Abstract
The development of bleomycin-induced pulmonary fibrosis in rats was studied over a period of 21 d after an intratracheal instillation of bleomycin. The expression of three small proteoglycans (biglycan, decorin, and fibromodulin), collagen III and TGF-beta 1 was studied by RNA-transfer blot analysis. The proteoglycans were also studied by SDS-polyacrylamide gel electrophoresis and Western blots. TGF-beta 1 mRNA increased threefold already on day 3 and remained elevated until day 10. After the increase of TGF-beta 1 mRNA the messages for biglycan and collagen III steadily increased to reach a maximum 10 d after bleomycin instillation. The mRNA for biglycan increased maximally fourfold and that of collagen III 2.5-fold. Decorin mRNA, in contrast to biglycan decreased and reached 20% of control on day 10. The message for fibromodulin remained constant throughout the study period. The amounts of biglycan and decorin in the tissue changed in accordance with the mRNA levels. The results corroborate and extend previous in vitro studies concerning the effect of TGF-beta 1 on the metabolism of small proteoglycans and show that these macromolecules are regulated differently also in vivo. The marked alterations of biglycan and decorin during the development of fibrosis suggests that these proteoglycans have a regulating role in this process.
Full text
PDF

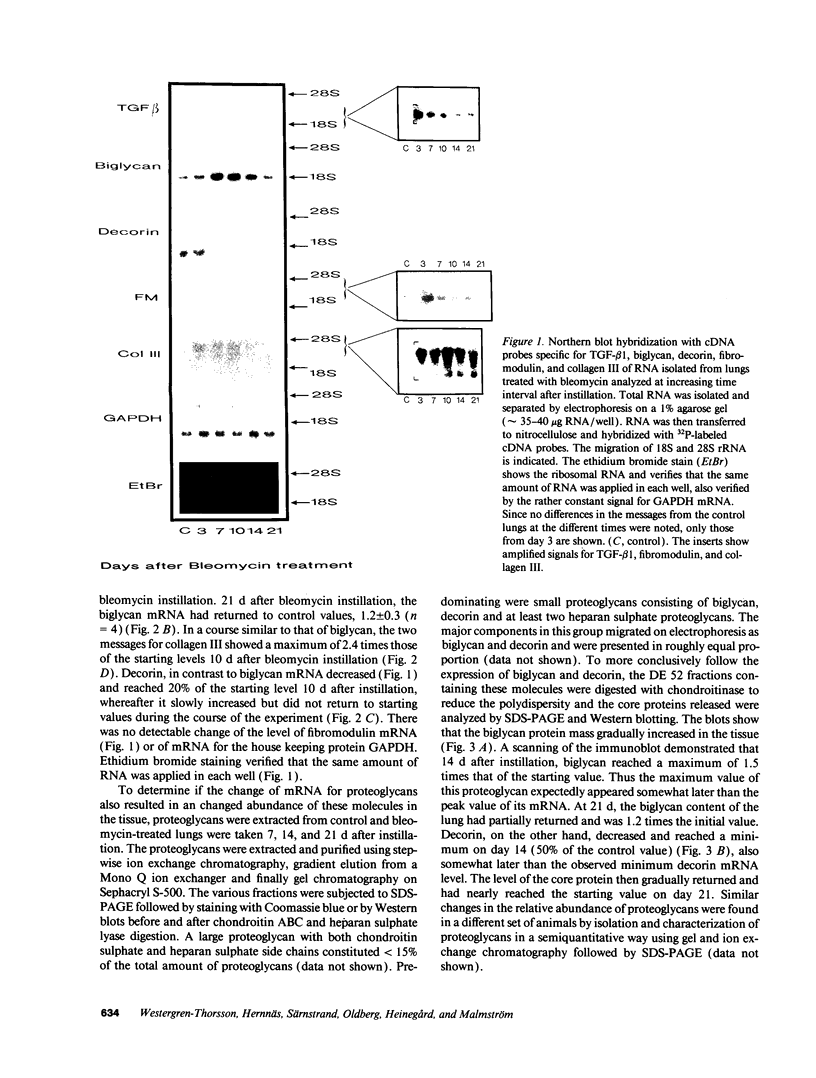
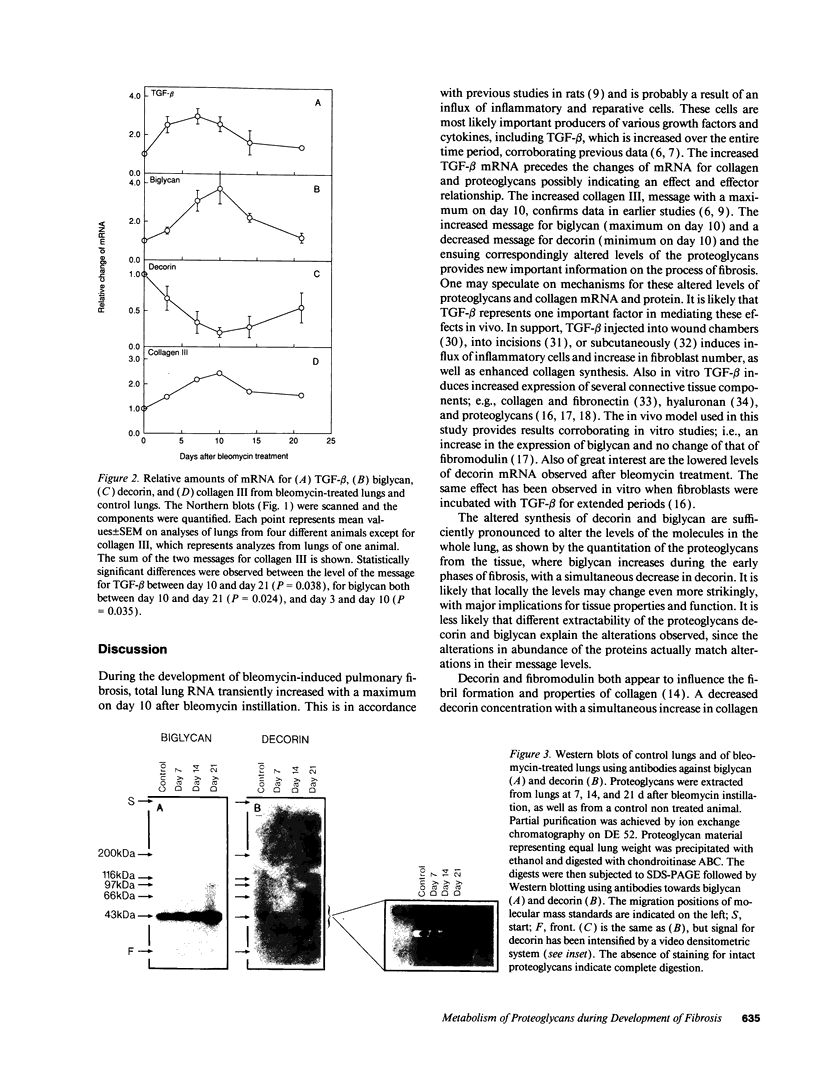


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Border W. A., Noble N. A., Yamamoto T., Tomooka S., Kagami S. Antagonists of transforming growth factor-beta: a novel approach to treatment of glomerulonephritis and prevention of glomerulosclerosis. Kidney Int. 1992 Mar;41(3):566–570. doi: 10.1038/ki.1992.83. [DOI] [PubMed] [Google Scholar]
- Broekelmann T. J., Limper A. H., Colby T. V., McDonald J. A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cromack D. T., Sporn M. B., Roberts A. B., Merino M. J., Dart L. L., Norton J. A. Transforming growth factor beta levels in rat wound chambers. J Surg Res. 1987 Jun;42(6):622–628. doi: 10.1016/0022-4804(87)90005-9. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Eaton D. H., Bell J. R., Assoian R. K., Roberts A. B., Sporn M. B., Goeddel D. V. Human transforming growth factor-beta complementary DNA sequence and expression in normal and transformed cells. Nature. 1985 Aug 22;316(6030):701–705. doi: 10.1038/316701a0. [DOI] [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernnäs J., Nettelbladt O., Bjermer L., Särnstrand B., Malmström A., Hällgren R. Alveolar accumulation of fibronectin and hyaluronan precedes bleomycin-induced pulmonary fibrosis in the rat. Eur Respir J. 1992 Apr;5(4):404–410. [PubMed] [Google Scholar]
- Hoyt D. G., Lazo J. S. Alterations in pulmonary mRNA encoding procollagens, fibronectin and transforming growth factor-beta precede bleomycin-induced pulmonary fibrosis in mice. J Pharmacol Exp Ther. 1988 Aug;246(2):765–771. [PubMed] [Google Scholar]
- Jensenius J. C., Andersen I., Hau J., Crone M., Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46(1):63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Kelley J., Chrin L., Shull S., Rowe D. W., Cutroneo K. R. Bleomycin selectively elevates mRNA levels for procollagen and fibronectin following acute lung injury. Biochem Biophys Res Commun. 1985 Sep 16;131(2):836–843. doi: 10.1016/0006-291x(85)91315-4. [DOI] [PubMed] [Google Scholar]
- Khalil N., Bereznay O., Sporn M., Greenberg A. H. Macrophage production of transforming growth factor beta and fibroblast collagen synthesis in chronic pulmonary inflammation. J Exp Med. 1989 Sep 1;170(3):727–737. doi: 10.1084/jem.170.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J. Fibrogenic cytokines: the role of immune mediators in the development of scar tissue. Immunol Today. 1991 Jan;12(1):17–23. doi: 10.1016/0167-5699(91)90107-5. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Larjava H., Uitto J. Differential regulation of extracellular matrix proteoglycan (PG) gene expression. Transforming growth factor-beta 1 up-regulates biglycan (PGI), and versican (large fibroblast PG) but down-regulates decorin (PGII) mRNA levels in human fibroblasts in culture. J Biol Chem. 1991 Jun 5;266(16):10608–10615. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazenby A. J., Crouch E. C., McDonald J. A., Kuhn C., 3rd Remodeling of the lung in bleomycin-induced pulmonary fibrosis in the rat. An immunohistochemical study of laminin, type IV collagen, and fibronectin. Am Rev Respir Dis. 1990 Jul;142(1):206–214. doi: 10.1164/ajrccm/142.1.206. [DOI] [PubMed] [Google Scholar]
- Lindblom A., Carlstedt I., Fransson L. A. Identification of the core proteins in proteoglycans synthesized by vascular endothelial cells. Biochem J. 1989 Jul 1;261(1):145–153. doi: 10.1042/bj2610145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe T. A., Pierce G. F., Thomason A., Gramates P., Sporn M. B., Deuel T. F. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987 Sep 11;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- Nettelbladt O., Bergh J., Schenholm M., Tengblad A., Hällgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989 Mar;139(3):759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- Norman M., Ekman G., Ulmsten U., Barchan K., Malmström A. Proteoglycan metabolism in the connective tissue of pregnant and non-pregnant human cervix. An in vitro study. Biochem J. 1991 Apr 15;275(Pt 2):515–520. doi: 10.1042/bj2750515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. A collagen-binding 59-kd protein (fibromodulin) is structurally related to the small interstitial proteoglycans PG-S1 and PG-S2 (decorin). EMBO J. 1989 Sep;8(9):2601–2604. doi: 10.1002/j.1460-2075.1989.tb08399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B., Assoian R. K., Smith J. M., Roche N. S., Wakefield L. M., Heine U. I., Liotta L. A., Falanga V., Kehrl J. H. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Vuorio E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J Cell Biol. 1987 Apr;104(4):1077–1084. doi: 10.1083/jcb.104.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall R. S., Barton R. W., D'Amato D. A., Sulavik S. B. Differential cellular analysis of bronchoalveolar lavage fluid obtained at various stages during the development of bleomycin-induced pulmonary fibrosis in the rat. Am Rev Respir Dis. 1982 Sep;126(3):488–492. doi: 10.1164/arrd.1982.126.3.488. [DOI] [PubMed] [Google Scholar]
- Varga J., Rosenbloom J., Jimenez S. A. Transforming growth factor beta (TGF beta) causes a persistent increase in steady-state amounts of type I and type III collagen and fibronectin mRNAs in normal human dermal fibroblasts. Biochem J. 1987 Nov 1;247(3):597–604. doi: 10.1042/bj2470597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M. Host immune factors regulating fibrosis. Ciba Found Symp. 1985;114:175–195. doi: 10.1002/9780470720950.ch12. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Antonsson P., Malmström A., Heinegård D., Oldberg A. The synthesis of a family of structurally related proteoglycans in fibroblasts is differently regulated by TFG-beta. Matrix. 1991 Jun;11(3):177–183. doi: 10.1016/s0934-8832(11)80156-3. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Onnervik P. O., Fransson L. A., Malmström A. Proliferation of cultured fibroblasts is inhibited by L-iduronate-containing glycosaminoglycans. J Cell Physiol. 1991 Jun;147(3):523–530. doi: 10.1002/jcp.1041470319. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Schmidtchen A., Särnstrand B., Fransson L. A., Malmström A. Transforming growth factor-beta induces selective increase of proteoglycan production and changes in the copolymeric structure of dermatan sulphate in human skin fibroblasts. Eur J Biochem. 1992 Apr 1;205(1):277–286. doi: 10.1111/j.1432-1033.1992.tb16778.x. [DOI] [PubMed] [Google Scholar]
- Westergren-Thorsson G., Särnstrand B., Fransson L. A., Malmström A. TGF-beta enhances the production of hyaluronan in human lung but not in skin fibroblasts. Exp Cell Res. 1990 Jan;186(1):192–195. doi: 10.1016/0014-4827(90)90227-2. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]





