the McGill Faculty of Medicine for Internal Medicine Studentship. A Kulamarva acknowledges a CGSD Scholarship NSERC, Canada. The authors would like to acknowledge the assistance provided by Ms Line Mongeon, McGill University, Canada for her assistance with the SEM studies.
References
- 1.Gradishar WJ. Albumin-bound nanoparticle paclitaxel. Clin Adv Hematol Oncol. 2005;3(5):348–349. [PubMed] [Google Scholar]
- 2.Lee MK, Lim SJ, Kim CK. Preparation, characterization and in vitro cytotoxicity of paclitaxel-loaded sterically stabilized solid lipid nanoparticles. Biomaterials. 2007;28(12):2137–2146. doi: 10.1016/j.biomaterials.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Damascelli B, Cantù G, Mattavelli F, et al. Intra-arterial chemotherapy with polyoxyethylated castor oil free paclitaxel, incorporated in albumin nanoparticles (ABI-007): Phase II study of patients with squamous cell carcinoma of the head and neck and anal canal: preliminary evidence of clinical activity. Cancer. 2001;92(10):2592–2602. doi: 10.1002/1097-0142(20011115)92:10<2592::aid-cncr1612>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Ke Y, Ye K, Grossniklaus HE, Archer DR, et al. Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunol Immunother. 2000;49(4–5):217–225. doi: 10.1007/s002620000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aneja R, Dhiman N, Idnani J, et al. Preclinical pharmacokinetics and bioavailability of noscapine, a tubulin-binding anticancer agent. Cancer Chemother Pharmacol. 2007;60(6):831–839. doi: 10.1007/s00280-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 6.Hiser L, Herrington B, Lobert S. Effect of noscapine and vincristine combination on demyelination and cell proliferation in vitro. Leuk Lymphoma. 2008;49(8):1603–1609. doi: 10.1080/10428190802213480. [DOI] [PubMed] [Google Scholar]
- 7.Jackson T, Chougule MB, Ichite N, Patlolla RR, Singh M. Antitumor activity of noscapine in human non-small cell lung cancer xenograft model. Cancer Chemother Pharmacol. 2008;63(1):117–126. doi: 10.1007/s00280-008-0720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dahlstrom B, Mellstrand T, Lofdahl C, Johansson M. Pharmacokinetic properties of noscapine. Eur J Clin Pharmacol. 1982;22(6):535–539. doi: 10.1007/BF00609627. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson MO, Dahlström B, Eckernäs SÅ, Johansson MA. Tufvesson Alm A. Pharmacokinetics of oral noscapine. Eur J Clin Pharmacol. 1990;39(3):275–279. doi: 10.1007/BF00315110. [DOI] [PubMed] [Google Scholar]
- 10.Winter CA, Flataker L. Toxicity studies on noscapine. Toxicol Appl Pharmacol. 1961;3(1):96–106. doi: 10.1016/0041-008x(61)90013-8. [DOI] [PubMed] [Google Scholar]
- 11.Newcomb EW, Lukyanov Y, Smirnova I, Schnee T, Zagzag D. Noscapine induces apoptosis in human glioma cells by an apoptosis-inducing factor-dependent pathway. Anticancer Drugs. 2008;19(6):553–563. doi: 10.1097/CAD.0b013e3282ffd68d. [DOI] [PubMed] [Google Scholar]
- 12.Aneja R, Vangapandu SN, Lopus M, et al. Synthesis of microtubule-interfering halogenated noscapine analogs that perturb mitosis in cancer cells followed by cell death. Biochem Pharmacol. 2006;72(4):415–426. doi: 10.1016/j.bcp.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Aneja R, Zhou J, Zhou B, Chandra R, Joshi HC. Treatment of hormone-refractory breast cancer: apoptosis and regression of human tumors implanted in mice. Mol Cancer Ther. 2006;5(9):2366–2377. doi: 10.1158/1535-7163.MCT-06-0205. [DOI] [PubMed] [Google Scholar]
- 14.Aneja R, Vangapandu SN, Lopus M, Chandra R, Panda D, Joshi HC. Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Mol Pharmacol. 2006;69(6):1801–1809. doi: 10.1124/mol.105.021899. [DOI] [PubMed] [Google Scholar]
- 15.Zhou J, Gupta K, Aggarwal S, et al. Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol. 2003;63(4):799–807. doi: 10.1124/mol.63.4.799. [DOI] [PubMed] [Google Scholar]
- 16.Hawkins MJ, Soon-Shiong P, Desai N. Protein nanoparticles as drug carriers in clinical medicine. Adv Drug Deliv Rev. 2008;60(8):876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Groneberg DA, Giersig M, Welte T, Pison U. Nanoparticle-based diagnosis and therapy. Curr Drug Targets. 2006;7(6):643–648. doi: 10.2174/138945006777435245. [DOI] [PubMed] [Google Scholar]
- 18.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54(5):631–651. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 19.Hung O. Drug transformation: Advances in drug delivery systems. Can J Anaesth. 2006;53(11):1074–1077. doi: 10.1007/BF03022873. [DOI] [PubMed] [Google Scholar]
- 20.Jiang W, Kim BY, Rutka JT, Chan WC. Advances and challenges of nanotechnology-based drug delivery systems. Expert Opin Drug Deliv. 2007;4(6):621–633. doi: 10.1517/17425247.4.6.621. [DOI] [PubMed] [Google Scholar]
- 21.Lazorthes Y, Sallerin-Caute B, Verdie JC, Bastide R. Advances in drug delivery systems and applications in neurosurgery. Adv Tech Stand Neurosurg. 1991;18:143–192. doi: 10.1007/978-3-7091-6697-0_5. [DOI] [PubMed] [Google Scholar]
- 22.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 23.Tokuda Y. Antibodies as molecular target-based therapy: trastuzumab. Int J Clin Oncol. 2003;8(4):224–229. doi: 10.1007/s10147-003-0334-8. [DOI] [PubMed] [Google Scholar]
- 24.Reddy KR. Controlled-release, pegylation, liposomal formulations: new mechanisms in the delivery of injectable drugs. Ann Pharmacother. 2000;34(7–8):915–923. doi: 10.1345/aph.10054. [DOI] [PubMed] [Google Scholar]
- 25.Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem. 2006;384(3):620–630. doi: 10.1007/s00216-005-0247-7. [DOI] [PubMed] [Google Scholar]
- 26.West JL, Halas NJ. Applications of nanotechnology to biotechnology commentary. Curr Opin Biotechnol. 2000;11(2):215–217. doi: 10.1016/s0958-1669(00)00082-3. [DOI] [PubMed] [Google Scholar]
- 27.Vogelson C. Advances in drug delivery systems. 2001. Available from: http://pubs.acs.org/subscribe/journals/mdd/v04/i04/html/MDD04FeatureVogelson.html.
- 28.Weber C, Kreuter J, Langer K. Desolvation process and surface characteristics of HSA-nanoparticles. Int J Pharm. 2000;196(2):197–200. doi: 10.1016/s0378-5173(99)00420-2. [DOI] [PubMed] [Google Scholar]
- 29.Lin W, Coombes AG, Davies MC, Davis SS, Illum L. Preparation of sub-100 nm human serum albumin nanospheres using a pH-coacervation method. J Drug Target. 1993;1(3):237–243. doi: 10.3109/10611869308996081. [DOI] [PubMed] [Google Scholar]
- 30.Lin W, Garnett MC, Davis SS, Schacht E, Ferruti P, Illum L. Preparation and characterisation of rose Bengal-loaded surface-modified albumin nanoparticles. J Control Release. 2001;71(1):117–126. doi: 10.1016/s0168-3659(01)00209-7. [DOI] [PubMed] [Google Scholar]
- 31.Merodio M, Arnedo A, Renedo MJ, Irache JM. Ganciclovir-loaded albumin nanoparticles: characterization and in vitro release properties. Eur J Pharm Sci. 2001;12(3):251–259. doi: 10.1016/s0928-0987(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 32.Yuan H, Miao J, Du YZ, You J, Hu FQ, Zeng S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int J Pharm. 2008;348(1–2):137–145. doi: 10.1016/j.ijpharm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 33.Ueno NT, Yu D, Hung MC. Chemosensitization of HER-2/neu-overexpressing human breast cancer cells to paclitaxel (Taxol) by adenovirus type 5 E1A. Oncogene. 1997;15(8):953–960. doi: 10.1038/sj.onc.1201250. [DOI] [PubMed] [Google Scholar]
- 34.Koziara JM, Lockman PR, Allen DD, Mumper RJ. Paclitaxel nanoparticles for the potential treatment of brain tumors. J Control Release. 2004;99(2):259–269. doi: 10.1016/j.jconrel.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Müller RH, Maassen S, Weyhers H, Mehnert W. Phagocytic uptake and cytotoxicity of solid lipid nanoparticles (SLN) sterically stabilized with poloxamine 908 and poloxamer 407. J Drug Target. 1996;4(3):161–170. doi: 10.3109/10611869609015973. [DOI] [PubMed] [Google Scholar]
- 36.Rubino OP, Kowalsky R, Swarbrick J. Albumin microspheres as a drug delivery system: relation among turbidity ratio, degree of cross-linking, and drug release. Pharm Res. 1993;10(7):1059–1065. doi: 10.1023/a:1018979126326. [DOI] [PubMed] [Google Scholar]