Abstract
In Alzheimer's disease brains morphological changes in the dendrites of pyramidal neurons of the prefrontal cortex (PFC) and hippocampus have been observed. These changes are particularly reflected in the decrement of both the dendritic tree and spine number. Donepezil is a potent and selective acetylcholinesterase inhibitor used in the treatment of Alzheimer's disease. We have studied the effect of oral administration of this drug on the morphology of neuronal cells from the brain of aged rats. We examined dendrites of pyramidal neurons of the PFC, dorsal or ventral hippocampus and medium spiny neurons of the nucleus accumbens (NAcc). Donepezil (1 mg/Kg, vo) was administrated every day for 60 days to rats aged 10 and 18 months. Dendritic morphology was studied by the Golgi-Cox stain procedure followed by Sholl analysis at 12 and 20 months ages, respectively. In all Donepezil treated-rats a significant increment of the dendritic spines number in pyramidal neurons of the PFC, dorsal hippocampus was observed. However, pyramidal neurons of the ventral hippocampus and medium spiny cells of the NAcc only showed an increase in the number of their spines in 12 months old-rats. Our results suggest that Donepezil prevents the alterations of the neuronal dendrite morphology caused by aging.
Keywords: dendritic morphology, prefrontal cortex, nucleus accumbens, hippocampus, Alzheimer, donepezil
Introduction
Alzheimer's disease (AD) is a neurodegenerative process characterized by memory loss and dementia. At the neural level, the cholinergic system is one of the most affected. The pyramidal neuronal cells in cortical and hippocampal areas are severely degenerated as well as those of the nucleus basalis of Maynert (Samuel et al., 1994). This structure provides approximately 80% of the cholinergic neurons in the CNS (Samuel et al., 1994). In AD, it is also observed a reduction in levels of choline acetyltransferase accompanied by a decrease in the number of the neuronal nicotinic acetylcholine receptors (AChRs) (Shimohana et al., 1986; Whitehouse et al., 1986; Araujo et al., 1988).
Donepezil is a potent and selective acetylcholinesterase inhibitor extensively used for the treatment of AD (Tsuno, 2009). Donepezil as well as other central-type acetylcholinesterase (AChE) inhibitors, including galantamine, tacrine, and rivastigmine, have been used for the treatment of AD because of their effects in the reactivation of the hypofunctional cholinergic systems improve the memory and cognitive deficits of the patients (Seow and Gauthier, 2007). Recently, it has been suggested that the therapeutic effects of donepezil (Wynn and Cummings, 2004) also appear to involve an increase in the communication between the neurons via the increase of the synaptic connections (Ginestet et al., 2007; Kotani et al., 2008).
In contrast with the large number of clinical trials using donepezil in AD patients (Knobloch and Mansuy, 2008), to date, the information regarding the effects of this drug on the neuronal morphology is limited (Ginestet et al., 2007; Oda et al., 2007). In AD, the β amyloid peptide is able to induce the loss or alteration of neuronal dendritic spines (Knobloch and Mansuy, 2008). Another study demonstrated that in AD and other dementias, there are morphological changes on dendritic spine density mainly observed in the prefrontal cortex and the hippocampus (Knobloch and Mansuy, 2008; Uylings and de Brabander, 2002). It has been also found than AD brains are characterized by a reduced cell proliferation in the CA1 area of the hippocampus (Ferrer and Gullotta, 1990; Einstein et al., 1994; Scheff et al., 2007), and prefrontal cortex (PFC) (Shim and Lubec, 2002). Recently, alterations have been reported in the neuronal morphology of an AD mouse model (Aoki et al., 2007; Spires-Jones et al., 2007; Knafo et al., 2009).
In the present study, we assessed the effect of donepezil on the dendritic morphology of neurons from different limbic regions associated with the process of the memory and learning in aged rats (12 and 20 months old). Our results showed that the donepezil lead to significant changes in the number of the dendritic spines in the PFC, hippocampus and NAcc neurons. These observations may be relevant to the understanding of the effect of the cholinergic transmission during the aging process.
Material and Methods
Animals and donepezil administration
Sprague-Dawley male rats of the 10 and 18 months age were obtained from our facilities (University of Puebla). Animals were individually housed in a temperature and humidity controlled environment on a 12-h light-dark cycle with free access to food and water. Animals were grouped and each rat was assigned to either a vehicle (control) or donepezil hydrochloride group. All procedures described in this study were approved by the BUAP Animal Care Committee and the governmental guidelines (Mexican Council for Animal Care, Norma Oficial Mexicana NOM-062-ZOO-1999) and by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used. Rats were then administrated with donepezil (1 mg/Kg from WYETH, S.A. de C.V of Mexico) or an equal volume of vehicle (0.5 % of gum tragacanth in 0.1 M phosphate-buffered saline, PBS, pH 7.4) was administrated by oral administration every day in the morning (10:00 to 12:00 am) for a period of 10 weeks.
Golgi-Cox stain method
The animals (n = 7 per groups for 12 months and 4 per groups for 20 months) were deeply anesthetized with sodium pentobarbital (75 mg/kg body weight, ip) and perfused intracardially with 0.9% saline solution. The brains were removed and stained by modified Golgi-Cox method following a previously described protocol (Silva-Gomez et al., 2003; Flores et al., 2005; Solis et al., 2007). Two hundred-μm thick coronal sections from the PFC, hippocampus and NAcc were obtained using a vibrotome (Campden Instrument, MA752, Leicester, UK). Sections were collected on clean gelatin-coated microscope slides and treated with ammonium hydroxide for 30 min, followed by 30 min in Kodak Film Fixer, and finally rinsed with distilled water and mounted with resinous medium (Robinson and Kolb, 1997; Gibb and Kolb, 1998).
Microscopic observation and Sholl analysis
Pyramidal cells from layer 3 and 5 of the PFC (area Cg1 and prelimbic cortex, plate 7- 9 of Paxinos and Watson Atlas, 1986), CA1 of the dorsal hippocampus (plate 27 – 32 of Paxinos and Watson Atlas, 1986), CA1 of the ventral hippocampus (plate 37 – 42 of Paxinos and Watson Atlas, 1986), and medium spiny neurons of the NAcc (plate 10 - 14 of Paxinos and Watson Atlas, 1986) were selected for study. Five neurons from each region of each hemisphere per animal were drawn using camera lucida at a magnification of 250× (DMLS, Leica Microscope) by a trained observer who was blind to the experimental conditions (Kolb et al., 1998). Pyramidal neurons were readily identified by their characteristic triangular soma-shape, apical dendrites extending toward the pial surface, and numerous dendritic spines. Medium spiny neurons of the NAcc core and shell were recognized by their soma size and dendritic extensions. In the case of CA1 and PFC pyramidal neurons, the present analyses were performed on the basal dendrites since these run parallel to the coronal plane. Sequential 2-dimensional reconstructions of the entire dendritic tree were generated for each neuron and the dendritic tracings were quantified by Sholl analysis (Sholl, 1953), as follows. A transparent grid with equi-distant (10 μm) concentric rings was centered over the dendritic tree tracings. The number of ring intersections was used to estimate the total dendritic length and dendritic arborization (Kolb et al., 1998; Silva-Gomez et al., 2003; Vega et al., 2004; Flores et al., 2005; Martínez-Tellez et al., 2005; Solis et al., 2007). Dendritic arborization was also measured by counting the total number of dendritic branches (branching, indicated, Y bifurcated) at each order away from the cell body or dendritic shaft. To calculate the spine number, a length of dendrite (at least ≥ 10-μm long) was traced (at 1000 x). The exact length of each dendritic branch was calculated, and the number of spines along the length counted (to yield spines/10 μm).
Statistical Analysis
The mean values from each brain region of each animal were treated as a single measurement for the data analysis. Data on dendritic length and the spine number were analyzed by two-way ANOVA, followed by the Newman-Keuls test for post-hoc comparisons, with donepezil and age as independent factors (P < 0.05 was considered significant). Data of the length per branch order also was analyzed by two-way ANOVA, followed by the Newman-Keuls test for post-hoc comparisons, with donepezil and branch order as independent factors (P < 0.05 being significant).
Results
Dendritic spine density
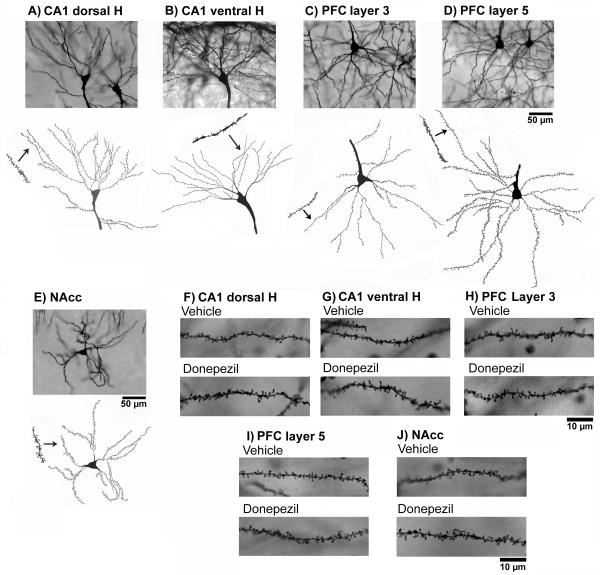
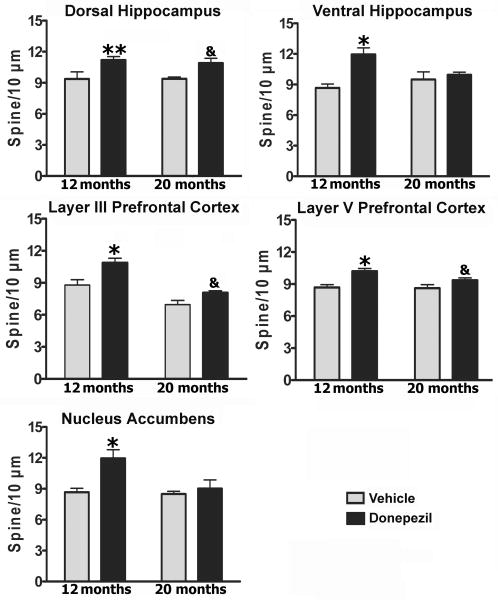
The morphological analysis presented here is based on a total of 1100 neurons from 22 animals. Estimates of dendritic length and spine number were obtained from 440 PFC and 440 hipocampal CA1 pyramidal neurons and from 220 NAcc medium spiny neurons. The effects of the donepezil on dendritic morphology in the prefrontal cortex, hippocampus and NAcc of the rats with 12 and 20 months of age are illustrated in Figs. 1 - 4. Dendritic branching and number of dendritic spines on neurons of layers 3 and 5 of the PFC, and CA1 of the dorsal and ventral hippocampus and the medium spiny neurons of the NAcc, were measured by Golgi-Cox stain. The dendritic length for each branching order, spine number, and total dendritic length was obtained as reported previously (Silva-Gomez et al., 2003; Flores et al., 2005; Alquicer et al., 2008; Juarez et al., 2008; Martínez-Tellez et al., 2009; Solis et al., 2009). Golgi-Cox staining clearly filled the dendritic shafts and the spines of neurons from layers 3 and 5 of the PFC (Fig. 1C, 1D, 1H and 1I) and CA1 of the dorsal and ventral hippocampus (Fig. 1A, 1B, 1F and 1G) and the medium spiny neurons (Fig. 1E and 1J) of the NAcc. The pyramidal neurons of the CA1 of the dorsal (2-way ANOVA analysis, donepezil; F1, 18 = 9.4, P < 0.01) and ventral hippocampus (2-way ANOVA analysis, donepezil; F1, 18 = 5.2, P < 0.05) response to the donepezil with an increase in the dendritic spine number in 12 months ages group compared with its corresponding control group (P < 0.05) (Fig. 2, up panel). While, spines of the pyramidal neurons of the CA1 dorsal hippocampus from animals of the 20 months age show a trend to increase in the number by donepezil compared to their corresponding control group (P = 0.07). Similarly, A two-way ANOVA analysis of the dendritic spine number of the pyramidal PFC neurons at the level of layer 3 (donepezil; F1, 18 = 11.86, P < 0.01, age; F1, 18 = 25.3, P < 0.01) and at the level of the layer 5 (donepezil; F1, 18 = 15.4, P < 0.01) revealed that the number of the dendritic spines was significantly increase in the pyramidal neurons of the PFC in rats with donepezil compared to their corresponding control group (P < 0.01) (Fig. 2, middle panel) at 12 months age. While, animal with donepezil at 20 months only present a trend to increase the number of spine (P = 0.07) compared to their corresponding age control group (Fig. 2, middle panel). Finally, dendritic spine number of the medium spiny neurons of the NAcc (2-way ANOVA analysis, donepezil; F1, 18 = 10.1, P < 0.01, age; F1, 18 = 6.6, P = 0.01; donepezil × age; F1, 18 = 5.3, P < 0.03) revealed that donepezil caused an increase in the dendritic spine number in 12 months aged group but not so in 18 month-old rats when compared with their corresponding control group (P < 0.01) (Fig. 2, low panel).
Fig 1.
Representative Golgi-Cox-impregnated pyramidal neurons in control rats. CA1 of the ventral and dorsal hippocampus (A, B, F and G); layer 3 and 5 of the prefrontal cortex (C, D, H and I); and medium spiny neurons of the NAcc (E and J).
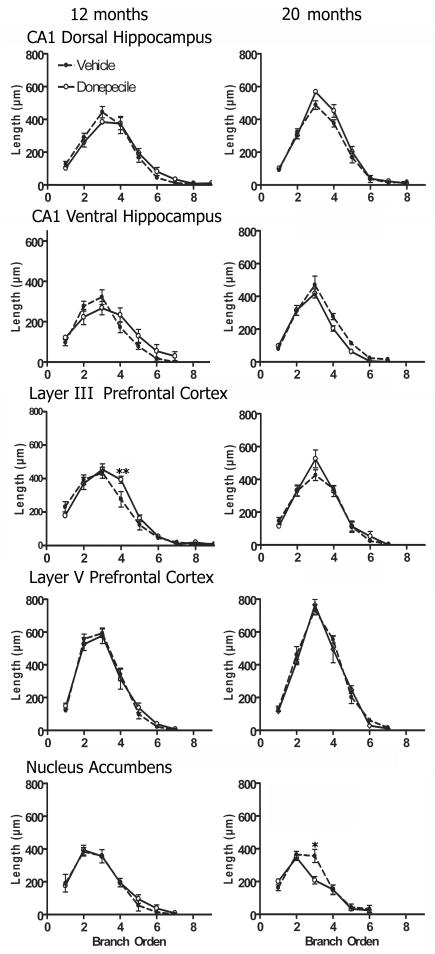
Fig 4.
Length of branch order analysis. The pyramidal cells of the layer III from the prefrontal cortex showed an increase in the dendritic length only at the level of the fourth order (** = P < 0.05) at 12 months old. The medium spiny neurons of the NAcc from 20 months old rats with donepezil showed a decreased in the dendritic length at third branch their corresponding control group (* = P < 0.01).
Fig 2.
Analysis of the donepezil effect on the dendritic spine density. The density of the dendritic spines increased in the 12 months old rats with donepezil compared to their corresponding age group (** = P < 0.05, CA1 dorsal hippocampus; * = P < 0.01, rest of the regions) in all the regions studied. At 20 months old, the number of dendritic spine from neurons of the dorsal hippocampus and prefrontal cortex showed a trend to increase after donepezil treatment. (& = P = 0.07).
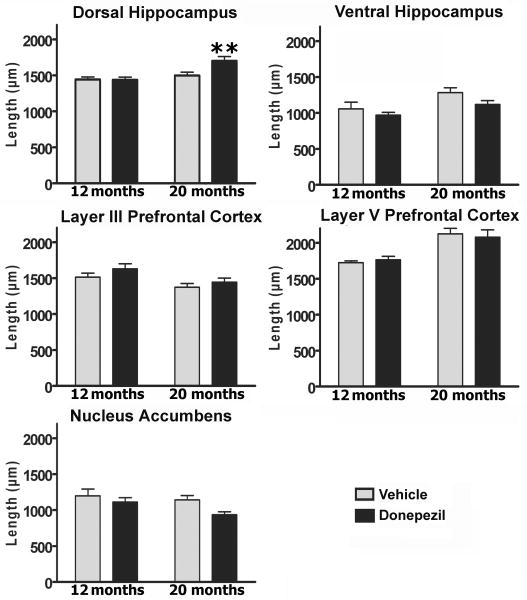
Dendritic length
The total dendritic length of the pyramidal CA1 dorsal hippocampus neurons (two-way ANOVA, donepezil; F1, 18 = 4.7, P < 0.05, age; F1, 18 = 12, P < 0.01; donepezil × age; F1, 18 = 5.2, P < 0.05) (Fig. 3, up panel) revealed that donepezil caused an increase in the dendritic length only in the group of animal at 20 months age compared to its corresponding control group (P < 0.05).There are not difference between groups at any other region.
Fig 3.
Analysis of the donepezil effect on the total dendritic length. The total dendritic length of the dorsal hippocampus was increased in the 20 months old donepezil treated-rats compared to their corresponding age group (** = P < 0.05).
Branch order
Another measure obtained from the Sholl analysis was the length per branch order. The branch-order analysis of the layer III of the pyramidal cells from the 12 months rats (two-way ANOVA, branch order; F8, 82 = 48 P < 0.001) revealed that donepezil produced an increase in the dendritic length only at the level of the fourth order (P < 0.05) (Fig. 4). In addition, the two-way ANOVA analysis of the branch-order of the medium spiny neurons of the NAcc from the 20 months rats revealed that there was a significant effect of age (F5, 30 = 46, P < 0.01) and donepezil × age interaction (F5, 30 = 2.8, P < 0.05) (Fig. 4). A post-hoc test revealed that the longitude of the third branch was significantly decrease in the medium spine neurons in 20 months rats with donepezil compared to their corresponding control group (P < 0.01) (Fig. 4). Finally there are no differences between groups at any point studied (Fig. 4).
Discussion
The major aim of the present study was to investigate the effects of 10 weeks of donepezil administration on the dendritic morphology of PFC, CA1 dorsal and ventral hippocampus, and NAcc neurons in aged rats. Interestingly, donepezil caused morphological changes in all the analyzed regions in the form of enhancement in the dendritic spine number at 12 months old with an increase in the dendritic length at 20 months old rats but restricted to the dorsal hippocampus neurons. In agreement with the present results, the assessment of the cortical neurons during aging in nonhuman primates showed loss of dendritic spines without differences in the total dendritic length when compared to old to adult animals (Dickstein et al., 2007). In addition, the spine loss on basal dendrites of the pyramidal neurons occurred mainly on distal branches in old animals (Page et al., 2002).
Dendritic morphology of individual neurons, including dendritic length, arborization and spine number can be assessed by using the Golgi-Cox-impregnation method (Gibb and Kolb, 1998). This technique is commonly used to determine the dendritic surface which receives more than 95% of the excitatory synapses of a given neuron (Kolb et al., 1998). Interestingly, adult cortical neurons receive approximately 15 000 synaptic inputs (Huttenlocher, 1984). In addition, dendritic length and spines number are related to the degree of connectivity and afferent activity (McAllister, 2000). Therefore, it is possible to make inferences about connectivity from dendritic structure by measuring dendritic length, dendritic length per branch order, arborization, and spine number. The bulk of our results suggest that donepezil may cause dendritic changes in old rats, especially in the pyramidal neurons of the PFC and the dorsal hippocampus with an increase in the connection concerning dendritic spine number at both ages. In contrast to this, in aged rats, donepezil does not appear to affect neurons from the ventral hippocampus and NAcc. This effect could be related with the level of the cholinergic transmission, which decreased with the age (Rylett and Williams, 1994; Billard, 2006). However, the enhancement in the communication between PFC and dorsal Hippocampus neurons, may be playing a role in the cognition, as previously suggested in donepezil—treated rats (Lee et al., 2007;Wise et al., 2007; Cutuli et al., 2008).
Donepezil hydrochloride is the most widely prescribed drug for AD. The main mechanism of action through which it influences cognition and function is presumed to be the inhibition of acetylcholinesterase enzyme in the brain. However, donepezil may also impact the pathophysiology of AD at several other levels. As this study suggests, donepezil may also have an effect on the dendritic spine number in old rats. At the present, this effect cannot be associated to an increase in the cholinergic transmission, however, some recent reports suggest that donepezil may be related with an increase in the neurotrophins such as nerve growth factor (NGF) (Oda et al., 2007) and brain derived neurotrophic factor (BDNF) (Leyhe et al., 2008). This drug also appears to be related with hipocampal neurogenesis (Kotani et al., 2008). Neurotrophins are expressed in almost all neuronal populations in the central and peripheral nervous system, and their effect can be neuron-specific, because neurons from each cortical layer respond in a distinct fashion on apical and basal dendrites to subsets of neurotrophins (McAllister et al., 1995). It is intriguing that donepezil treatment appears to induce the increase in the spine number in all the analyzed regions at 12 months old-rats but restricted to PFC and dorsal hippocampal neurons in 20 months old-rats. The latter areas are critical for the memory and learning (Mimura, 2008). In addition, donepezil has also been shown to be effective in vascular dementia, Parkinson's disease dementia/Lewy body disease and cognitive symptoms associated with multiple sclerosis. Contrasting with AD, in this group of neuropathologies the cholinergic transmission is not critical. However, the increase in the communication among neurons may be, at least in part, beneficial for the brain function in all these degenerative process.
Recent in vitro studies in rat primary cultured neurons, have demonstrated that donepezil has protective effects against ischemic damage, glutamate excitotoxicity and Aβ-induced toxicity (Akasofu et al., 2008a). Interestingly, none of the acetylcholinesterase inhibitiors such as galatamine, tacrine or rivastigmine, or NMDA receptor antagonists such as MK-801 or memantine, had neuroprotective effects (Akasofu et al., 2008b) as donepezil did. This last result indicates that donepezil has a specific effect on neuronal morphology in aged rat brains. Therefore, the synaptic spine number observed in the rats with donepezil may in part be caused by these neuroprotective effects. The mechanism(s) by which donepezil inhibits the alterations of the neuronal dendrite morphology caused by the age, has not been determined up to now. However, it is know that cerebral ischemia causes neurotoxicity via increases of intracellular sodium concentration ([Na+]i) and calcium concentration ([Ca2+]i) with a consequent increase of glutamate release (Choi, 1988; Lynch et al., 1995) and a recent report has been demonstrated that donepezil may exert its neuroprotective effect by blockade the rise of [Na+]i induced by veratridine (Akasofu et al., 2008a), suggesting that donepezil may imply a direct effect on Na+ channels.
Contrary to our results, a recent report (Garret et al., 2006) suggests that disruption of the cholinergic corticopetal nucleus basalis causes an increase in spine density in pyramidal neurons of the prefrontal cortex. It also suggests that this effect was prevented by treatment with the N-methyl-d-aspartate (NMDA) antagonist, MK-801. In addition several reports have demonstrated the critical role of the cholinergic-glutamatergic interactions in synaptic plasticity (Fernandez de Sevilla et al., 2008). Therefore, inhibition of cholinesterase with donepezil, and disruption of the cholinergic corticopetal system, may affect differently tonic and phasic acetyl-choline release, apparently affecting the system in the same direction in spite of the former being cholinomimetic and the latter anticholinergic.
Finally, recent data in molluscan neurons (Solntseva et al., 2006, 2007 and 2009) and rat hipocampal neurons (Yu and Hu, 2005) have shown that donepezil has an inhibitory effect on K+-current. In addition, several reports suggest that small-conductance Ca2+-activated K+ channels (SK channels) are also important in regulating dendritic excitability (Cai et al., 2004), synaptic transmission and plasticity (Ngo-Ahn et al., 2005; Faber et al., 2005; Hammond et al., 2006). SK2 channels are activated solely by intracellular Ca2+ ions, with submicromolar Ca2+ affinity (Köhler, et al., 1996) and are selectively blocked by the peptide toxin apamin. SK2 channels are expressed throughout the dendritic arbor of CA1 neurons and in dendritic spines (Ngo-Anh, et al., 2005; Sailer et al., 2004). Interestingly, the administration of apamin to rodents facilitates the induction of synaptic plasticity and hippocampal-dependent memory encoding (Stackman et al., 2008). In contrast, over-expression of SK2 channels in transgenic mice reduced long-term potentiation after high-frequency stimulation and severely impaired learning in hippocampus-dependent tasks (Hammond et al., 2006; Stackman et al., 2008). Furthermore, our preliminary results indicate that apamin may also produce alterations in the dendritic morphology of pyramidal neurons of dentate gyrus, CA1 ventral and dorsal hippocampus of old rats (A Curiel-Romero and G. Flores, unpublished data).
In summary, donepezil may decrease the alterations of the neuronal dendrite morphology caused by the age, suggesting that improvement of the cholinergic transmission may help to maintain the functional synaptic morphology in hippocampus and prefrontal cortex, limbic structures related with the cognition. In conclusion, donepezil has a potent and specific effect on the neuronal cell plasticity in the brain of aged rats. Therefore, donepezil has a potential use not only in treatment of AD symptoms but in the aging process as well.
Acknowledgments
This study was supported by VIEP-BUAP grant (No. FLAG/SAL08/G) to G Flores and Conacyt grant (No. 47630-M) to R Mena. We are grateful to WYETH, S.A. de C.V of Mexico for the Donepezil gift. We also want to thank Dr. Carlos Escamilla for his help in the animal care. IMT, IJ, ICA and OS acknowledge the CONACYT for the scholarship. Thanks to Stephanie Newton for editing the English language text.
Abbreviations
- AChE
acetylcholinesterase
- AChRs
acetylcholine receptors
- AD
Alzheimer's disease
- BDNF
brain derived neurotrophic factor
- CNS
central nervous system
- NAcc
nucleus accumbens
- NGF
nerve growth factor
- PFC
prefrontal cortex
- DH
dorsal hippocampus
- VH
ventral hippocampus
References
- Akasofu S, Sawada K, Kosasa T, Hihara H, Ogura H, Akaike A. Donepezil attenuates excitotoxic damage induced by membrane depolarization of cortical neurons exposed to veratridine. Eur J Pharmacol. 2008a;588:189–197. doi: 10.1016/j.ejphar.2008.03.064. [DOI] [PubMed] [Google Scholar]
- Akasofu S, Kimura M, Kosasa T, Sawada K, Ogura H. Study of neuroprotection of donepezil, a therapy for Alzheimer's disease. Chem Biol Interact. 2008b;175:222–226. doi: 10.1016/j.cbi.2008.04.045. [DOI] [PubMed] [Google Scholar]
- Alquicer G, Morales-Medina JC, Quirion R, Flores G. Postweaning social isolation enhances morphological changes in the neonatal ventral hippocampal lesión rat model of psychosis. J Chem Neuroanat. 2008;35:179–187. doi: 10.1016/j.jchemneu.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Aoki C, Mahadomrongkul V, Fujisawa S, Habersat R, Shirao T. Chemical and morphological alterations of spines within the hippocampus and entorhinal cortex precede the onset of Alzheimer's disease pathology in double knock-in mice. J Comp Neurol. 2007;505:352–362. doi: 10.1002/cne.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Lapchak PA, Robitaille Y, Gauthier S, Quirion R. Differential alteration of various cholinergic markers in cortical and subcortical regions of human brain in Alzheimer's disease. J Neurochem. 1988;50:1914–1923. doi: 10.1111/j.1471-4159.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19:199–215. [PubMed] [Google Scholar]
- Cai X, Liang CW, Muralidharan S, Kao JP, Tang CM, Thompson SM. Unique roles of SK and Kv4.2 potassium channels in dendritic integration. Neuron. 2004;44:351–364. doi: 10.1016/j.neuron.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–469. doi: 10.1016/0166-2236(88)90200-7. [DOI] [PubMed] [Google Scholar]
- Cutuli D, Foti F, Mandolesi L, De Bartolo P, Gelfo F, Federico F, Petrosini L. Cognitive performance of healthy young rats following chronic donepezil administration. Psychopharmacology (Berl) 2008;197:661–673. doi: 10.1007/s00213-008-1084-0. [DOI] [PubMed] [Google Scholar]
- Dickstein DL, Kabaso D, Rocher AB, Luebke JI, Wearne SL, Hof PR. Changes in the structural complexity of the aged brain. Aging Cell. 2007;6:275–284. doi: 10.1111/j.1474-9726.2007.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein G, Buranosky R, Crain BJ. Dendritic pathology of granule cells in Alzheimer's disease is unrelated to neuritic plaques. J Neurosci. 1994;14:5077–5088. doi: 10.1523/JNEUROSCI.14-08-05077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci. 2008;28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Gullotta F. Down's syndrome and Alzheimer's disease: dendritic spine counts in the hippocampus. Acta Neuropathol. 1990;79:680–685. doi: 10.1007/BF00294247. [DOI] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, Srivastava LK. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Garrett JE, Kim I, Wilson RE, Wellman CL. Effect of N-methyl-d-aspartate receptor blockade on plasticity of frontal cortex after cholinergic deafferentation in rat. Neuroscience. 2006;140:57–66. doi: 10.1016/j.neuroscience.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Gibb R, Kolb B. A method for vibratome sectioning of Golgi-Cox stained whole rat brain. J Neurosci Methods. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- Ginestet L, Ferrario JE, Raisman-Vozari R, Hirsch EC, Debeir T. Donepezil induces a cholinergic sprouting in basocortical degeneration. Neurochem. 2007;102:434–440. doi: 10.1111/j.1471-4159.2007.04497.x. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttenlocher PR. Synapse elimination and plasticity in developing human cerebral cortex. Am J Ment Defic. 1984;88:488–496. [PubMed] [Google Scholar]
- Juarez I, Gratton A, Flores G. Ontogeny of altered dendritic morphology in the rat prefrontal cortex, hippocampus and nucleus accumbens following Caesarean delivery and birth anoxia. J Comparative Neurol. 2008;507:1734–1747. doi: 10.1002/cne.21651. [DOI] [PubMed] [Google Scholar]
- Knafo S, Alonso-Nanclares L, Gonzalez-Soriano J, Merino-Serrais P, Fernaud-Espinosa I, Ferrer I, Defelipe J. Widespread Changes in Dendritic Spines in a Model of Alzheimer's Disease. Cereb Cortex. 2009;19:586–592. doi: 10.1093/cercor/bhn111. [DOI] [PubMed] [Google Scholar]
- Knobloch M, Mansuy IM. Dendritic spine loss and synaptic alterations in Alzheimer's disease. Mol Neurobiol. 2008;37:73–82. doi: 10.1007/s12035-008-8018-z. [DOI] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Kolb B, Forgie M, Gibb R, Gorny G, Rowntree S. Age, experience and the changing brain. Neurosci Biobehav Rev. 1998;22:143–159. doi: 10.1016/s0149-7634(97)00008-0. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem Biol Interact. 2008;175:227–230. doi: 10.1016/j.cbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park SY, Shin YW, Kim CD, Lee WS, Hong KW. Concurrent administration of cilostazol with donepezil effectively improves cognitive dysfunction with increased neuroprotection after chronic cerebral hypoperfusion in rats. Brain Res. 2007;1185:246–255. doi: 10.1016/j.brainres.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Leyhe T, Stransky E, Eschweiler GW, Buchkremer G, Laske C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 2008;258:124–128. doi: 10.1007/s00406-007-0764-9. [DOI] [PubMed] [Google Scholar]
- Lynch JJ, Yu SP, Canzoniero LM, Sensi SL, Choi DW. Sodium channel blockers reduce oxygen–glucose deprivation-induced cortical neuronal injury when combined with glutamate receptor antagonists. J Pharmacol Exp Ther. 1995;273:554–560. [PubMed] [Google Scholar]
- Martínez-Tellez R, Gómez-Villalobos MJ, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005;1048:108–115. doi: 10.1016/j.brainres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Martínez-Téllez RI, Hernández-Torres E, Gamboa C, Flores G. Prenatal stress alters spine density and dendritic length of nucleus accumbens and hippocampus neurons in rat offspring. Synapse. 2009;63:794–804. doi: 10.1002/syn.20664. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Cellular and molecular mechanisms of dendrite growth. Cereb Cortex. 2000;10:963–973. doi: 10.1093/cercor/10.10.963. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Lo DC, Katz LC. Neurotrophins regulate dendritic growth in developing visual cortex. Neuron. 1995;15:791–803. doi: 10.1016/0896-6273(95)90171-x. [DOI] [PubMed] [Google Scholar]
- Mimura M. Memory impairment and awareness of memory deficits in early-stage Alzheimer's disease. Tohoku J Exp Med. 2008;215:133–140. doi: 10.1620/tjem.215.133. [DOI] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+ -mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Oda T, Kume T, Katsuki H, Niidome T, Sugimoto H, Akaike A. Donepezil potentiates nerve growth factor-induced neurite outgrowth in PC12 cells. J Pharmacol Sci. 2007;104:349–354. doi: 10.1254/jphs.fp0070563. [DOI] [PubMed] [Google Scholar]
- Page TL, Einstein M, Duan H, He Y, Flores T, Rolshud D, Erwin JM, Wearne SL, Morrison JH, Hof PR. Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett. 2002;317:37–41. doi: 10.1016/s0304-3940(01)02428-4. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. Academic Press; New York: 1986. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rylett RJ, Williams LR. Role of neurotrophins in cholinergic-neurone function in the adult and aged CNS. Trends Neurosci. 1994;17:486–490. doi: 10.1016/0166-2236(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Samuel W, Terry RD, DeTeresa R, Butters N, Masliah E. Clinical correlates of cortical and nucleus basalis pathology in Alzheimer dementia. Arch Neurol. 1994;51:772–778. doi: 10.1001/archneur.1994.00540200048015. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, DeKosky ST, Mufson EJ. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007;68:1501–1508. doi: 10.1212/01.wnl.0000260698.46517.8f. [DOI] [PubMed] [Google Scholar]
- Seow D, Gauthier S. Pharmacotherapy of Alzheimer disease. Can J Psychiatry. 2007;52:620–629. doi: 10.1177/070674370705201003. [DOI] [PubMed] [Google Scholar]
- Shim KS, Lubec G. Drebrin, a dendritic spine protein, is manifold decreased in brains of patients with Alzheimer's disease and Down syndrome. Neurosci Lett. 2002;324:209–212. doi: 10.1016/s0304-3940(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Shimohama S, Taniguchi T, Fujiwara M, Kameyama M. Changes in nicotinic and muscarinic cholinergic receptors in Alzheimer-type dementia. J Neurochem. 1986;46:288–293. doi: 10.1111/j.1471-4159.1986.tb12960.x. [DOI] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Silva-Gomez AB, Rojas D, Juarez I, Flores G. Decreased dendritic spine density on prefrontal cortical and hippocampal pyramidal neurons in postweaning social isolation rats. Brain Res. 2003;983:128–136. doi: 10.1016/s0006-8993(03)03042-7. [DOI] [PubMed] [Google Scholar]
- Solis O, Limón DI, Flores-Hernández J, Flores G. Alterations in dendritic morphology of the prefrontal cortical and striatum neurons in the unilateral 6-OHDA-rat model of Parkinson's disease. Synapse. 2007;61:450–458. doi: 10.1002/syn.20381. [DOI] [PubMed] [Google Scholar]
- Solis O, Vázquez-Roque RA, Camacho-Abrego I, Gamboa C, De La Cruz F, Zamudio S, Flores G. Decreased dendritic spine density of neurons of the prefrontal cortex and nucleus accumbens and enhanced amphetamine sensitivity in postpubertal rats after a neonatal amygdala lesion. Synapse. 2009;63:1143–1153. doi: 10.1002/syn.20697. [DOI] [PubMed] [Google Scholar]
- Solntseva EI, Bukanova YV, Skrebitskii VG. Interaction of acetylcholinesterase inhibitor donepezil with ionic channels of the neuronal membrane. Bull Exp Biol Med. 2006;142:387–390. doi: 10.1007/s10517-006-0372-0. [DOI] [PubMed] [Google Scholar]
- Solntseva EI, Bukanova JV, Marchenko E, Skrebitsky VG. Donepezil is a strong antagonist of voltage-gated calcium and potassium channels in molluscan neurons. Comp Biochem Physiol C Toxicol Pharmacol. 2007;144:319–326. doi: 10.1016/j.cbpc.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Solntseva EI, Bukanova JV, Marchenko EV, Rossokhin AV, Skrebitsky VG. The binding of donepezil with external mouth of K+-channels of molluscan neurons. Cell Mol Neurobiol. 2009;29:219–224. doi: 10.1007/s10571-008-9314-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires-Jones TL, Meyer-Luehmann M, Osetek JD, Jones PB, Stern EA, Bacskai BJ, Hyman BT. Impaired spine stability underlies plaque-related spine loss in an Alzheimer's disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Jr, Bond CT, Adelman JP. Contextual memory deficits observed in mice overexpressing small conductance Ca2+-activated K+ type 2 (KCa2.2, SK2) channels are caused by an encoding deficit. Learn Mem. 2008;15:208–213. doi: 10.1101/lm.906808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuno N. Donepezil in the treatment of patients with Alzheimer's disease. Expert Rev Neurother. 2009;9:591–598. doi: 10.1586/ern.09.23. [DOI] [PubMed] [Google Scholar]
- Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- Vega E, Gomez-Villalobos MJ, Flores G. Alteration in dendritic morphology of pyramidal neurons from the prefrontal cortex of rats with renovascular hypertension. Brain Res. 2004;1021:112–118. doi: 10.1016/j.brainres.2004.06.042. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Martino AM, Antuono PG, Lowenstein PR, Coyle JT, Price DL, Kellar KJ. Nicotinic acetylcholine binding sites in Alzheimer's disease. Brain Res. 1986;371:146–451. doi: 10.1016/0006-8993(86)90819-x. [DOI] [PubMed] [Google Scholar]
- Wise LE, Iredale PA, Stokes RJ, Lichtman AH. Combination of rimonabant and donepezil prolongs spatial memory duration. Neuropsychopharmacology. 2007;32:1805–1812. doi: 10.1038/sj.npp.1301297. [DOI] [PubMed] [Google Scholar]
- Wynn ZJ, Cummings JL. Cholinesterase inhibitor therapies and neuropsychiatric manifestation of Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;17:100–108. doi: 10.1159/000074281. [DOI] [PubMed] [Google Scholar]
- Yu B, Hu GY. Donepezil blocks voltage-gated ion channels in rat dissociated hippocampal neurons. Eur J Pharmacol. 2005;508:15–21. doi: 10.1016/j.ejphar.2004.12.004. [DOI] [PubMed] [Google Scholar]