Abstract
The nuclear lamina is thought to be a steric barrier to the herpesvirus capsid. Disruption of the lamina accompanied by phosphorylation of lamina proteins is a conserved feature of herpesvirus infection. In HSV-1-infected cells, protein kinase C (PKC) alpha and delta isoforms are recruited to the nuclear membrane and PKC delta has been implicated in phosphorylation of emerin and lamin B. We tested two critical hypotheses about the mechanism and significance of lamina disruption. First, we show that chemical inhibition of all PKC isoforms reduced viral growth five-fold and inhibited capsid egress from the nucleus. However, specific inhibition of either conventional PKCs or PKC delta does not inhibit viral growth. Second, we show hyperphosphorylation of emerin by viral and cellular kinases is required for its disassociation from the lamina. These data support hypothesis that phosphorylation of lamina components mediates lamina disruption during HSV nuclear egress.
Keywords: HSV-1, nuclear egress, protein kinase C, nuclear lamina, emerin
INTRODUCTION
All herpesviruses assemble newly formed capsids in the nucleus of the host cell. To escape the nucleus, the capsids must traverse the inner and outer nuclear membranes via an envelopment/de-envelopment process at the nuclear envelope (NE) to release un-enveloped capsids into the cytoplasm {Mettenleiter, 2006 #251}.
The inner nuclear membrane (INM) is not, however, freely accessible to large macromolecular complexes like a herpesvirus capsid. The INM is supported by a complex meshwork of proteins called the nuclear lamina (Aebi et al., 1986; Gruenbaum et al., 2005; Worman and Courvalin, 2005). The lamina mesh is primarily composed of intermediate filament-related proteins called lamins of which there are three types: A, B, and C. The lamin A gene is alternatively spliced to generate A and C types while the two B types are encoded by separate genes (Gruenbaum et al., 2003). The lamin meshwork is anchored to the INM via interactions with lamin associated proteins (LAPs) (Worman and Courvalin, 2005). Many LAPs such as emerin, the lamin B receptor (LBR), MAN1, and LAP2β are inner nuclear membrane-bound proteins that bind both lamins and chromatin (Holmer and Worman, 2001). The organization of the nuclear periphery suggests that it may present multiple barriers to herpesvirus envelopment. The presence of chromatin attached to the lamina and the organization of the lamin proteins themselves may each present a steric barrier. The spacing of structural elements of the lamin lattice (about 50 nm) is too small to allow passage of a herpesvirus capsid, and physical measurements suggest that the lamin network is quite stiff and resistant to deformation and therefore unlikely to bend around a capsid during envelopment (Aebi et al., 1986; Panorchan et al., 2004). It is likely that the lamina must be disrupted in order for capsids to gain access to the INM.
Infection with wild-type herpesviruses results in changes in nuclear architecture consistent with disruption of the nuclear lamina, including: (i) enlargement of the nucleus demonstrated for HSV-1 and HCMV (Bjerke and Roller, 2006a; Radsak, Brucher, and Georgatos, 1991; Simpson-Holley et al., 2005); (ii) change in the shape of the nucleus from a smooth ovoid to something that more closely resembles a raisin in contour, demonstrated for HSV and HCMV (Bjerke and Roller, 2006a; Hamirally et al., 2009; Radsak, Brucher, and Georgatos, 1991; Simpson-Holley et al., 2004; Simpson-Holley et al., 2005); (iii) changes in the localization of both A and B type lamin proteins from a smooth, even lining of the INM to an uneven distribution showing gross thickening of the lamin layer at some sites and small perforations in the layer at other sites, demonstrated for HSV-1, HSV-2, HCMV, MCMV, and EBV (Bjerke and Roller, 2006a; Camozzi et al., 2008; Cano-Monreal et al., 2009; Hamirally et al., 2009; Lee et al., 2008; Radsak, Brucher, and Georgatos, 1991; Reynolds, Liang, and Baines, 2004; Simpson-Holley et al., 2004; Simpson-Holley et al., 2005); (iv) masking and unmasking of monoclonal antibody epitopes on the lamin proteins that indicate a change in the conformation or associations of the lamin proteins, seen with HSV-1 and HSV-2 {Reynolds, 2004 #22;Cano-Monreal, 2009 #67}; (v) redistribution of LAPs including LBR, LAP2β, and emerin in herpes simplex infections (Bjerke and Roller, 2006b; Leach et al., 2007; Morris, Hofemeister, and O'Hare, 2007; Scott and O'Hare, 2001; Simpson-Holley et al., 2004).
Mitosis requires disruption of the nuclear lamina (reviewed in (Margalit et al., 2005) which is mediated by phosphorylation of lamins and LAPs by cellular kinases including cyclin dependent kinase 1 (Cdk1), protein kinase C (PKC), mitogen-activated protein kinase (MAPK), protein kinase A (PKA), casein kinase II, and AKT (Gruenbaum et al., 2003; Gruenbaum et al., 2005; Margalit et al., 2005). Phosphorylation disrupts the lamina protein-protein and protein-DNA interactions. A growing body of evidence suggests that herpesviruses adapt this mechanism and induce phosphorylation of nuclear lamina components to gain access to the INM. HSV-1, HSV-2 and HCMV infections induce phosphorylation of all three types of lamins (Cano-Monreal et al., 2009; Marschall et al., 2005; Mou, Forest, and Baines, 2007; Mou et al., 2008; Park and Baines, 2006). EBV and MCMV infections induce phosphorylation of at least lamin A/C (Lee et al., 2008; Muranyi et al., 2002). LAPs are also phosphorylated during herpesvirus infection. Emerin is hyperphosphorylated and disconnected from the lamina during HSV −1 and −2 infections however the mechanism and significance of this modification remains untested (Leach et al., 2007; Morris, Hofemeister, and O'Hare, 2007). It is unknown which emerin residues are modified during HSV infection and if these modifications are required for its disassociation from the NE.
Phosphorylation of lamina components during infection is mediated by both viral and cellular kinases. Herpesviruses encode a conserved protein kinase called CHPK (Conserved Herpesvirus Protein Kinase), which shows similarities to Cdk1 in function and substrate specificity (Hume et al., 2008; Kawaguchi and Kato, 2003). Cdk1, also known as cdc2, has also been implicated in HSV-2 lamina disruption via its putative role in emerin phosphorylation (Morris, Hofemeister, and O'Hare, 2007). Involvement of the beta- and gamma-herpesvirus CHPKs in nuclear lamina disruption and nuclear egress has been established (Krosky, Baek, and Coen, 2003; Lee et al., 2008), and the HCMV CHPK phosphorylates lamin proteins on previously defined CDK-dependent sites (Hamirally et al., 2009). While there is no evidence that HSV-1 CHPK, pUL13, can directly phosphorylate nuclear lamina proteins, the HSV-2 pUL13 can disrupt lamin localization in transiently transfected cells and directly phosphorylate lamins in vitro (Cano-Monreal et al., 2009). Alpha-herpesviruses encode a second serine/threonine protein kinase designated pUS3 in HSV. pUS3 mediates phosphorylation of lamina components, including lamin A/C, and emerin, and regulates the degree of lamina disruption (Bjerke and Roller, 2006b; Leach et al., 2007; Mou et al., 2008).
During HSV-1, MCMV, and HCMV infections, PKC isoforms are recruited to the NE by viral proteins that are required for lamina disruption, suggesting that PKC activity may contribute to lamina-disrupting phosphorylation events (Muranyi et al., 2002; Park and Baines, 2006). There are ten PKC isoforms divided into three groups that differ in their activation mechanisms and all isoforms may be involved in herpesvirus-mediated lamina disruption. Conventional PKC isoforms (cPKCs), such as protein kinase c alpha (PKC alpha), require an efflux of calcium and diacylglycerol (DAG) for activation. Novel PKC (nPKC) family members, such as PKC delta (encoded by the PRKCD gene), are activated by DAG in a calcium-independent manner. Atypical PKCs (aPKC) such as PKC zeta do not require either for activation (Reyland, 2009b).
Recruitment of PKCs to the NE appears to be isoform specific. Although not all ten isoforms were tested, both PKC alpha and PKC delta, but not PKC zeta, were recruited to the NE upon HSV-1 infection (Park and Baines, 2006). Treatment of HSV infected cultures with Rottlerin, a widely used putative PKC delta inhibitor, blocked lamin B phosphorylation (Park and Baines, 2006). These data suggested a role for PKC alpha and delta but not zeta in nuclear egress.
Recruitment of PKCs to the NE in herpesvirus infections requires expression of the conserved proteins of the virus nuclear egress complex. In HSV, these proteins are called pUL31 and pUL34, and they form a complex that is required for events in lamina disruption including redistribution of lamin proteins, masking and unmasking of lamin epitopes during infection and full hyperphosphorylation and redistribution of emerin (Leach et al., 2007; Reynolds, Liang, and Baines, 2004). In HSV-1 infection, recruitment of both PKC alpha and PKC delta depends on pUL34 expression (Park and Baines, 2006). In MCMV, M50/p38, the pUL34 homolog, is required to recruit cPKCs to the NE (Muranyi et al., 2002). HSV-1 induced emerin phosphorylation is dependent upon both pUS3 kinase activity and pUL34 expression. The pUL34 dependent component of emerin hyperphosphorylation is sensitive to inhibition by Rottlerin suggesting that PKC delta mediates emerin hyperphosphorylation (Leach et al., 2007).
Despite the evidence for herpesvirus-dependent lamina disruption, it should be emphasized that the hypothesis that lamina disruption is necessary for herpesvirus egress has not yet been rigorously tested largely because all of the viral and cellular activities that mediate lamina disruption may also have other functions in infection and in nuclear egress. Experiments that specifically isolate the effects of virus-induced lamina disruption on virus growth have not been performed. Also, no study has yet directly demonstrated that the observed phosphorylation of lamina components causes their disconnection from other components of the lamina. The data presented in this article support both of these hypotheses by showing that (i) PKC family function is required both for efficient replication of HSV-1 and for nuclear egress, and (ii) emerin localization in the infected cell is determined by phosphorylation state. Emerin hyperphosphorylation is not sensitive to pan-PKC or DN-PKC delta inhibition suggesting a role for a non-PKC isoform in emerin phosphorylation. This non-PKC isoform or Rottlerin sensitive kinase (RttSK) is sensitive to Rottlerin but not BIM I treatment.
MATERIALS AND METHODS
Cells, viruses, and chemicals
HEp-2 and Vero cells were maintained as previously described (Roller et al., 2000). MCF-7 and BT-549 cells were maintained in RPMI containing 10% fetal calf serum. BT-549 cells were derived from the NCI-60 cell collection and were kindly provided by Jack Stapleton. The properties of HSV-1(F), vRR1072(tk+) (UL34-null mutant virus), vRR1202 (US3-null virus), vRR1204 (US3 kinase-dead virus K220A), and repair viruses for vRR1072(tk+) (vRR1072Rep) and vRR1202 (vRR1202Rep) were previously described and characterized (Reynolds et al., 2001; Roller et al., 2000; Ryckman and Roller, 2004). Rottlerin (Santa Cruz Biotechnology) was diluted in DMSO to a stock concentration of 10 mM and used to treat cells at 10 uM. Ro-31–7539 (Calbiochem) was diluted in DMSO to a stock concentration of 1mM and used to treat cells at 1 uM while bisindolylmaleimide I (BIM I-LC Laboratories) was diluted in DMSO to a stock concentration of 500 uM and used at 10 uM. For IFNβ (Interferon β) treatment, HEp-2 cells were serum starved in DMEM without serum overnight, and then exposed to 1000U of Human recombinant IFNβ (IBL) for 30 minutes. Phorbol 12-myristate 13-acetate (PMA) was kindly provided by Jeffery Meier (Sigma). PMA was used at 20 nM to activate PKCs for 30 minutes.
Plasmids and cell lines
pRR1072Rep, which expresses wild-type HSV-1 UL34 from its own promoter, has been previously described (Roller et al., 2000). The FLAG epitope-tagged rat wild-type and K376M (dominant negative) PKC delta expression vectors were kindly provided by U. Kikkawa (Konishi et al., 1997). pRR1342 (PRKCDwt-UL34 Duo) and pRR1343 (PRKCDdn-UL34 Duo), which express either wild-type or K376M FLAG epitope tagged rat PKC delta (respectively) and wild-type HSV-1 UL34 were constructed by ligation of the 3.05 kb XbaI-NgoMIV fragment of pRR1072Rep and the 5.44 kb fragment of the corresponding PKC delta expression vectors. In pRR1342 and pRR1343, PKC delta is constitutively expressed from the human cytomegalovirus major immediate early promoter (HCMV MIEP), and pUL34 is expressed in a HSV infection-dependent manner from its own promoter. The HuSH shRNA constructs against PKC delta (product number TR320468) were purchased from OriGene. In order to construct stable clonal cells lines expressing HuSH shRNAs against PKC delta, HEp-2 cells were transfected with individual HuSH shRNA constructs and then selected with 0.5 ug/mL puromycin for one week. Colonies were then scraped from the plate, transferred to individual cultures, and amplified. The HuSH cell lines shRNA-control, shRNA-PRKCD-1, shRNA-PRKCD-2, and shPRKCD-PRKCD-3 constitutively express the following HuSH plasmids respectively: TR30003, TI379089, TI379090, and TI379090. To create the Strep-His-EGFP-emerin plasmid, PCR amplification of EGFP from the EGFP-emerin-C1 plasmid from Y. Hiraoka (Leach et al., 2007) was performed with the following primers: 5’ CACAGCTAGCCATGGCGTGGAGCCACCCCCAGTTCGAAAAGGCGCACCATCACCAT CACCATCACCATATGGTGAGCAAGGGCGAGGA 3’ and 5’ AGTTGTCCATGGATCTGAGTCCGGA 3’. The 0.8kb PCR product and EGPF-emerin-C1 were digested with Nhe1 and BspE1 and the PCR product was ligated into the 4.6 kb product of the EGFP-emerin-C1 digestion to create pRR1315. To create the retroviral expression plasmid for Strep-His-EGFP-emerin, Strep-His-EGFP-emerin was PCR amplified from pRR1315 using the following primers: 5’-CACAGTTAACGCTAGCCATGGCGTGGAGC-3’ and 5’- CACAGCTCGAGATCCTAGAAGGGGTTGC-3’. This 1.6kb PCR product and 5.9kb pLXSN vector were digested with Xho1 and Hpa1 and ligated to create pRR1319. pLXSN was kindly provided by A. Klingelhutz. The University of Iowa Vector Core used pRR1319 to generate a MoMLV pseudotyped retroviral transduction particle. These particles were used to transduce HEp-2 cells. 48 hr post transduction, cells were placed under G-418 selection for two weeks. Colonies were then scraped from the plate, transferred to individual cultures, and amplified to create the HEp-2 Strep-His-EFGP-emerin B2 cell line.
Indirect Immunofluorescence
Indirect immunofluorescence (IF) was performed for pUL34, emerin, lamin A/C and FLAG-epitope-tagged PKC delta as previously described (Bjerke and Roller, 2006b; Reynolds et al., 2001). Briefly, cells were fixed with 4% formaldehyde for 10 minutes, washed with phosphate-buffered saline (PBS), and then permeabilized and blocked in the same step with IF buffer (0.5% Triton X-100, 0.5% Sodium Deoxycholate, 1% BSA, 0.05% Sodium Azide diluted in PBS) for 1 hour. Primary antibodies were diluted in IF buffer as follows: chicken anti-UL34, 1:1000 (Reynolds et al., 2001); mouse monoclonal anti-lamin A/C, 1:1000 (Santa Cruz Biotechnology, sc-7292); mouse monoclonal anti-emerin, 1:500 (Santa Cruz Biotechnology, sc-25284); mouse monoclonal anti-FLAG M2, 1:1000 (Sigma, F1804). The procedure for STAT3-pTyr705 localization differed in that cells were fixed in cold methanol for 30 minutes, dried, and rehydrated in IF buffer. Rabbit polyclonal anti-STAT3-pTyr705 (Cell Signaling, #1938), was diluted 1:400.
Secondary antibodies were diluted 1:1000 in IF buffer as follows: Alexa Fluor goat anti-chicken IgG (Invitrogen, A11001), Alexa Fluor goat anti-mouse IgG (Invitrogen, A1104), Alexa Flour donkey anti-rabbit IgG (Invitrogen, A21206). Slo-fade II (Invitrogen, Molecular Probes) was used to mount cover slips on glass slides. All confocal microscopy work was done with a Zeiss 510 microscope except Figure 5 D and Supplemental Figure 2, which were performed on a BioRad Multiphoton microscope.
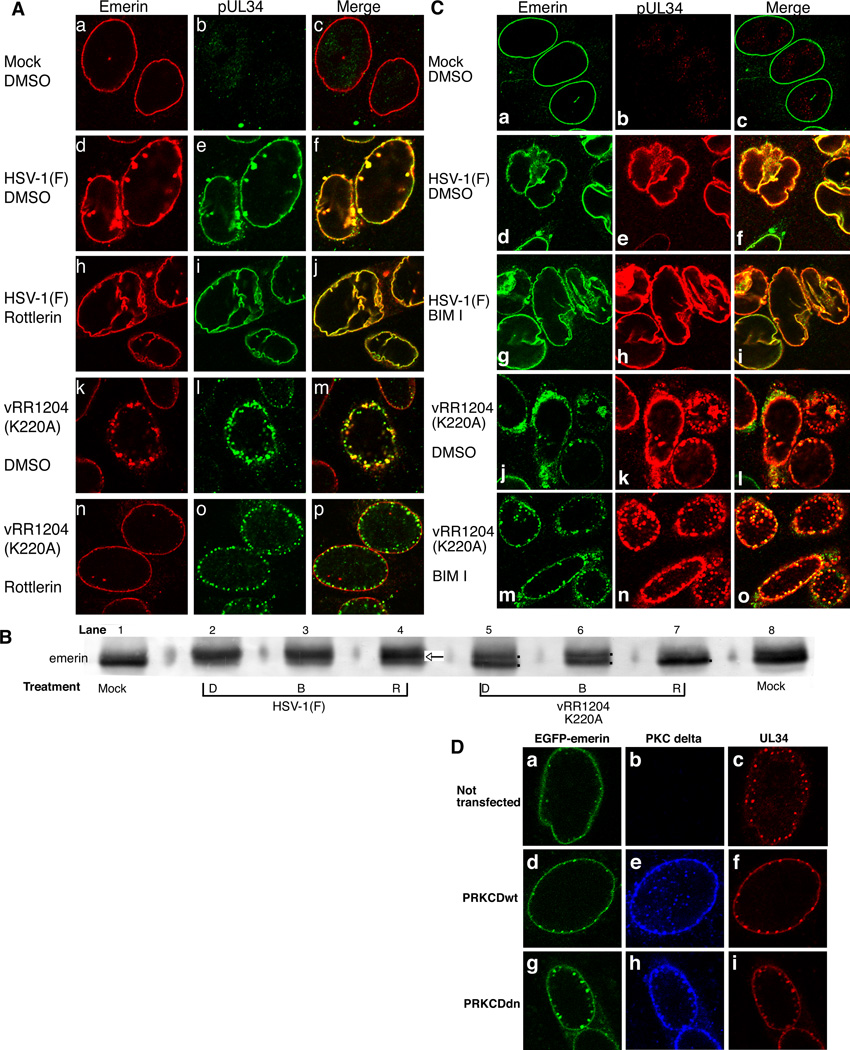
Figure 5. Disruption of emerin localization in HSV-1-infected HEp-2 cells is sensitive to pUS3-mediated and Rottlerin sensitive kinase phosphorylation events but not BIM I treatment or PKC delta activity.
A) Shown are digital confocal images of optical sections taken near the middle of the nuclei of HEp-2 cells that were uninfected (a–c) or infected with WT HSV-1(F) (d–i) or pUS3 kinase-dead (vRR1204) (j–o) with five PFU/cell for 20 hr and treated with vehicle (a–f and j–l) or Rottlerin (g–i and m–o) beginning at five hpi. Cells were stained with antibodies directed against emerin (left column) or pUL34 (center column). Emerin is represented in red and pUL34 in green. B) Digital image of western blot for emerin in HEp-2 cell nuclear lamina preparations. Cells were mock infected or infected with five PFU/cell of either WT or pUS3 kinase-dead (vRR1204) HSV-1(F). At five hpi, cells began treatment with vehicle (D), BIM I (B), or Rottlerin (R) and were harvested for lamina preparations at 16 hpi. The arrow in lane four indicates the predominant middle species, while the dots in lanes five, six, and seven indicate double or single emerin species. C) Shown are digital confocal images of optical sections taken near the middle of the nuclei of HEp-2 cells that were uninfected (a–c), HSV-1(F) (d–i), or pUS3 kinase-dead (vRR1204) (j–o) infected with five PFU/cell for 20 hr and treated with vehicle (a–f and j–k) or BIM I (g–i, and m–o) beginning at five hpi. Cells were stained with antibodies directed against emerin (left column) or pUL34 (center column). Emerin is represented in green and pUL34 in red. D) Digital confocal images taken near the center of nuclei of EGFP-Emerin HEp-2 B2 cells. Cells were un-transfected (a, b, c) or transfected with PRKCDwt-UL34 Duo (d, e, f) or PRKCDdn-UL34 Duo (g, h, i) for 24 hr. Cells were then infected with pUS3 kinase-dead (vRR1204) at an MOI of 20 for 20 hr. Cells were fixed with formaldehyde and stained with antibodies directed against FLAG-PKC delta (middle column) or pUL34 (right column). Emerin is represented in green, PKC delta in blue, and pUL34 in red.
SDS-PAGE sample preparations
For analysis of viral protein expression and PKC delta protein expression, infected or uninfected cultures containing 2 × 106 cells were washed twice with PBS, scraped into PBS, pelleted at low speed, total soluble protein was extracted with acetic acid, and precipitated with acetone as previously described (Roller et al., 1996). Total protein concentration was determined using BioRad Dc Protein Assay (BioRad Laboratories). MEK 1/2 lysates were prepared differently in that after collecting the treated cultures containing 2 × 106 cells after with a low speed spin, total soluble protein was extracted with SDS-PAGE sample buffer and PBS. This total cell lysate was denatured at 70C, sonicated twice for 30 seconds each, and then total protein concentration was determined with BioRad Dc Protein Assay (BioRad Laboratories).
Preparation of nuclear fractions
Nuclear-lamina fractions were prepared as described in (Leach et al., 2007).
Western blot
The antibodies and dilutions used were: mouse monoclonal anti-emerin, 1:500 (Santa Cruz, sc-25284); rabbit polyclonal anti-PKC delta, 1:400 (Santa Cruz, sc-937); mouse monoclonal anti-US11, 1:1000 (Roller and Roizman, 1992); chicken monoclonal anti-UL34, 1:1000 (Reynolds et al., 2001); mouse monoclonal anti-VP5, 1:1000 (Meridian Life Science, #C05014M); mouse anti-actin, 1:1000 (Sigma, A3853); mouse monoclonal anti-gC, 1:1000 (Virusys, H1A022M); rabbit monoclonal anti-phos-MEK 1/2 Ser 217/221, 1:1000 (Cell Signaling, 9154); rabbit monoclonal anti-MEK 1/2 (Cell Signaling, 9122). Alkaline phosphatase conjugated anti-mouse, anti-rabbit, and anti-chicken secondary antibodies were incubated with the appropriate blot for 30 minutes (Sigma, mouse: A3562, rabbit: A3687 Aves Lab, chicken: AP1001).
Virus Growth
Single-step growth analysis was performed as previously described (Reynolds et al., 2002; Roller et al., 2000). Briefly, replicate cultures of cells were infected at an MOI of five, and residual virus was removed or inactivated with a low-pH-buffer wash. Cells were untreated or treated with DMSO, Ro-31–7549 (1 uM), or BIM I (10 uM) at five hpi. At 24 hpi, virus was harvested and total culture PFU were calculated by titration on Vero cells.
Transmission Electron Microscopy
Vero cells were infected with HSV-1(F) at an MOI of five and at five hpi cells were treated with DMSO or BIM I. At 16 hpi, cells were fixed with glutaraldehyde, processed, and analyzed by electron microscopy as described previously (Roller et al., 2000).
35-S-methionine Labeling
2 × 106 HEp-2 cells were uninfected or infected with five PFU/cell of HSV-1(F) and beginning at five hpi, the cells were treated with DMSO (D) or BIM I (B). At 9 hpi, the cells were starved for methionine by replacing the media with methionine minus media (Gibco, 21013) with DMSO or BIM I. Beginning at 14 hpi, each sample was labeled for two hours (hr) with 20 uCi of 35S-methionine. Total cell lysates were prepared at 16 hpi by the acid preparation as described above (Roller et al., 1996). Total protein from equal numbers of cells was separated on an SDS-PAGE gel and visualized by autoradiography.
Complementation assays
Twelve-well cultures of Vero cells containing 4 × 105 cells were transfected with a total of 650 ng of plasmid DNA using 5 µl of LipofectAmine Reagent according to the manufacturer’s instructions. Complementation assays were performed on the transfected cultures as previously described (Bjerke et al., 2003).
Inhibitor toxicity assays
2 × 105 HEp-2 and Vero cells were plated in a 48 well dish in triplicate. 12 hr later, cells were treated with vehicle (DMSO), 10 uM Rottlerin, 10 uM BIM I, and 1 uM Ro-31–3549. 24 hr post-treatment, the ATPLite assay (Perkin-Elmer, #6016739) was followed per manufacture’s directions. This was repeated three independent times to determine that at the indicated dosage, the kinase inhibitors did not induce significant toxicity compared to vehicle (DMSO).
Inhibitor signaling assays
2 × 106 HEp-2 cells were placed into the presence of 10uM BIM I, 1uM Ro-31–7549 or vehicle for two hr, followed by a 30 minute activation of PKCs with 20nM PMA. The cells were washed twice with PBS, then pelleted at low speed, resuspened in PBS and SDS-PAGE buffer. Protein concentration was determined using the BioRad Dc Assay described above. These lysates were subjected to western blot analysis for MEK 1/2 and phos-MEK 1/2.
RESULTS
Inhibition of cellular kinases involved in lamina disruption inhibits viral replication
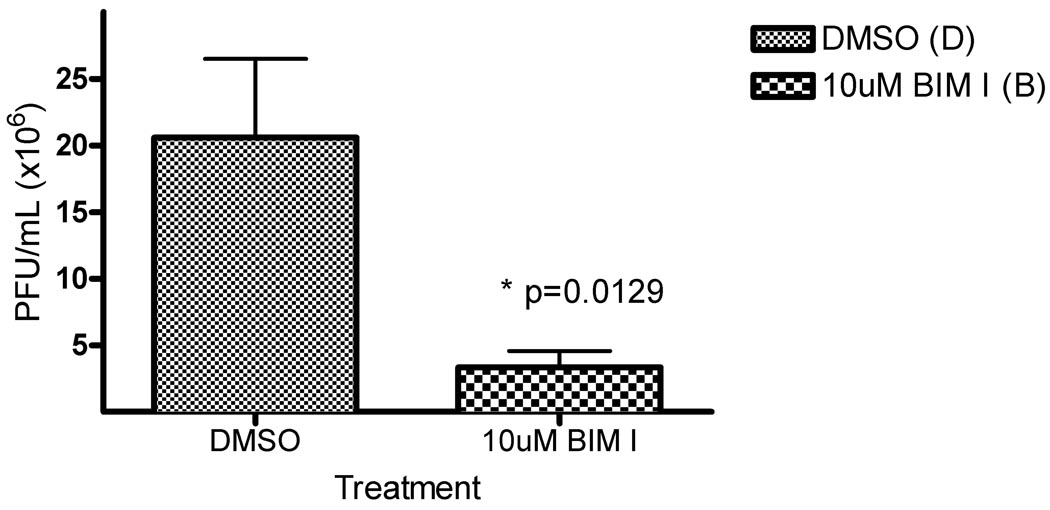
To test the hypothesis that PKC activity is required for late events of HSV-1 replication, specifically nuclear egress, activity of all PKC isoforms was blocked with the pan-PKC small molecule inhibitor bis-indolylmaleimide I (BIM I) (Toullec et al., 1991). HEp-2 cells were infected with five plaque forming units (PFU)/cell of WT HSV-1(F), treated with 10 uM BIM I or vehicle (DMSO) beginning at five hours post infection (hpi), and production of infectivity was measured at 24 hpi (Figure 1). This time of drug addition was chosen to allow at least the entry and IE (immediate early) gene expression phases to occur un-inhibited. 10 uM BIM I has been previously shown to inhibit all PKC isoform activity (Toullec et al., 1991). BIM I treatment reduced the infectivity compared to DMSO by nearly five-fold. Similar results were obtained in Vero cells and at 16 hpi (not shown).
Figure 1. PKC activity required for HSV-1 replication.
Replicate cultures of HEp-2 cells were infected at an MOI of 5 with HSV-1(F) A) Cells were treated with DMSO or 10uM pan-PKC inhibitor bis-indolylmaleimide (BIM I) beginning at five hours post infection (hpi). Titer was determined at 24 hpi. Virus yields are expressed as plaque forming units (PFU) per milliliter. Each data point represents the mean of five experiments. Error bars indicate standard deviations. p-values were determined via a paired Student T-Test.
Toxicity, determined using the ATPLite system, was not observed at the concentrations used for any of the inhibitors used in this study (data not shown). BIM I inhibits PKC activity as previously reported, since BIM I treatment blocked PKC activation of the MAPK pathway (Supplemental Figure 1) (Reyland, 2009a; Wang, Rolfe, and Proud, 2003). Similar results were obtained in Vero cells (data not shown). Collectively, Figures 1 and Supplemental Figure 1 suggest a significant role for PKC function in the late phase of HSV-1 replication.
PKC activity is required for extra-nuclear capsid accumulation
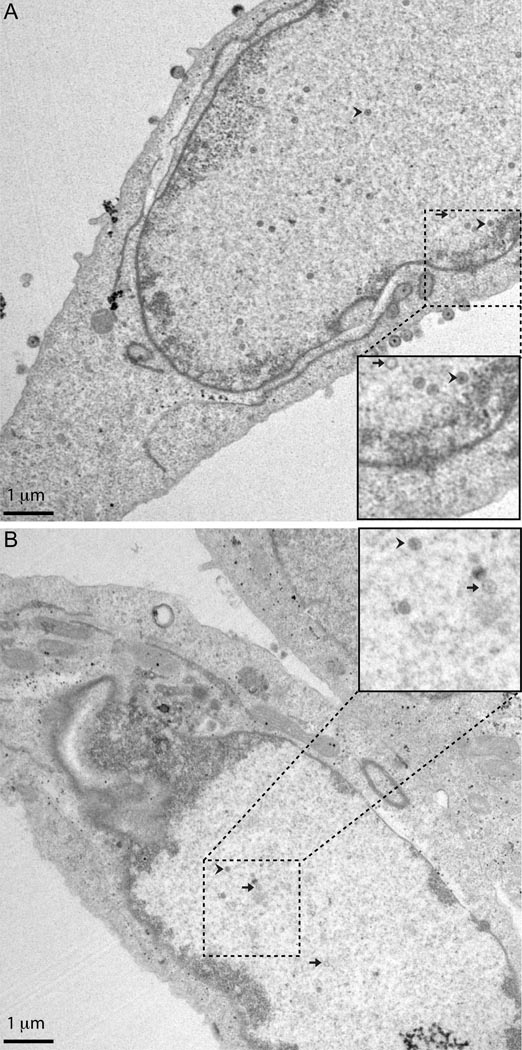
To determine if the decrease in viral infectivity produced by PKC inhibition is due to a block to primary envelopment, Vero cells were infected with five PFU/cell of WT HSV-1(F), treated with vehicle (Figure 2 A) or 10 uM BIM I (Figure 2 B) beginning at five hpi, fixed at 16 hpi, and processed for TEM analysis. DMSO-treated, WT virus-infected cells contained empty (A and B capsids (without DNA)) and full capsids (C capsids (with DNA)) in four distinct sub-cellular compartments: nucleus, perinuclear space\ER, cytoplasm (either as naked or membrane-enclosed capsids), and cell surface (membrane-enclosed capsids) (Figure 2). Capsids were counted in the four distinct compartments in 20 cells for each treatment condition and represented as a percentage of total capsids (Table 1). In DMSO-treated cells, 33% of virions were found in the nucleus. BIM I treatment increased the number of nuclear virions to 77% of total. While 46% of virions in DMSO-treated infected cells were found at the cell surface, only 9% of BIM I-treated virions were at the cell surface. BIM I-treated cells either had no or few capsids in the entire cell or the majority of the capsids were located inside the nucleus (Table 1). The ratio of full to empty capsids did not dramatically change with BIM I treatment (DMSO-7.90:1 vs BIM I-7.92:1).
Figure 2. Inhibition of PKC blocks extra-nuclear capsid accumulation.
Shown are digital images of transmission electron micrographs (TEM) of Vero cells infected with five PFU/cell HSV-1(F). Cells were treated with vehicle beginning at five hpi with vehicle (A) or BIM I (B) fixed with glutaraldehyde at 16 hpi, and thin sectioned for TEM. The inset picture is approximately twice the magnification and shows an example of an empty capsid (A and B capsids) (arrow) and a full capsid (C capsid) (arrow head).
Table 1.
Assembly and egress intermediates in BIM I-treated cells
| Capsid number (% of total capsids) |
||
|---|---|---|
| Cellular compartment | DMSO-treated | BIM I-treated |
| Nucleus | 221 (33%) | 159 (71%) |
| Perinuclear space/ER | 1 (0%) | 7 (3%) |
| Cytoplasm | 129 (20%) | 38 (17%) |
| Cell surface | 290 (46%) | 19 (9%) |
| Total | 631 (100%) | 223 (100%) |
| Mean Capsid/Cell | 31.6 | 11.2 |
| Median Capsid/Cell | 29.0±13.3a | 1.5±11.1 |
Average absolute deviation from median
Many of the WT virus-infected, BIM I-treated cells, displayed distinct characteristics of infection such as marginated chromatin (Figure 2 B), and yet had few or no capsids in the cell. Furthermore, the total number of capsids found in or on BIM I-treated cells was decreased when compared to vehicle-treated cells (Table 1, total DMSO-631 vs BIM I-223). To determine if there was a statistical difference in capsid accumulation, we compared the number of capsids per cell in DMSO- and BIM I-treated cells using an unpaired Student T-Test (Table 1, mean DMSO-31.6 vs BIM I-11.2) (http://www.physics.csbsju.edu/stats/t-test.html). Inhibition of PKC with BIM I changed the mean number of capsids per cell significantly as evaluated by the T-Test (p=0.001). Collectively, these data suggest BIM I inhibition of PKC inhibits (i) accumulation of capsids and (ii) the ability of the capsids that are formed to egress from the nucleus.
PKC inhibition does not inhibit viral protein translation
The significant reduction in the fraction of extra-nuclear capsids produced in BIM I- treated, HSV-1(F)-infected cells compared to vehicle control cells, indicated that BIM I inhibits nuclear egress (Table 1). However, the reduction in the total number of capsids observed in the presence of the pan-PKC inhibitor suggested its effect on nuclear egress might result from inhibition of events preceding capsid assembly, including expression of genes required for capsid assembly and nuclear envelopment (Table 1).
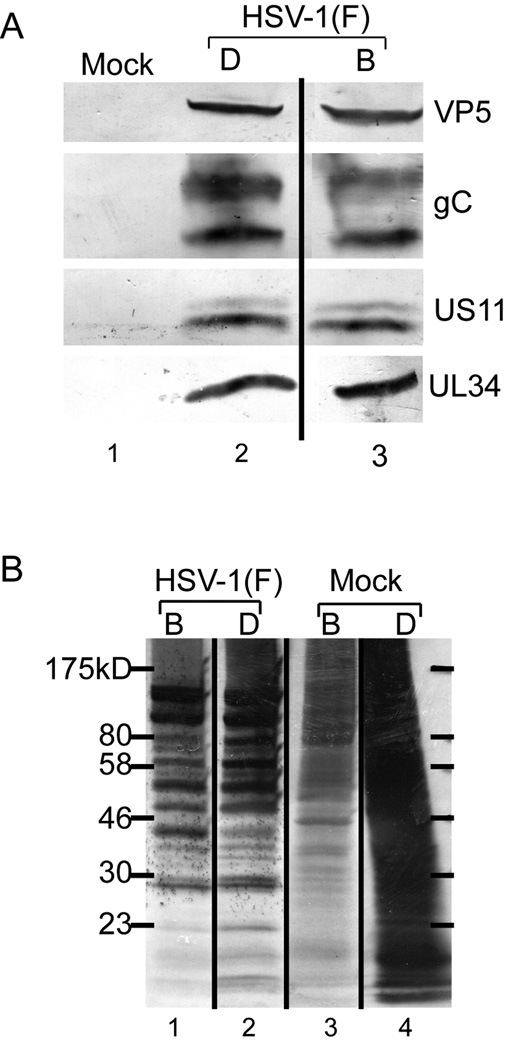
To test the hypothesis that BIM I treatment inhibits late gene expression necessary for efficient capsid assembly and nuclear egress, accumulation of viral proteins was tested in cells infected in the presence of vehicle and BIM I. HEp-2 cells were infected with five PFU/cell of HSV-1(F) and at five hpi, the cells began treatment with DMSO (D) or BIM I (B). Total cell lysates were prepared at 16 hpi and protein from equal numbers of cells was separated on an SDS-PAGE gel and transferred to nitrocellulose (Figure 3 A). These samples were subjected to western blot analysis for the following proteins: VP5 (leaky-late gene product and capsid protein), pUS11 and gC (archetype true-late gene products), and pUL34 (leaky-late and required for nuclear egress). BIM I treatment in infected cells had little to no effect on the accumulation of VP5, gC, pUS11, or pUL34 compared to DMSO treatment (compare lanes three to two). The same results were achieved when equivalent amounts of total protein are loaded (data not shown).
Figure 3. PKC inhibitor inhibits cellular but not viral protein synthesis.
A) Digital images of western blots of HEp-2 unlabeled total cell lysates prepared from mock-infected cells or cells infected with five PFU/cell of HSV-1(F). At 16 hpi, total cell lysates were prepared from equal numbers of cells. Cells were DMSO (D) or BIM I (B) treated beginning at five hpi. Blots were probed with monoclonal antibodies to VP5, pUL34, gC, and US11. B) Autoradiogram of 35S-methionine labeled HEp-2 cell lysates from A.
To test the hypothesis that BIM I treatment inhibits global late gene expression, synthesis of late viral proteins was tested in cells infected in the presence of vehicle or BIM I. HEp-2 cells were infected with five PFU/cell of HSV-1(F) and beginning at five hpi, the cells were treated with DMSO (D) or BIM I (B). Beginning at 14 hpi, cells were labeled for two hr with 35S-methionine. Total cell lysates were prepared at 16 hpi and protein from equal numbers of cells was separated on an SDS-PAGE gel and visualized by autoradiography (Figure 3 B). Upon infection with HSV-1(F), host protein synthesis was turned off and viral protein synthesis was evident in DMSO-treated samples (compare lanes two to four). When infected cells were treated with BIM I little or no effect on total viral protein synthesis was observed (compare lanes one to two). In individual experiments we noted differences in the intensities of specific bands (e.g., at 80K, 58K, and 40K in this experiment). These were not reproducible between experiments. BIM I treatment reduced mock-infected HEp-2 protein synthesis (compare lanes three to four) as previously observed (Soltoff, 2007; Wang, Rolfe, and Proud, 2003). These data suggest that BIM I mediated inhibition of nuclear egress is not due to inhibition of late gene expression, and are consistent with the hypothesis that inhibition of PKCs has specific inhibitory effects on both (i) accumulation of capsids and (ii) egress of accumulated capsids from the nucleus.
Neither conventional PKCs nor PKC delta kinase activity are uniquely required for HSV-1 replication
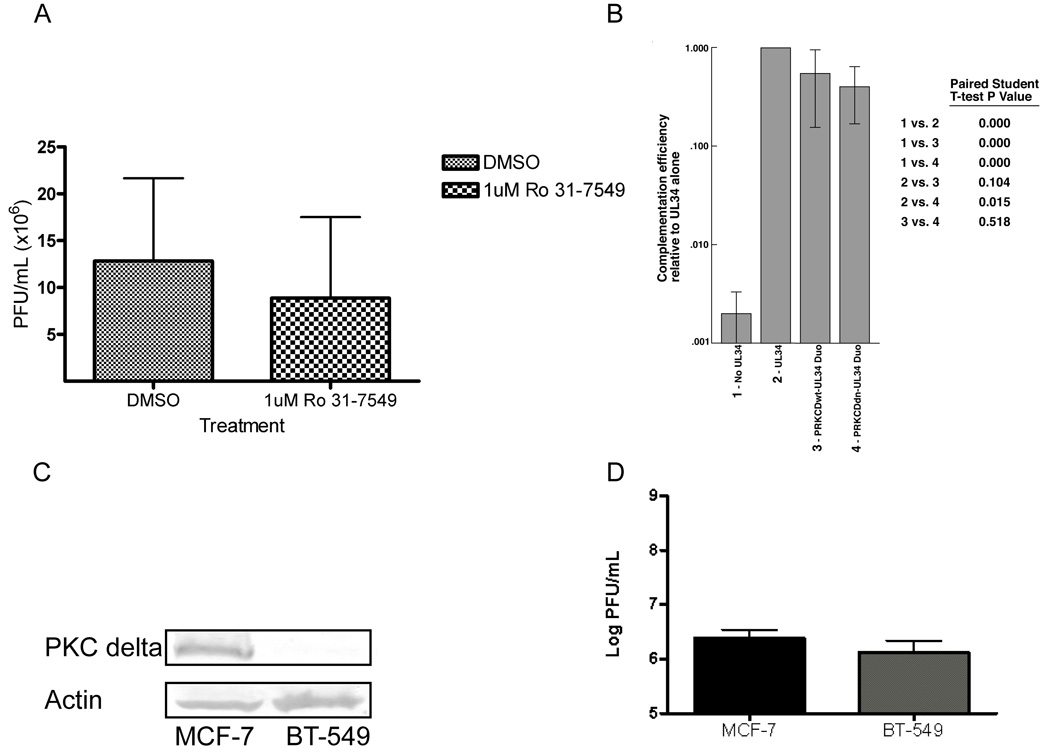
PKC alpha, a conventional (cPKC) isoform, is recruited to the NE during HSV-1 infection and the entire class of cPKCs is recruited to the rim during MCMV infection suggesting a conserved and specific role for cPKCs in herpesvirus nuclear egress (Muranyi et al., 2002; Park and Baines, 2006). To test the hypothesis that cPKC isoforms are required for optimal HSV-1 growth, cPKCs were inhibited with the small molecule inhibitor Ro-31–7549 (Zhang, Hirasawa, and Beaven, 1997). HEp-2 cells were infected with five PFU/cell of WT HSV-1, treated with 1 uM Ro-31–7549 or vehicle (DMSO) beginning at five hpi, and production of infectivity was measured at 24 hpi (Figure 4 A). Treatment with Ro-31–7549 had no significant effect on replication compared to vehicle. Ro-31–7549 inhibits cPKC (PKC alpha) signaling, as previously reported, since it partially blocked PKC signaling and activation of the MAPK pathway (Supplemental Figure 2 A). Collectively, Figure 4 A and Supplemental Figure 2 A do not suggest a unique, significant role for cPKC function in the late phase of HSV-1 replication.
Figure 4. cPKCs nor PKC delta are uniquely required for HSV-1 replication.
A) Cells were treated vehicle (DMSO) or 1 uM Ro-31-7549 beginning at five hpi. Titer was determined at 24 hpi. Virus yields are expressed as plaque forming units (PFU) per milliliter. Each data point represents the mean of six experiments. B) Vero cells were co-transfected for 24 hr with pCMVβ and either: pRR1072, pRR1072Rep, PRKCDwt-UL34 Duo or PRKCDdn-UL34 Duo. Complementation of the vRR1072(tk+) was performed five times as previously described (Bjerke et al., 2003). Error bars indicate standard deviations. p-values were determined via a paired Student T-Test. C) Digital images of western blots probed with polyclonal antibody to PKC delta (top) and monoclonal antibody to actin (bottom). Equal amounts of total protein from total cell lysates from MCF-7 or BT-549 cells were probed. D) Replicate cultures of MCF-7 and BT-549 cells were infected at an MOI of 5 with HSV-1(F). At 24 hpi total culture virus was titrated on Vero cells. Virus yields are expressed as plaque forming units (PFU) per milliliter. Each data point represents the mean of three experiments. Error bars indicate standard deviations.
HSV-1 also recruits PKC delta to the NE and treatment with Rottlerin, a reported PKC delta inhibitor, reduces infection-induced phosphorylation of lamin B and emerin (Leach et al., 2007; Park and Baines, 2006). These data suggested that PKC delta contributed to lamina disruption and might be required for nuclear egress. It has become apparent with increasing research, however, that there are many PKC delta-independent Rottlerin-dependent phenotypes (Lim et al., 2009; Soltoff, 2007; Tillman et al., 2003; Wang, Rolfe, and Proud, 2003), necessitating the use of more specific methods to probe PKC delta function. We used three approaches to reduce PKC delta activity to test the hypothesis that PKC delta activity is specifically required for viral replication.
First, we inhibited PKC delta activity by over-expression of dominant-negative (DN) PKC delta (K376M) (Konishi et al., 1997). Our strategy was to place a DN-PRKCD gene on the same plasmid as an essential viral gene and then perform a complementation assay with the virus lacking that essential gene. Two plasmids were created expressing both FLAG-PKC delta (WT or DN) and the viral gene UL34. These constructs are termed PRKCDwt-UL34 Duo or PRKCDdn-UL34 Duo. These plasmids allow for co-expression of both genes in the transfected cell, however UL34 will only be expressed upon infection with the UL34-null virus (vRR1072(tk+)) since the UL34 gene is under the control of its viral promoter. If PKC delta activity is required for HSV-1 replication, then the PRKCDdn-UL34 Duo plasmid should inhibit complementation of the UL34-null virus. We tested for efficient gene expression from the Duo plasmids (Supplemental Figure 2 B). We also confirmed that DN-PKC delta (K376M–PRKCD) expressed from the Duo system retains the dominant negative function (Supplemental Figure 2 C).
To test the hypothesis that PKC delta activity is required for viral growth, PKC delta activity was blocked with PRKCDdn-UL34 Duo in a complementation assay. Vero cells were co-transfected for 24 hr with a transfection efficiency control plasmid, pCMVβ, and no UL34 (pRR1072), WT UL34 (pRR1072Rep), PRKCDwt-UL34 Duo, or PRKCDdn-UL34 Duo. These Vero cells were infected with five PFU/cell of UL34-null virus (vRR1072(tk+)) and at 18 hpi the amount of infectivity produced was measured by plaque assay on the UL34 complementing cell line RepAC (Figure 4 B). The complementation index for WT UL34 is set to 1.000 and the other conditions are calculated relative to that. No pUL34 results in little virus production, while pUL34 provided in trans supports replication as previously shown. Co-expression of either WT-or DN-PKC delta appears to slightly reduce complementation (Figure 4 B). However, there was not a significant difference between WT- and DN-PKC delta suggesting that DN-PKC delta did not have a specific inhibitory effect on complementation. These data in Figure 4 suggest that neither cPKCs nor PKC delta kinase function is required for efficient HSV replication.
Our second approach was to determine if reduction of PKC delta protein levels would depress viral replication. HEp-2 clonal cell lines constitutively expressing shRNA against human PKC delta were generated (Supplemental Figure 3 A). There was not a significant difference between replication of HSV-1(F) in any of the shRNA-PKC delta cell lines and replication in the shRNA-control or untransfected control (Supplemental Figure 3 B).
Our third approach was to evaluate HSV-1 replication in a cell line with little or no endogenous PKC delta protein. It is possible that 70% knockdown in HEp-2 cells was not sufficient to reduce the level of PKC delta total kinase activity to inhibit viral replication (Supplemental Figure 3 A and B) or that the requirement for PKC delta during HSV-1 replication may be a cell type specific event. Using the NCI-60 microarray database, we identified a breast cancer epithelial cell line, BT-549, with reduced mRNA expression levels of PRKCD. The NCI-60 data indicated that BT-549 cells express low levels of PRKCD mRNA as compared to another breast cancer epithelial line, MCF-7, which have a median expression profile for PRKCD (http://dtp.nci.nih.gov/mtweb/search.jsp). MCF-7 cells express readily detectable amounts of PKC delta while BT-549 cells have almost undetectable levels of PKC delta before or after infection (Figure 4 C and data not shown) (Jackson et al., 2005). The breast cancer cells were infected with five PFU/cell of WT HSV-1(F) and infectivity was determined at 24 hpi (Figure 4 D). There was not a significant difference in viral infectivity between MCF-7 and BT-549 cells (Figure 4 D). Collectively, results from shRNA-expressing and breast cancer cell comparisons suggest that protein level of PKC delta does not significantly alter WT HSV-1 replication (Supplemental Figure 3 and Figure 4 D).
Emerin hyperphosphorylation is necessary for disassociation from the lamina during HSV-1 infection
Another goal of this study was to determine if phosphorylation of lamina components regulates their association with the nuclear lamina during herpesvirus infection. It has been previously hypothesized that recruitment of kinases to the NE and concurrent phosphorylation of nuclear lamina proteins is one mechanism of generating the necessary flexibility in the lamina for primary envelopment. However, it remains unknown if phosphorylation of nuclear lamina proteins, such as emerin, is required for their re-localization during HSV-1 infection. It also remains unknown if phosphorylation of lamina components, LAPs and lamins, is required for nuclear egress. Since HSV-1 induced emerin hyperphosphorylation is blocked in the absence of pUS3 kinase activity and in the presence of Rottlerin (Leach et al., 2007), these conditions were used as a tool to induce known phosphorylation states in emerin and to determine whether hyperphosphorylation of emerin is required for disassociation from the lamina.
HEp-2 cells were mock infected or infected with five PFU/cell of WT or pUS3 kinase-dead virus (vRR1204) and treatment with vehicle (DMSO) or Rottlerin began at five hpi. At 16 hpi, cells were fixed and stained for emerin and pUL34 and visualized by confocal microscopy (Figure 5 A). Mock-infected HEp-2 cells exhibited smooth emerin localization around the NE (Figure 5 Aa). WT-infected, vehicle-treated cells demonstrated two changes in the appearance of emerin reactivity (Leach et al., 2007). (i) Most emerin is still smoothly distributed in the nuclear membrane and reveals the infection-dependent alteration of the contour of the NE from a smooth ovoid to a wrinkled ovoid. (ii) Some emerin is localized to blebs that appear both inside and outside the NE. In both places it co-localizes with pUL34 (Figure 5 Ad,e,f). One of the two changes in emerin appearance is lost in WT virus-infected, Rottlerin-treated cells. All emerin is smoothly distributed in the nuclear membrane and the interior and exterior membrane but the blebs are absent (Figure 5 A compare g to d). The contour of the NE is still altered to an irregular ovoid in the presence of Rottlerin (Figure 5 Ah,i), suggesting that the NE contour change is not dependent on Rottlerin-sensitive phosphorylation events.
It has been previously demonstrated that in the absence of pUS3 activity and in the presence of Rottlerin treatment, emerin modification is indistinguishable from that in uninfected cells (Leach et al., 2007). Based on the results from Figure 5 Aa–i, our hypothesis would predict that under these same conditions (pUS3-kinase dead and presence of Rottlerin), emerin localization would resemble that seen in mock-infected cells. pUS3 kinase-dead (vRR1204)-infected, vehicle-treated cells showed pUL34 and emerin co-localization in puncta around the NE indicating dissociation from the nuclear lamina (Leach et al., 2007) (Figure 5 Aj,k,l). As predicted, pUS3 kinase-dead (vRR1204)-infected, Rottlerin-treated cells showed emerin localization indistinguishable from mock (Figure 5 A compare m to a). Interestingly, although pUL34 and emerin are binding partners, pUL34 still localized in puncta (Figure 5 An) and no longer co-localized with emerin (Figure 5 Ao) (Leach et al., 2007). These results suggest that emerin hyperphosphorylation is necessary for its disassociation from the lamina and for its association with pUL34.
We previously reported pUL34-dependent, pUS3-independent emerin phosphorylation events are sensitive to Rottlerin treatment. These data suggested that pUL34-dependent, phosphorylation is mediated by the cellular kinase PKC delta (Leach et al., 2007). However, the usefulness of Rottlerin as a PKC delta inhibitor has since been brought into question. Additionally, data presented in Figures 1, 2, and 4 suggest that while inhibition of the entire PKC family leads to a nuclear egress block, PKC delta nor cPKCs are not uniquely required for HSV replication. To test if the pUL34-dependent emerin phosphorylation event relies on the activity of a PKC isoform, HEp2 cells were mock infected or infected with five PFU/cell of WT or pUS3 kinase-dead (vRR1204) viruses. At five hpi, treatment began with vehicle (DMSO) (D), Rottlerin (R), or BIM I (B). At 16 hpi, lamina preparations were performed, proteins were separated on an SDS-PAGE gel, transferred to nitrocellulose, and then probed for emerin (Figure 5 B). As shown previously, infection with WT HSV-1 induces hyperphosphorylation of emerin, resulting in the appearance of multiple bands that migrate more slowly than the single major band seen in uninfected cells (compare lanes one and two) (Leach et al., 2007). Emerin hyperphosphorylation is reduced in the absence of pUS3 kinase activity (lane five, two bands) or in the presence of Rottlerin (lane four, middle band is most prominent), and is completely eliminated in cells infected with vRR1204 and treated with Rottlerin (compare lanes seven (one species) and eight). Treatment with BIM I, however, does not result in any change in hyper-phosphorylated species present in cells infected with either WT (compare lanes two and three) or pUS3 kinase-dead virus (compare lanes five and six, two species in each lane). Similar results were observed in Vero cells (data not shown). The lack of mobility shift upon BIM I treatment suggests that BIM I inhibition of PKC isoforms late in HSV-1 infection does not affect HSV-1 induced emerin hyperphosphorylation.
Emerin localization in the infected cell nuclear membrane depends upon its phosphorylation state yet BIM I treatment does not alter emerin’s phosphorylation state (Figure 5 A and B). Therefore, we would predict BIM I treatment would not alter emerin localization. To test this prediction, the same experimental conditions were used as in Figure 5 A except PKCs were inhibited by treatment with vehicle (DMSO) or BIM I beginning at five hpi. At 20 hpi, the cells were fixed in formaldehyde and prepared for confocal microscopy (Figure 5 C). Consistent with our hypothesis, emerin mislocalization and co-localization with pUL34 were not appreciably changed by treatment with BIM I in cells infected with either WT virus (Figure 5 C, compare panels d–f and g–i) or pUS3 kinase-dead virus (Figure 5 C compare panels j–l and m–o).
PKC delta is recruited to the nuclear envelope during HSV-1 infection (Supplemental Figure 2 Bd) (Park and Baines, 2006). Emerin appears to be re-localized in a phosphorylation dependent manner (Figure 5 A), however, inhibition of the entire PKC family did not block emerin hyperphosphorylation (Figure 5 B). To test for a specific role for PKC delta activity in HSV-1-induced emerin re-localization, cells from a stable EGFP-emerin-expressing HEp-2 cell line were transfected with PRKCDwt-UL34 Duo or PRKCDdn-UL34 Duo. One day later, the cells were infected with 20 PFU/cell pUS3 kinase-dead virus. At 20 hpi, cells were fixed and processed for detection of FLAG and pUL34 by immunofluorescence (Figure 5 D). If PKC delta activity is required for emerin re-localization in the absence of pUS3 activity, then PRKCDdn-UL34 Duo transfected cells should be unable to localize emerin to puncta. Contrary to our hypothesis, emerin re-localization to puncta was observed in cells that express both PRKCDwt and PRKCDdn (Figure 5 D, compare panels d–f and g–i).
DISCUSSION
The involvement of PKCs in nuclear egress of herpesviruses appears to be a conserved property of herpesviruses. Both PKC alpha and delta, but not zeta, are recruited to the NE in HSV-1 infection while cPKCs are recruited upon HCMV infection (Muranyi et al., 2002; Park and Baines, 2006). For HSV, HCMV and MCMV, PKC recruitment depends on expression of pUL34 (or its HCMV and MCMV homologs pUL50 and M50), further suggesting a role in nuclear egress of capsids (Muranyi et al., 2002; Park and Baines, 2006). In the case of HCMV, recruitment of cPKCs to the NE also requires cPKC activity, since recruitment can be inhibited by the specific cPKC inhibitor Ro-31–7549 (Muranyi et al., 2002). Data presented here support the hypothesis that the PKC activity is required for replication of HSV and specifically, for nuclear egress of the virus capsid. Inhibition of all PKC isoforms by the pan-PKC inhibitor BIM I beginning at five hr post-infection, results in a five-fold reduction in new virus production (Figure 1). The reduction in new virus production is accompanied by retention of capsids in the cell nucleus (Table 1) suggesting that it is at least partially attributable to inhibition of nuclear egress. This is the first evidence that PKC function is required for efficient nuclear egress and supports the hypothesis that phosphorylation-mediated nuclear lamina breakdown is important for herpesvirus nuclear egress.
Interestingly, our data also suggest a role for PKC function in formation or stability of capsids (Table 1). BIM I inhibition of PKC activity reduced the overall number of capsids in infected cells (DMSO-631 vs BIM I-223). The reduction in capsid number is not apparently due to overall diminished viral protein synthesis late in infection since we observed no inhibition of overall protein synthesis (Figure 3). The decrease in capsid number could be attributable an important function of PKCs in capsid assembly or stability, directly or indirectly through signaling events. These data suggest that of PKC activity is involved in (i) accumulation of capsids and (ii) egress of capsids that do accumulate from the nucleus.
The recruitment of conventional PKCs to the NE during HSV and CMV infection suggests specific roles for cPKCs in herpesvirus nuclear egress (Muranyi et al., 2002; Park and Baines, 2006). We tested for a unique role for cPKCs in HSV infection by use of a specific chemical inhibitor, and were surprised to observe that inhibition of cPKCs with Ro-31–7549 had no effect on replication (Figure 4 A). This suggests that cPKCs do not have a unique, specific role in HSV replication. Recruitment of PKC delta to the NE in a pUL34-dependent manner in HSV-infected cells suggested that PKC delta might be required for HSV replication due to its proposed function in lamina disruption prior to nuclear egress (Park and Baines, 2006). Similar to cPKCs, inhibition of PKC delta had no effect on viral replication (Figure 4 B and Supplemental Figure 3). These results for cPKCs and PKC delta, along with our findings that inhibition of the entire family (Figure 1) does inhibit HSV-1 replication are consistent with two hypotheses. First, it is possible that the functions of cPKCs and PKC delta are redundant. Second, it is also possible that these kinases are in fact irrelevant and that some other PKC isoform (perhaps another of the nPKCs or aPKC) plays an important role in viral replication and nuclear egress.
Emerin and lamin B have both been reported to be phosphorylated by PKC delta in HSV-1-infected cells (Leach et al., 2007; Park and Baines, 2006). However, this conclusion was based on sensitivity of phosphorylation to Rottlerin. Since it has become clear that Rottlerin is not a specific PKC delta inhibitor in intact cells, we re-examined the dependence of emerin hyperphosphorylation on PKC activity. HSV-1-induced emerin hyperphosphorylation does not appear to be dependent on activity of a PKC isoform since its mobility in SDS-PAGE was not altered by the inhibition of all PKC isoforms with BIM I (Figure 5 B), and its infected cell localization was not altered by expression of DN-PKC delta (Figure 5 D). This creates a class of enzymes we termed Rottlerin sensitive kinases (RttSK) since they are sensitive to Rottlerin but not specific PKC delta inhibition. The RttSK responsible for pUL34-dependent emerin phosphorylation remains unknown at this time, but possible candidates include: AKT, PDK1, JNK1alpha, GSK3beta, PKA, and/or MSK1 (Soltoff, 2007).
Our data also show that protein synthesis in uninfected HEp-2 and Vero cells is dependent on PKC activity, but protein synthesis in wild-type HSV-infected cells is not (Figure 3). Therefore, HSV infection apparently protects the infected cell protein synthesis machinery from the effect of PKC inhibition. PKC isoforms have been identified mostly as positive regulators of translation in different cell types and in response to different stimuli. Positive effects on translation have mostly been attributed to indirect signaling effects through Erk, MAPK or mTOR signaling pathways (Aeder et al., 2004; Akimoto et al., 1998; Herbert et al., 2000; Kumar et al., 2000; Romanelli et al., 1999; Wang, Rolfe, and Proud, 2003), but cPKCs have also been reported to directly phosphorylate eIF4e (Whalen et al., 1996). The difference between infected and uninfected cells in PKC dependence of protein synthesis suggests that HSV infection provides a functional substitute for PKC in maintenance of protein synthesis. HSV-1 is known to protect host protein synthesis from protein kinase R (PKR)-induced shut-off through pUS11 and ICP34.5 (Mohr, 2006). It may be that the virus encodes or induces other activities to maintain robust synthesis of viral proteins.
Many lines of evidence suggest that localization and function of emerin is regulated by its phosphorylation state. Studies in a Xenopus oocyte cell-free system, demonstrated that emerin binding to barrier to auto-integration factor (BAF) was un-coupled by a mitotic phosphorylation event on Ser 175 (Hirano et al., 2005). Emerin is phosphorylated in a cell cycle-dependent manner, with maximal phosphorylation observed during mitosis (Ellis et al., 1998). Some Emery-Dreifuss Muscular Dystrophy (EDMD) patients with mutant emerin have increased emerin phosphorylation that correlates with emerin mislocalization away from the NE suggesting that phosphorylation regulates emerin localization and function and aberrant phosphorylation may contribute to EDMD pathogenesis (Ellis et al., 1998; Manilal et al., 1998; Somech et al., 2005). Emerin is hyperphosphorylated in HSV-1 infected cells, and this is correlated with disconnection of emerin from lamin A/C (Leach et al., 2007). From these data we hypothesized that emerin disassociation from the lamina is caused by its phosphorylation state (Figure 5 A). Using conditions shown previously to completely ablate emerin hyperphosphorylation, we observed that, in fact, these same conditions result in maintenance of emerin connections to the lamina but not to pUL34 (Figure 5 A). Although PKC activity appears important for HSV-1 growth due to a block in (i) nuclear egress and (ii) capsid accumulation, it does not appear to be involved in the hyperphosphorylation of emerin (Figure 5 B). This was surprising since emerin hyperphosphorylation is sensitive to Rottlerin treatment (Figure 5 B) (Leach et al., 2007). Emerin localization also is insensitive to PKC delta activity (Figure 5 D), although this enzyme is recruited to the NE (Supplemental Figure 2 B) (Park and Baines, 2006). It remains unknown which cellular enzyme mediates emerin hyperphosphorylation, and if phosphorylation of emerin is required for nuclear egress.
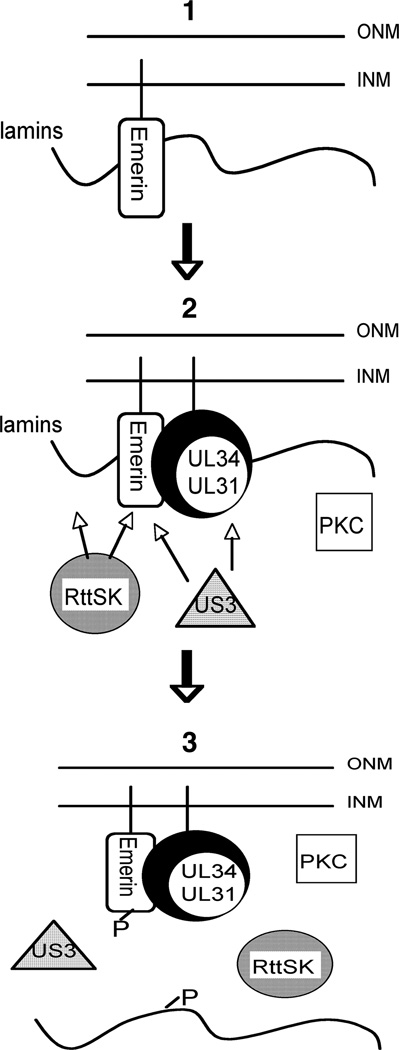
All of these data are consistent with the following model (Figure 6). (1) Emerin is bound to lamin A/C in the uninfected cell. It is also anchored to the INM via its transmembrane domain. (2) Upon HSV-1 infection, pUL34 and pUL31 are expressed and assemble a complex. pUL34 anchors the complex at the INM via its transmembrane domain. The pUL34/pUL31 complex recruits PKC isoforms, pUS3, and non-PKC Rottlerin sensitive kinase(s) to the NE. Emerin, lamin A, lamin C, and lamin B are phosphorylated. Independently of pUL34/pUL31, pUS3 can be recruited to phosphorylate emerin (3). Phosphorylated emerin no longer interacts with phosphorylated lamin A/C creating the flexibility necessary for the capsid to bypass the lamina and access INM for envelopment. In this model, emerin is the prototype LAP and it remains to be determined if the localization and interactions of other LAPs, such as MAN1 or LBR, are regulated by phosphorylation in the infected cell as emerin appears to be.
Figure 6. Model of HSV-1 induced nuclear lamina disruption.
(1) In the un-infected cell, emerin is bound to the lamins via interaction with lamin A/C. Emerin is intended to be a prototype LAP protein and may behave in the cell similarly to MAN1 or other LAPs. (2) Upon infection with HSV-1, pUL34 and pUL31 are expressed and accumulated as a complex at the inner nuclear membrane (INM). pUL34/pUL31 recruits pUS3, the alpha-herpesvirus viral kinase, PKCs, and Rottlerin Sensitive Kinases (RttSK) to the NE to phosphorylate emerin, lamins and pUL34/pUL31. Arrows indicate that pUS3 and RttSK are known to be necessary for phosphorylation of emerin, pUL34/pUL31, and lamin B. (3) Phosphorylation of lamins and emerin, prevents there binding and creates flexibility within the lamina necessary for nuclear egress. PKCs, RttSK, and pUS3 are depicted near the INM due to their localization at the NE during infection.
AKNOWLEDGMENTS
We would like to thank Alison Haugo and Jean Ross for excellent technical assistance with confocal and transmission electron microscopy, respectively. We would like to thank Dr. Wendy Maury, Dr. Mark Stinski, Alison Haugo, Andrew Kondratowicz, and Martina Maric for critical reading of the manuscript. This work was supported by the University of Iowa and by PHS award AI 41478. N. R. L. also received support form PHS grant T32 AI 007533.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aebi U, Cohn J, Buhle L, Gerace L. The nuclear lamina is a meshwork of intermediate-type filaments. Nature. 1986;323(6088):560–564. doi: 10.1038/323560a0. [DOI] [PubMed] [Google Scholar]
- Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene. 2004;23(56):9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- Akimoto K, Nakaya M, Yamanaka T, Tanaka J, Matsuda S, Weng QP, Avruch J, Ohno S. Atypical protein kinase Clambda binds and regulates p70 S6 kinase. Biochem J. 1998;335(Pt 2):417–424. doi: 10.1042/bj3350417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke SL, Cowan JM, Kerr JK, Reynolds AE, Baines JD, Roller RJ. Effects of charged cluster mutations on the function of herpes simplex virus type 1 UL34 protein. J Virol. 2003;77(13):7601–7610. doi: 10.1128/JVI.77.13.7601-7610.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke SL, Roller R. Roles for herpes simplex type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006a;347(2):261–276. doi: 10.1016/j.virol.2005.11.053. 261–76(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjerke SL, Roller RJ. Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress. Virology. 2006b;347(2):261–276. doi: 10.1016/j.virol.2005.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camozzi D, Pignatelli S, Valvo C, Lattanzi G, Capanni C, Dal Monte P, Landini MP. Remodelling of the nuclear lamina during human cytomegalovirus infection: role of the viral proteins pUL50 and pUL53. J. Gen. Virol. 2008;89(3):731–740. doi: 10.1099/vir.0.83377-0. [DOI] [PubMed] [Google Scholar]
- Cano-Monreal GL, Wylie KM, Cao F, Tavis JE, Morrison LA. Herpes simplex virus 2 UL13 protein kinase disrupts nuclear lamins. Virology. 2009;392(1):137–147. doi: 10.1016/j.virol.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JA, Craxton M, Yates JR, Kendrick-Jones J. Aberrant intracellular targeting and cell cycle-dependent phosphorylation of emerin contribute to the Emery-Dreifuss muscular dystrophy phenotype. J Cell Sci. 1998;111(Pt 6):781–792. doi: 10.1242/jcs.111.6.781. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, Fridkin A, Dayani Y, Prokocimer M, Enosh A. The nuclear lamina and its functions in the nucleus. Int Rev Cytol. 2003;226:1–62. doi: 10.1016/s0074-7696(03)01001-5. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y, Margalit A, Goldman RD, Shumaker DK, Wilson KL. The nuclear lamina comes of age. Nat Rev Mol Cell Biol. 2005;6(1):21–31. doi: 10.1038/nrm1550. [DOI] [PubMed] [Google Scholar]
- Hamirally S, Kamil JP, Ndassa-Colday YM, Lin AJ, Jahng WJ, Baek MC, Noton S, Silva LA, Simpson-Holley M, Knipe DM, Golan DE, Marto JA, Coen DM. Viral mimicry of Cdc2/cyclin-dependent kinase 1 mediates disruption of nuclear lamina during human cytomegalovirus nuclear egress. PLoS Pathog. 2009;5(1):e1000275. doi: 10.1371/journal.ppat.1000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TP, Kilhams GR, Batty IH, Proud CG. Distinct signalling pathways mediate insulin and phorbol ester-stimulated eukaryotic initiation factor 4F assembly and protein synthesis in HEK 293 cells. J Biol Chem. 2000;275(15):11249–11256. doi: 10.1074/jbc.275.15.11249. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Segawa M, Ouchi FS, Yamakawa Y, Furukawa K, Takeyasu K, Horigome T. Dissociation of emerin from barrier-to-autointegration factor is regulated through mitotic phosphorylation of emerin in a xenopus egg cell-free system. J Biol Chem. 2005;280(48):39925–39933. doi: 10.1074/jbc.M503214200. [DOI] [PubMed] [Google Scholar]
- Holmer L, Worman HJ. Inner nuclear membrane proteins: functions and targeting. Cell Mol Life Sci. 2001;58(12–13):1741–1747. doi: 10.1007/PL00000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume AJ, Finkel JS, Kamil JP, Coen DM, Culbertson MR, Kalejta RF. Phosphorylation of retinoblastoma protein by viral protein with cyclin-dependent kinase function. Science. 2008;320(5877):797–799. doi: 10.1126/science.1152095. [DOI] [PubMed] [Google Scholar]
- Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI, Humphries MJ, Reyland ME, Foster DA. Suppression of cell migration by protein kinase Cdelta. Oncogene. 2005;24(18):3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kato K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev Med Virol. 2003;13(5):331–340. doi: 10.1002/rmv.402. [DOI] [PubMed] [Google Scholar]
- Konishi H, Tanaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94(21):11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J. Virol. 2003;77(2):905–914. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Pandey P, Sabatini D, Kumar M, Majumder PK, Bharti A, Carmichael G, Kufe D, Kharbanda S. Functional interaction between RAFT1/FRAP/mTOR and protein kinase cdelta in the regulation of cap-dependent initiation of translation. EMBO J. 2000;19(5):1087–1097. doi: 10.1093/emboj/19.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach N, Bjerke SL, Christensen DK, Bouchard JM, Mou F, Park R, Baines J, Haraguchi T, Roller RJ. Emerin is hyperphosphorylated and redistributed in herpes simplex virus type 1-infected cells in a manner dependent on both UL34 and US3. J Virol. 2007;81(19):10792–10803. doi: 10.1128/JVI.00196-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CP, Huang YH, Lin SF, Chang Y, Chang YH, Takada K, Chen MR. Epstein-Barr virus BGLF4 kinase induces disassembly of the nuclear lamina to facilitate virion production. J Virol. 2008;82(23):11913–11926. doi: 10.1128/JVI.01100-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Park JW, Choi KS, Park YB, Kwon TK. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis. 2009;30(5):729–736. doi: 10.1093/carcin/bgn265. [DOI] [PubMed] [Google Scholar]
- Manilal S, Recan D, Sewry CA, Hoeltzenbein M, Llense S, Leturcq F, Deburgrave N, Barbot J, Man N, Muntoni F, Wehnert M, Kaplan J, Morris GE. Mutations in Emery-Dreifuss muscular dystrophy and their effects on emerin protein expression. Hum Mol Genet. 1998;7(5):855–864. doi: 10.1093/hmg/7.5.855. [DOI] [PubMed] [Google Scholar]
- Margalit A, Vlcek S, Gruenbaum Y, Foisner R. Breaking and making of the nuclear envelope. J Cell Biochem. 2005;95(3):454–465. doi: 10.1002/jcb.20433. [DOI] [PubMed] [Google Scholar]
- Marschall M, Marzi A, aus dem Siepen P, Jochmann R, Kalmer M, Auerochs S, Lischka P, Leis M, Stamminger T. Cellular p32 recruits cytomegalovirus kinase pUL97 to redistribute the nuclear lamina. J Biol Chem. 2005;280(39):33357–33367. doi: 10.1074/jbc.M502672200. [DOI] [PubMed] [Google Scholar]
- Mohr I. Phosphorylation and dephosphorylation events that regulate viral mRNA translation. Virus Res. 2006;119(1):89–99. doi: 10.1016/j.virusres.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Morris JB, Hofemeister H, O'Hare P. Herpes simplex virus infection induces phosphorylation and delocalization of emerin, a key inner nuclear membrane protein. J Virol. 2007;81(9):4429–4437. doi: 10.1128/JVI.02354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Forest T, Baines JD. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol. 2007;81(12):6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou F, Wills EG, Park R, Baines JD. Effects of lamin A/C, lamin B1, and viral US3 kinase activity on viral infectivity, virion egress, and the targeting of herpes simplex virus U(L)34-encoded protein to the inner nuclear membrane. J Virol. 2008;82(16):8094–8104. doi: 10.1128/JVI.00874-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranyi W, Haas J, Wagner M, Krohne G, Koszinowski UH. Cytomegalovirus recruitment of cellular kinases to dissolve the nuclear lamina. Science. 2002;297(5582):854–857. doi: 10.1126/science.1071506. [DOI] [PubMed] [Google Scholar]
- Panorchan P, Schafer BW, Wirtz D, Tseng Y. Nuclear envelope breakdown requires overcoming the mechanical integrity of the nuclear lamina. J. Biol. Chem. 2004;279(42):43462–43467. doi: 10.1074/jbc.M402474200. [DOI] [PubMed] [Google Scholar]
- Park R, Baines JD. Herpes simplex virus type 1 infection induces activation and recruitment of protein kinase C to the nuclear membrane and increased phosphorylation of lamin B. J Virol. 2006;80(1):494–504. doi: 10.1128/JVI.80.1.494-504.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radsak KD, Brucher KH, Georgatos SD. Focal nuclear envelope lesions and specific nuclear lamin A/C dephosphorylation during infection with human cytomegalovirus. Eur J Cell Biol. 1991;54(2):299–304. [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009a;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009b;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Liang L, Baines JD. Conformational changes in the nuclear lamina induced by herpes simplex virus type 1 require genes U(L)31 and U(L)34. J Virol. 2004;78(11):5564–5575. doi: 10.1128/JVI.78.11.5564-5575.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Ryckman BJ, Baines JD, Zhou Y, Liang L, Roller RJ. U(L)31 and U(L)34 proteins of herpes simplex virus type 1 form a complex that accumulates at the nuclear rim and is required for envelopment of nucleocapsids. J Virol. 2001;75(18):8803–8817. doi: 10.1128/JVI.75.18.8803-8817.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34, and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76(17):8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Monk LL, Stuart D, Roizman B. Structure and function in the herpes simplex virus 1 RNA-binding protein U(s)11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J Virol. 1996;70(5):2842–2851. doi: 10.1128/jvi.70.5.2842-2851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. Journal of Virology. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller RJ, Zhou Y, Schnetzer R, Ferguson J, DeSalvo D. Herpes simplex virus type 1 U(L)34 gene product is required for viral envelopment. J Virol. 2000;74(1):117–129. doi: 10.1128/jvi.74.1.117-129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli A, Martin KA, Toker A, Blenis J. p70 S6 kinase is regulated by protein kinase Czeta and participates in a phosphoinositide 3-kinase-regulated signalling complex. Mol Cell Biol. 1999;19(4):2921–2928. doi: 10.1128/mcb.19.4.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckman BJ, Roller RJ. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J Virol. 2004;78(1):399–412. doi: 10.1128/JVI.78.1.399-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott ES, O'Hare P. Fate of the inner nuclear membrane protein lamin B receptor and nuclear lamins in herpes simplex virus type 1 infection. J Virol. 2001;75(18):8818–8830. doi: 10.1128/JVI.75.18.8818-8830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holley M, Baines J, Roller R, Knipe DM. Herpes simplex virus 1 U(L)31 and U(L)34 gene products promote the late maturation of viral replication compartments to the nuclear periphery. J Virol. 2004;78(11):5591–5600. doi: 10.1128/JVI.78.11.5591-5600.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson-Holley M, Colgrove RC, Nalepa G, Harper JW, Knipe DM. Identification and functional evaluation of cellular and viral factors involved in the alteration of nuclear architecture during herpes simplex virus 1 infection. J Virol. 2005;79(20):12840–12851. doi: 10.1128/JVI.79.20.12840-12851.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff SP. Rottlerin: an inappropriate and ineffective inhibitor of PKCdelta. Trends Pharmacol Sci. 2007;28(9):453–458. doi: 10.1016/j.tips.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Somech R, Shaklai S, Amariglio N, Rechavi G, Simon AJ. Nuclear envelopathies--raising the nuclear veil. Pediatr Res. 2005;57(5 Pt 2) doi: 10.1203/01.PDR.0000159566.54287.6C. 8R–15R. [DOI] [PubMed] [Google Scholar]
- Tillman DM, Izeradjene K, Szucs KS, Douglas L, Houghton JA. Rottlerin sensitizes colon carcinoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis via uncoupling of the mitochondria independent of protein kinase C. Cancer Res. 2003;63(16):5118–5125. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266(24):15771–15781. [PubMed] [Google Scholar]
- Wang L, Rolfe M, Proud CG. Ca(2+)-independent protein kinase C activity is required for alpha1-adrenergic-receptor-mediated regulation of ribosomal protein S6 kinases in adult cardiomyocytes. Biochem J. 2003;373(Pt 2):603–611. doi: 10.1042/BJ20030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen SG, Gingras AC, Amankwa L, Mader S, Branton PE, Aebersold R, Sonenberg N. Phosphorylation of eIF-4E on serine 209 by protein kinase C is inhibited by the translational repressors, 4E-binding proteins. J Biol Chem. 1996;271(20):11831–11837. doi: 10.1074/jbc.271.20.11831. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Courvalin JC. Nuclear envelope, nuclear lamina, and inherited disease. Int Rev Cytol. 2005;246:231–279. doi: 10.1016/S0074-7696(05)46006-4. [DOI] [PubMed] [Google Scholar]
- Zhang C, Hirasawa N, Beaven MA. Antigen activation of mitogen-activated protein kinase in mast cells through protein kinase C-dependent and independent pathways. J. Immunol. 1997;158(10):4968–4975. [PubMed] [Google Scholar]