Abstract
Dynamic contrast-enhanced magnetic resonance imaging has been recently shown to be effective for diagnostic urography. High-resolution urographic images can be acquired with T1 contrast agents for the kidney and urinary tract with minimal noise in the abdomen. Currently, clinical contrast agents are low molecular weight agents and can rapidly extravasate from blood circulation, leading to slow contrast agent elimination through kidney and consequently providing limited contrast enhancement in urinary tract. In this study, a new biodegradable macromolecular contrast agent, nanoglobule-G4-cystamine-(Gd-DO3A), was prepared by conjugating Gd-DO3A chelates on the surface of a generation 4 nanoglobule, poly-l-lysine octa(3-aminopropyl)silsesquioxane dendrimer, via a disulfide spacer, where the carrier had a precisely defined nanosize that is far smaller than the renal filtration threshold. The in vivo contrast enhancement and dynamic imaging of the urinary tract of the agent was evaluated in nude mice using a low molecular weight agent Gd(DTPA-BMA) as a control. The agent eliminated rapidly from blood circulation and accumulated more abundantly in urinary tract than Gd(DTPA-BMA). The fast elimination kinetics is ideal for functional evaluation of the kidneys. The morphology of the kidneys and urinary tract was better visualized by the biodegradable nanoglobular contrast agent than Gd(DTPA-BMA). The agent also resulted in low liver contrast enhancement, indicating low nonspecific tissue deposition. These features render the G4 nanoglobule-cystamine-(Gd-DO3A) conjugate a promising contrast agent for magnetic resonance urography.
Keywords: dendrimer, gadolinium(III) contrast agent, disulfide bond, nanoparticle
Introduction
Morphological and functional imaging of the kidneys and urinary tract is crucial for accurate diagnosis of diseases in the urinary system and for better therapeutic decisions to treat patients with urological disorders. Ultrasonography and computed tomography (CT) are the commonly used diagnostic modalities for morphological imaging of the urinary system.1–4 CT with a large dose of iodinated contrast agents and diuretic renal scintigraphy using radioactive probes containing Tc-99m are often used for functional renal imaging.5–7 However, these modalities provide limited information for accurate diagnosis due to poor image qualities. Magnetic resonance (MR) urography produces morphological images with excellent spatial and contrast resolution.8–10 However, the fluid in the abdomen severely interferes the imaging contrast in the ureter and the bladder in T2-weighted MR urography. T1-weighted dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) using gadolinium-based contrast agents can improve the contrast enhancement in the urinary tract with minimized interference from the fluid in the abdomen.11–15 Meanwhile, the time course of signal intensity generated by the contrast agents in DCE MRI can be correlated to the excretory function of the kidneys.16–20 Currently, clinical contrast agents are low molecular weight molecules and extravasate rapidly from blood circulation and distribute throughout the extracellular space of surrounding tissues.21 Consequently, their rapid extravasation slows down the renal excretory rate leading to unfavorable pharmacokinetics in the urinary systems.
Macromolecular contrast agents, eg, gadolinium-bound albumin, were tested in MRI of urinary system.22 These agents have a long blood circulation suitable for kidney angiography. However, they are not suitable for urography because their renal excretion is limited by their large hydrodynamic size. Dendrimers are the macromolecules with defined structures and sizes and have been used to develop MRI contrast agents with different sizes. For example, poly-amidoamine (PAMAM)-based Gd(III) complexes have shown size-dependent pharmacokinetics and renal filtration.23 However, the PAMAM-based agents with the positively charged cores are associated with high acute systemic toxicity.24 In our previous study, a series of nanoglubular dendrimers, poly-l-lysine octa(3-aminopropyl) silsesquioxane (OAS) dendrimers, were developed, which displayed 3-dimensional (3D) symmetric structures, single molecular weight distributions, and low cytotoxicity.25 The nanoglobule-based MRI contrast agents showed relatively rapid renal filtration and low liver uptake,26 indicating the nanoglobular dendrimers as suitable carriers for urographic contrast agents.
In this study, we report the preparation and in vivo evaluation of a generation 4 (G4) nanoglobule-cystamine-(Gd-DO3A) conjugate as an extracellular degradable nano-sized contrast agent for DCE-MR urography. A disulfide spacer (–SS–) was introduced to accelerate the renal excretion of gadolinium chelates by reducing the disulfide spacer with the free endogenous thiols, including cysteine, glutathione (GSH), cysteinylglycine (Cys–Gly), and homocysteine in plasma.24,27–29 Since the concentration of thiols is relatively low (approximately 50 μM in mice), the spacer is gradually reduced in the circulation. The stable Gd-DO3A chelates released from the biodegradable nanoglobular contrast agent can rapidly transit through renal filtration and accumulate in the urinary tract with sufficient concentration for effective DCE-MR urography. The efficacy of the G4 nanoglobule-cystamine-(Gd-DO3A) conjugate for dynamic MRI of the urinary tract was evaluated in nude mice using a low molecular weight agent Gd(DTPA-BMA) as a control.
Materials and methods
Materials
Gadolinium acetate (Alfa Aesar, Ward Hill, MA), ethylenediaminetetraacetic acid (EDTA) dipotassium salt (Sigma-Aldrich, St. Louis, MO), Gd(DTPA-BMA; GE Healthcare, Amersham, UK), Spectra/Por 6 dialysis membrane (molecular weight cutoff 2 kDa; Spectrum Laboratories, Rancho Dominguez, CA, USA) were used as purchased. The G4 nanoglobule, G4 poly-l-lysine OAS dendrimer, was prepared according to a reported procedure.25 1,4,7,10-Tetraazacyclododecane-1,4,7-tris-(acetic acid)-10-(acetic acid isothiocyanatocystamine monoamide) (DO3A-SS-NCS) was synthesized according to a reported method.24,30 The nu/nu mice were purchased from the NCI (Frederick, MD) and were cared under an animal protocol approved by the University of Utah Institutional Animal Care and Use Committee.
Synthesis procedure
The G4 poly-l-lysine OAS dendrimer was dissolved in water at a concentration of 10 mg/mL. DO3A-SS-NCS (128 molar excess) was then added into the solution with pH adjusted to 9 by 1.0 M NaOH. The reaction mixture was stirred at room temperature (25°C) for 24 hours. Another 128 molar excess of DO3A-SS-NCS was added to the mixture, which was stirred for additional 24 hours. The product, G4 nanogobule-cystamine-DO3A conjugate, was purified by dialysis against deionized water for 48 hours and colorless solid was obtained after lyophilization. The nanoglobular ligand was then complexed with a 512-fold excess of Gd(OAc)3 based on the amount of nanoglobular conjugate in citric acid buffer (pH 5) for 72 hours at room temperature. EDTA was added to the mixture, which was stirred for additional 1 hour to remove free Gd(III) ions associated with the conjugate. The mixture was dialyzed against deionized water for 48 hours. The final product was obtained after lyophilization. The gadolinium contents in the conjugates were measured by inductively coupled plasma optical emission spectroscopy (ICP-OES; Perkin-Elmer Optima 3100XL, Norwalk, CT) at 342 nm. The size of the contrast agent was estimated by dynamic light scattering.
Relaxivities
The T1 relaxation time was measured using a standard inversion recovery-prepared turbo spin echo sequence on a Siemens Trio 3T MRI scanner at room temperature. Three different concentrations of each sample were prepared in triplicate and placed inside a birdcage human head coil and scanned with a series of inversion times (TIs) from 22 ms to 1,600 ms. The net magnetization (MTI) at each TI was calculated from the region of interest (ROI) using Osirix (http://www.osirix-viewer.com) software. T1 relaxation time was calculated by nonlinear regression fitting of Equation 1:
| (1) |
where MTI is the net magnetization at time TI; TI is the inversion time; M0 is the initial magnetization. Relaxivity r1 was determined as the slope of the plot of 1/T1 vs [Gd3+].
MR imaging
The MRI contrast enhancement of the biodegradable nano-globular agent was evaluated in female nu/nu mice. The mice (10–12 weeks, 25–30 g) were anesthetized by intraperitoneal administration of a mixture of xylazine (12 mg/kg) and ketamine (80 mg/kg). The biodegradable nanoglobular contrast agent was administered intravenously through a tail vein at a dose of 0.033 mMol Gd/kg, whereas Gd(DTPA-BMA) was injected at a dose of 0.1 mMol Gd/kg. Three mice were used for each agent. The mice were placed in a wrist coil and the MR images were acquired before and 2, 5, 10, 15 minutes after administration of the contrast agents on a Siemens Trio 3T scanner. A 3D-FLASH pulse sequence was used to acquire whole body images. The parameters of 3D-FLASH sequence were 2.74 ms echo time, 7.75 ms repetition time, 25 flip angle, 120 mM field of view, and 0.4 mM coronal slice thickness. MR images were analyzed with Osirix (http://homepage.mac.com/rossetantoine/osirix/) software. The ROIs in each mouse were set at the left ventricle of the heart, liver, and bladder. The contrast to noise ratio (CNR) was calculated by the equation: CNR = (SPost − SPre)/((δpost + δpre)/2), where SPost and SPre represent the mean signal intensities at the ROI before and after administration of contrast agents, and the δpost and δpre are the standard deviation of background noises before and after contrast agent administration.
Student’s t-test was performed with Prism software (GraphPad Software, San Diego, CA), with significance level set at P < 0.05.
Results
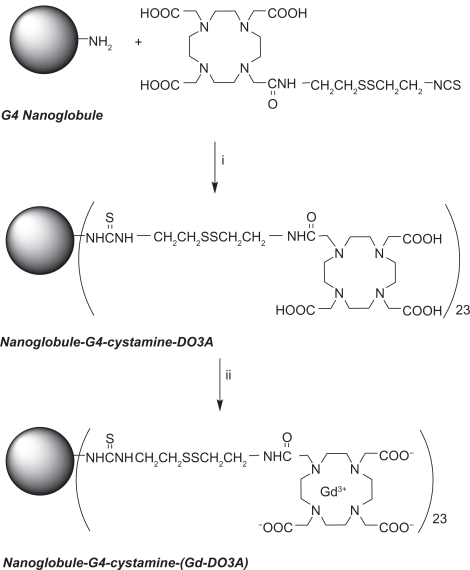
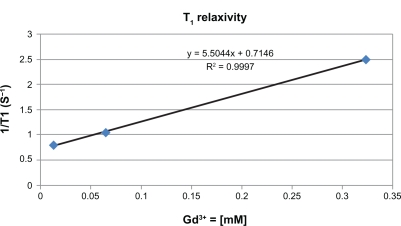
The G4 nanoglobule has 4 layers of lysine residues rooted from each of the amine groups at the 8 corners of the cubic OAS core. It has a compact and relatively symmetric 3D molecular architecture and is a suitable carrier for macromolecular MRI contrast agent with a precisely defined structure.25 The mass (m/z) of the G4 nanoglobule was 16,314 as determined by MALDI-TOF mass spectrometry, slightly greater than the calculated mass (16,263) possibly due to the incorporation of solvent molecules in the structure.25 The synthesis of the G4 nanoglobular contrast agent is described in Scheme 1. The gadolinium(III) content in nanoglobule-G4-cystamine-(Gd-DO3A) is 0.742 mMol/g with a conjugation degree of 18.0%. The calculated molecular weight of the nanoglobular contrast agent was 33287.96 Da based on the measured Gd(III) contents in the agent. The size of the agent was approximately 6.0 nm as determined by light scattering. The T1 relaxivity of the agent was 5.5 mM−1 s−1 at 3 T (Figure 1).
Scheme 1.
Synthesis of nanoglobule-G4-cystamine-(Gd-DO3A). (i) H2O, pH 9.0, 72 hours; (ii) gadolinium acetate, pH 5.0, 72 hours; EDTA, 1 hour.
Figure 1.
T1 relaxivity of nanoglobule-G4-cystamine-(Gd-DO3A).
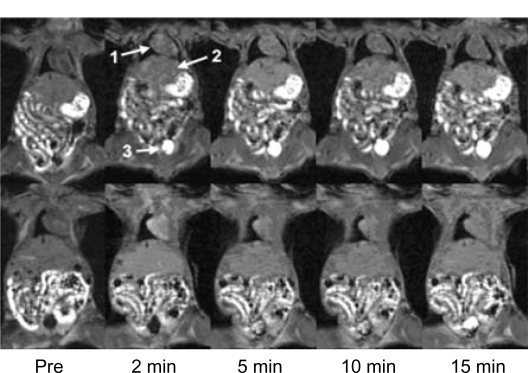
The DCE-MR urography was evaluated in female nude mice. Figure 2 shows the 2-dimensional (2D) coronal images crossing the heart and bladder before contrast and at various time points after contrast for nanoglobule-G4-cystamine-(Gd-DO3A) and Gd(DTPA-BMA). The bladder was visualized as a dark area with vague margins before the administration of the contrast agents. Strong signal intensities were observed in the bladder from 2 minutes and throughout the period of experiments after the injection of nanoglobule-G4-cystamine-(Gd-DO3A) at 0.033 mMol Gd/kg. The enhanced volume of the bladder gradually increased over time. The agent provided sufficient contrast enhancement for MRI of the urinary systems that overcame the noise from the fat tissue and gut in the abdomen. In comparison, Gd(DTPA-BMA) at 0.1 mmol/kg could not result in clear visualization of the bladder. The bladder was barely visible at 5 and 10 minutes after the injection of Gd(DTPA-BMA). Strong bladder enhancement was only observed at 15 minutes after administration, whereas the margin of the bladder was still vague. The low contrast enhancement in the bladder indicates low concentration of Gd(DTPA-BMA) in the urinary collecting system, resulting in poor image qualities for anatomic urological diagnosis.
Figure 2.
Contrast-enhanced coronal magnetic resonance images of nude mice before (pre) and at various time points after intravenous injection of nanoglobule-G4-cystamine-(Gd-DO3A; top panel) and Gd(DTPA-BMA; lower panel). Arrows point to the heart (1), liver (2), and bladder (3).
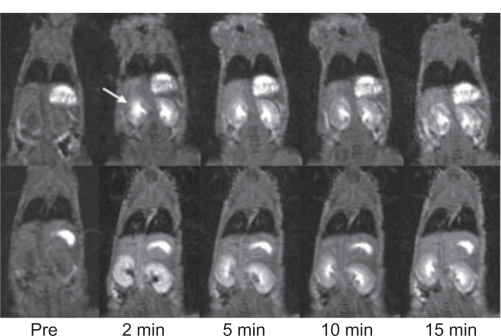
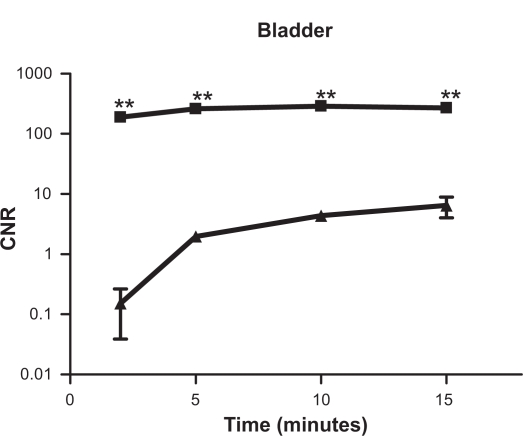
The functional information of renal excretion can be obtained from the DCE-MR images in ROIs in the urinary system. Figure 3 shows the 2D coronal images cross the kidneys. Both nanoglobule-G4-cystamine-(Gd-DO3A) and Gd(DTPA-BMA) resulted in significant contrast enhancement in the kidneys. However, the contrast enhancement by Gd(DTPA-BMA) limited to the renal cortex in early minutes postinjection and gradually shifted into the renal pelvis, whereas the enhancement with nanoglobule-G4-cys tamine-(Gd-DO3A) mainly located in the renal pelvis since 2 minutes postinjection, indicating faster renal excretion rate. The renal transit time, the time between the arrival of contrast materials in the cortex and its arrival in the ureter, is often used in scintigraphy to characterize diuretic renal function and to correlate to the washout half-life.31 Figure 3 clearly visualizes the shorter renal transit time of nanoglobule-G4-cystamine-(Gd-DO3A). Figure 4 shows the time course of CNRs in the bladder. The nanoglobule-G4-cystamine-(Gd-DO3A) resulted in much stronger signal intensities in the bladder at a much lower dose than Gd(DTPA-BMA) soon after the injection, validating faster renal elimination kinetics of nanoglobule-G4-cystamine-(Gd-DO3A).
Figure 3.
Contrast-enhanced coronal magnetic resonance images of nude mice before (pre) and at various time points after intravenous injection of nanoglobule-G4-cystamine-(Gd-DO3A; top panel) and Gd(DTPA-BMA; lower panel). Arrows point to the kidney.
Figure 4.
Semilogarithmic plots of contrast-enhanced signal intensities in the bladder before and at various time points after the injection of (▴) Gd(DTPA-BMA) and (▪) nanoglobule-G4-cystamine-(Gd-DO3A; n = 3, Student’s t-test, **P < 0.002).
Abbreviation: CNR, contrast to noise ratio.
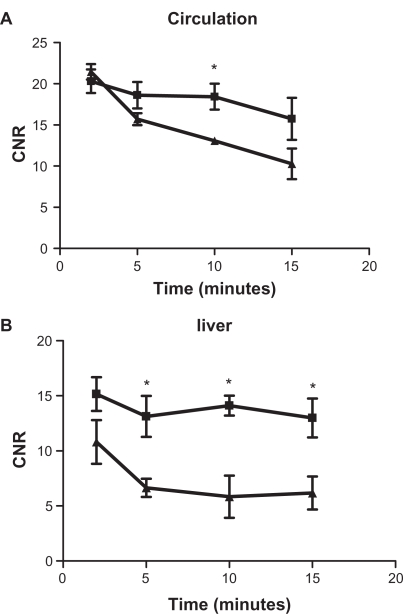
Figure 5 displays the time courses of CNR in blood and the liver before and after the injection of the contrast agents. Nanoglobule-G4-cystamine-(Gd-DO3A) eliminated from blood circulation at a relatively slower rate than Gd(DTPA-BMA; Figure 5A). Nanoglobule-G4-cystamine-(Gd-DO3A) resulted in higher contrast enhancement than Gd(DTPA-BMA) in the liver (Figure 5B). However, the difference of the CNRs between 2 agents in the liver was still in the same order of magnitude and much smaller than their CNR difference in the bladder (Figure 4). The results indicated that nanoglobule-G4-cystamine-(Gd-DO3A) extravasated slowly from blood circulation and had minimal nonspecific liver uptake. Therefore, majority of injected nanoglobule-G4-cystamine-(Gd-DO3A) excreted rapidly via renal filtration, resulting in strong signal intensities in the kidneys and the urinary system.
Figure 5.
Contrast-enhanced signal intensities in the blood of left ventricle (A) and liver (B) before and at various time points after the injection of (▴) Gd(DTPA-BMA) and (▪) nanoglobule-G4-cystamine-(Gd-DO3A; n = 3, Student’s t-test, *P < 0.05).
Abbreviation: CNR, contrast to noise ratio.
Discussion
The nanoglobules, polylysine dendrimers with the cubic silsesquioxane core, has a compact and 3D symmetric molecular architecture and precisely defined nanosizes.25 They may be advantageous over the conventional dendrimers, such as PAMAM, as carriers for macromolecular MRI contrast agents. Although PAMAM dendrimers have precisely defined structures and sizes, and have been used as carriers for MRI contrast agents,32 their structure is still flexible and the contrast agents based on PAMAM have a highly positively charged interior structure, which may affect their in vivo biological properties. We have recently observed that a macromolecular contrast agent based on PAMAM, PAMAM-G6-(Gd-DO3A), had high liver uptake, and its corresponding biodegradable macromolecular agent, PAMAM-G6-cystamine-(Gd-DO3A), resulted in death of experimental animal within 24 hours postadministration possibly due to the systemic toxicity of the PAMAM dendrimer.24 The G1, G2, and G3 nanoglobular MRI contrast agents have neutral interior structure and have shown size-dependent in vivo contrast enhancement, minimal liver enhancement, and significant tumor enhancement in a mouse tumor model. They showed size-dependent renal filtration and elimination rate decreased with increasing size from G1 to G3 agents.26 The G4 nanoglobule was chosen in this study as the carrier for the biodegradable nanoglobular contrast agents to take advantage of its high surface-loading capability.
Effective DCE-MR urography needs a sufficient amount of contrast agents passing through the urinary tract to be eliminated in urine in a relatively short timeframe. Low molecular weight MRI contrast agents rapidly extravasate into extracellular interstitium and, therefore, have a slow renal excretion rate. Macromolecular contrast agents generally have limited extravasation, but they also result in limited enhancement in the urinary tract because their large sizes lead to poor renal filtration. The conjugation of Gd(III)-DO3A chelates on the surface of the G4 nanoglobular carrier via the cleavable disulfide spacer substantially facilitate elimination of Gd(III)-chelate via renal filtration, resulting in significant dynamic enhancement in the urinary tract. The renal clearance of Gd-DO3A chelates from the nanoglobule-G4-cystamine-(Gd-DO3A) conjugate was faster than the nondegradable G2 and G3 nanoglobule-(Gd-DO3A) conjugates of smaller sizes.26 The cleavage disulfide spacer in the conjugate by the endogenous thiols in the plasma resulted in fast blood-pool clearance, less liver deposit, and enhanced renal filtration of Gd(III) chelates. As a result, strong enhancement was shown in the urinary tract with nanoglobule-G4-cystamine-(Gd-DO3A) conjugate as fast as 2 minutes postadministration. The G4 biodegradable nanoglobular contrast agent also demonstrated faster renal excretion kinetics than low molecular weight Gd(DTPA-BMA). Since Gd(III) chelates of the biodegradable nanoglobular contrast agent can be rapidly excreted via renal filtration, it may minimize tissue Gd(III) accumulation of the agent. Since the size of the nanoglobular agent is smaller than the renal filtration threshold (8 nm),33 the remaining nanoglobules can be gradually excreted via renal filtration. The biodegradable nanoglobular contrast agent is promising for further development for clinical MR urography.
This is the first attempt to develop biodegradable nanoglobular contrast agents with fast renal elimination for effective DCE-MR urography. The study is limited in several areas. The conjugation efficiency of Gd-DO3A to the surface of nanoglobules was relatively low in the agent. Further optimization is needed to increase the conjugation efficiency on the agent. The elimination mechanism of the biodegradable nanoglobular contrast agent needs to be further elucidated to better understand the correlation of the elimination of the agent with renal functions and to establish criteria for contrast-enhanced MR urography with the contrast agent. Further detailed preclinical studies, including pharmacokinetics, biodistribution, and toxicity, of the agent and the clearance of G4 nanoglobule are needed before further consideration for clinical development.
Conclusion
In summary, the biodegradable nanoglobular MRI contrast agent, nanoglobule-G4-cystamine-(Gd-DO3A), was synthesized for contrast-enhanced MR urography. The preliminary study has shown that the agent had faster elimination kinetics in the urinary tract than a low molecular weight clinical agent Gd(DTPA-BMA). The fast elimination kinetics is suitable for functional evaluation of the kidneys. The high concentration of the contrast agent in the urinary tract resulted in strong enhancement for effective morphological imaging of the tract with minimal noise from fluid in the abdomen. These features render nanoglobule-G4-cystamine-(Gd-DO3A) a suitable contrast agent for effective contrast-enhanced MR urography without diuretics.
Acknowledgments
We thank Dr Yongen Sun for his support in animal handling. This work was supported in part by the NIH R01 CA097465.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Browne RF, Zwirewich C, Torreggiani WC. Imaging of urinary tract infection in the adult. Eur Radiol. 2004;14(Suppl 3):E168–E183. doi: 10.1007/s00330-003-2050-1. [DOI] [PubMed] [Google Scholar]
- 2.Jankowski JT, Spirnak JP. Current recommendations for imaging in the management of urologic traumas. Urol Clin North Am. 2006;33(3):365–376. doi: 10.1016/j.ucl.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 3.Kawashima A, Sandler CM, Corl FM, et al. Imaging of renal trauma: a comprehensive review. Radiographics. 2001;21(3):557–574. doi: 10.1148/radiographics.21.3.g01ma11557. [DOI] [PubMed] [Google Scholar]
- 4.Palmer LS. Pediatric urologic imaging. Urol Clin North Am. 2006;33(3):409–423. doi: 10.1016/j.ucl.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Hilson AJ. Functional renal imaging with nuclear medicine. Abdom Imaging. 2003;28(2):176–179. doi: 10.1007/s00261-001-0184-7. [DOI] [PubMed] [Google Scholar]
- 6.Meller J, Sahlmann CO, Becker W. Nuclear medicine studies in the dialysis patient. Semin Dial. 2002;15(4):269–276. doi: 10.1046/j.1525-139x.2002.00069.x. [DOI] [PubMed] [Google Scholar]
- 7.Sfakianakis GN, Sfakianaki E. Renal scintigraphy in infants and children. Urology. 2001;57(6):1167–1177. doi: 10.1016/s0090-4295(01)01009-3. [DOI] [PubMed] [Google Scholar]
- 8.O’Malley ME, Soto JA, Yucel EK, et al. MR urography: evaluation of a three-dimensional fast spin-echo technique in patients with hydronephrosis. AJR Am J Roentgenol. 1997;168(2):387–392. doi: 10.2214/ajr.168.2.9016213. [DOI] [PubMed] [Google Scholar]
- 9.Rothpearl A, Frager D, Subramanian A, et al. MR urography: technique and application. Radiology. 1995;194(1):125–130. doi: 10.1148/radiology.194.1.7997538. [DOI] [PubMed] [Google Scholar]
- 10.Hussain S, O’Malley M, Jara H, et al. MR urography. Magn Reson Imaging Clin N Am. 1997;5(1):95–106. [PubMed] [Google Scholar]
- 11.Rohrschneider WK, Haufe S, Wiesel M, et al. Functional and morphologic evaluation of congenital urinary tract dilatation by using combined static-dynamic MR urography: findings in kidneys with a single collecting system. Radiology. 2002;224(3):683–694. doi: 10.1148/radiol.2243011207. [DOI] [PubMed] [Google Scholar]
- 12.Nolte-Ernsting CC, Bucker A, Adam GB, et al. Gadolinium-enhanced excretory MR urography after low-dose diuretic injection: comparison with conventional excretory urography. Radiology. 1998;209(1):147–157. doi: 10.1148/radiology.209.1.9769826. [DOI] [PubMed] [Google Scholar]
- 13.Kikinis R, von Schulthess GK, Jager P, et al. Normal and hydronephrotic kidney: evaluation of renal function with contrast-enhanced MR imaging. Radiology. 1987;165(3):837–842. doi: 10.1148/radiology.165.3.3685363. [DOI] [PubMed] [Google Scholar]
- 14.Laissy JP, Faraggi M, Lebtahi R, et al. Functional evaluation of normal and ischemic kidney by means of gadolinium-DOTA enhanced Turbo-FLASH MR imaging: a preliminary comparison with 99Tc-MAG3 dynamic scintigraphy. Magn Reson Imaging. 1994;12(3):413–419. doi: 10.1016/0730-725x(94)92534-8. [DOI] [PubMed] [Google Scholar]
- 15.Semelka RC, Hricak H, Tomei E, et al. Obstructive nephropathy: evaluation with dynamic Gd-DTPA-enhanced MR imaging. Radiology. 1990;175(3):797–803. doi: 10.1148/radiology.175.3.2343131. [DOI] [PubMed] [Google Scholar]
- 16.Carvlin MJ, Arger PH, Kundel HL, et al. Use of Gd-DTPA and fast gradient-echo and spin-echo MR imaging to demonstrate renal function in the rabbit. Radiology. 1989;170(3 Pt 1):705–711. doi: 10.1148/radiology.170.3.2916024. [DOI] [PubMed] [Google Scholar]
- 17.Choyke PL, Frank JA, Girton ME, et al. Dynamic Gd-DTPA-enhanced MR imaging of the kidney: experimental results. Radiology. 1989;170(3 Pt 1):713–720. doi: 10.1148/radiology.170.3.2916025. [DOI] [PubMed] [Google Scholar]
- 18.Frank JA, Choyke PL, Austin HA, et al. Functional MR of the kidney. Magn Reson Med. 1991;22(2):319–323. doi: 10.1002/mrm.1910220233. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda Y, Watanabe H, Tomita T, et al. Evaluation of glomerular function in individual kidneys using dynamic magnetic resonance imaging. Pediatr Radiol. 1996;26(5):324–328. doi: 10.1007/BF01395707. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Valtuille R, Garcia-Valtuille AI, Abascal F, et al. Magnetic resonance urography: a pictorial overview. Br J Radiol. 2006;79(943):614–626. doi: 10.1259/bjr/21075982. [DOI] [PubMed] [Google Scholar]
- 21.Grenier N, Pedersen M, Hauger O. Contrast agents for functional and cellular MRI of the kidney. Eur J Radiol. 2006;60(3):341–352. doi: 10.1016/j.ejrad.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Choyke PL, Kobayashi H. Functional magnetic resonance imaging of the kidney using macromolecular contrast agents. Abdom Imaging. 2006;31(2):224–231. doi: 10.1007/s00261-005-0390-9. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57(15):2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Xu R, Wang Y, Wang X, et al. In vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp Biol Med (Maywood) 2007;232(8):1081–1089. doi: 10.3181/0702-RM-33. [DOI] [PubMed] [Google Scholar]
- 25.Kaneshiro TL, Wang X, Lu ZR. Synthesis, characterization, and gene delivery of poly-L-lysine octa(3-aminopropyl)silsesquioxane dendrimers: nanoglobular drug carriers with precisely defined molecular architectures. Mol Pharm. 2007;4(5):759–768. doi: 10.1021/mp070036z. [DOI] [PubMed] [Google Scholar]
- 26.Kaneshiro TL, Jeong EK, Morrell G, et al. Synthesis and evaluation of globular Gd-DOTA-monoamide conjugates with precisely controlled nanosizes for magnetic resonance angiography. Biomacromolecules. 2008;9(10):2742–2748. doi: 10.1021/bm800486c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andersson A, Lindgren A, Hultberg B. Effect of thiol oxidation and thiol export from erythrocytes on determination of redox status of homocysteine and other thiols in plasma from healthy subjects and patients with cerebral infarction. Clin Chem. 1995;41(3):361–366. [PubMed] [Google Scholar]
- 28.Lu ZR, Mohs AM, Zong Y, et al. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. Int J Nanomedicine. 2006;1(1):31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zong Y, Wang X, Jeong EK, et al. Structural effect on degradability and in vivo contrast enhancement of polydisulfide Gd(III) complexes as biodegradable macromolecular MRI contrast agents. Magn Reson Imaging. 2009;27(4):503–511. doi: 10.1016/j.mri.2008.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke T, Feng Y, Guo J, et al. Biodegradable cystamine spacer facilitates the clearance of Gd(III) chelates in poly(glutamic acid) Gd-DO3A conjugates for contrast-enhanced MR imaging. Magn Reson Imaging. 2006;24(7):931–940. doi: 10.1016/j.mri.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Jones RA, Perez-Brayfield MR, Kirsch AJ, et al. Renal transit time with MR urography in children. Radiology. 2004;233(1):41–50. doi: 10.1148/radiol.2331031117. [DOI] [PubMed] [Google Scholar]
- 32.Venditto VJ, Regino CA, Brechbiel MW. PAMAM dendrimer based macromolecules as improved contrast agents. Mol Pharm. 2005;2(4):302–311. doi: 10.1021/mp050019e. [DOI] [PubMed] [Google Scholar]
- 33.Longmire M, Choyke P, Kobayashi H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine. 2008;3(5):703–717. doi: 10.2217/17435889.3.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]