Abstract
Pompe disease is caused by an inherited deficiency of acid α-glucosidase (GAA), a lysosomal enzyme that catalyzes the breakdown of glycogen to glucose. In the absence of GAA, enlarged, glycogen-laden lysosomes accumulate in multiple tissues, although the major clinical manifestations are seen in cardiac and skeletal muscle. For many years, it was believed that the rupture of glycogen-filled lysosomes was the major cause of the profound muscle damage observed in patients with Pompe disease. Here, we present evidence that a failure of productive autophagy in muscle tissue contributes strongly to disease pathology in both patients with Pompe disease and GAA-knockout mice. In the GAA-knockout mouse model, progressive accumulation of autophagic vesicles is restricted to Type II-rich muscle fibers. Not only does this build-up of autophagosomes disrupt the contractile apparatus in the muscle fibers, it also interferes with enzyme replacement therapy by acting as a sink for the recombinant enzyme and preventing its efficient delivery to the lysosomes. Our data indicate that a re-examination of the presumed pathological mechanism in Pompe disease is necessary, and suggest that successful treatment of patients with Pompe disease will require consideration of the dramatic failure of autophagy that occurs in this disease.
Keywords: autophagy, enzyme replacement therapy, glycogen, Pompe disease, skeletal muscle
Introduction
Pompe disease is caused by an inherited deficiency of the lysosomal enzyme acid α-glucosidase (GAA) [Hirschhorn and Reuser 2001]. This enzyme degrades glycogen to glucose in lysosomes, and its deficiency results in the accumulation of lysosomal glycogen in multiple tissues. Pompe disease is a systemic disorder, but the symptoms are mainly due to skeletal and cardiac muscle involvement [Hirschhorn and Reuser 2001]. There is, however, extreme clinical and genetic heterogeneity, with more than 300 variants identified to date (see www.pompecenter.nl). It is also the only lysosomal storage disease in which muscle is the primary target of enzyme replacement therapy (ERT). In 2006, a Chinese hamster ovary-derived form of recombinant human GAA (rhGAA), alglucosidase-α (Myozyme®, Genzyme Corporation, Framingham, MA, USA), became available for patients, making Pompe disease the first inherited muscle disorder to be treated by ERT.
Pompe disease has been studied extensively in the past decades, but it is still unclear how the primary defect – glycogen storage in enlarged lysosomes – leads to profound destruction of skeletal muscle. The prevailing view of pathogenesis, which was put forward more than 20 years ago, is that lysosomes in muscle cells have little space to expand; thus, muscle damage occurs when glycogen-loaded lysosomes are subjected to mechanical stress, leading to membrane rupture [Griffin 1984]. In the last couple of years we have gathered considerable evidence that this view of the pathology is incomplete. The majority of the findings presented here were obtained while studying the effect of ERT in our mouse model of the disease.
ERT in Pompe disease
ERT takes advantage of the fact that in normal cells a small fraction of newly synthesized lysosomal enzymes is secreted. The majority of secreted lysosomal enzymes can be taken up from the extracellular space by cation-independent mannose-6-phosphate receptor (CI-MPR)-mediated endocytosis and delivered to lysosomes [Ghosh et al. 2003, Kornfeld 1992]. In patients with Pompe disease, the missing enzyme is delivered as a mannose-6-phosphate-tagged precursor that binds to the CI-MPR on the plasma membrane and enters the cell in clathrin-coated vesicles. From this point on, the enzyme undergoes stepwise proteolytic processing/activation as it passes through the endocytic pathway. The receptor-enzyme complex traffics through early endosomes to late endosomes, where the complex dissociates at the acidic pH. The receptor recycles back to the plasma membrane and the enzyme is delivered to the lysosomes, where it is converted to its mature form and catalyzes the breakdown of accumulated glycogen [Moreland et al. 2005, Wisselaar et al. 1993].
In clinical trials in infants and in laboratory trials using our GAA-knockout mice, treatment with rhGAA successfully reversed cardiac abnormalities [Amalfitano et al. 2001, Kishnani et al. 2006, 2007, Raben et al. 2003, 2005, Van den Hout et al. 2000, 2004]. The situation in skeletal muscle, however, was more complicated. In mice, it was quickly apparent that glycolytic, fast-twitch Type II skeletal muscle fibers (particularly Type IIb) responded poorly to therapy. After months of treatment with high dosages of rhGAA, we observed only a very modest glycogen reduction in Type II fibers. On the other hand, glycogen was cleared very efficiently from oxidative, slow-twitch Type I fibers. The poor response of Type II muscle fibers to therapy is puzzling because in untreated animals, Type II fibers accumulate significantly less glycogen than cardiac or Type I fibers. We noted lower levels of trafficking proteins (including CI-MPR, clathrin and adaptor protein-2) in Type II-rich muscle than in Type I-rich muscle, suggesting that rhGAA uptake and delivery might be less efficient in the therapy-resistant fibers [Raben et al. 2003, 2005]. The elephant in the room, however, was the presence of large autophagic areas in Type II fibers.
Autophagic accumulation in Pompe disease
Autophagy (self-eating) is a process of lysosomal degradation of long-lived proteins and damaged organelles. In macroautophagy (hereafter referred to as autophagy), double-membrane vesicles known as autophagosomes sequester cytoplasm and damaged organelles. The autophagosomes fuse with endosomes, giving rise to amphisomes, followed by fusion with lysosomes where degradation of autophagosomal contents is completed [Berg et al. 1998, Liou et al. 1997].
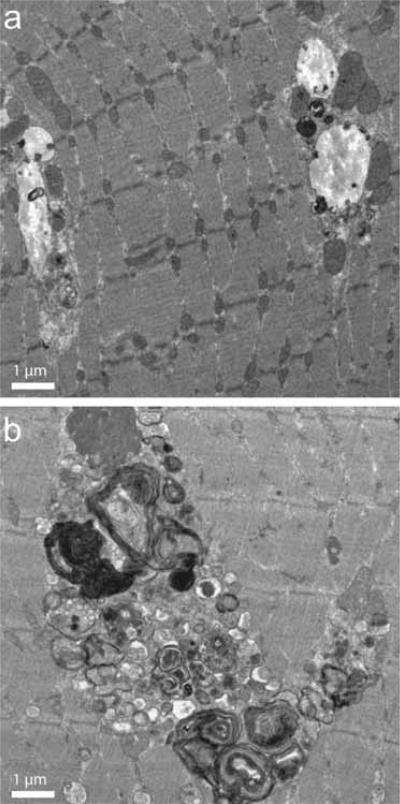
Autophagy is a critical survival mechanism under conditions of nutrient deprivation and appears to play a role in basal protein turnover as well (reviewed in [Mizushima 2005]). Under normal conditions, autophagosomes and amphisomes are quickly degraded by lysosomes, and their content is recycled. By contrast, in Type II muscle fibers from GAA-knockout mice, significant accumulation of autophagosomes was observed. Large non-contractile regions containing abundant double-membrane autophagosomes were clearly visible in Type II-rich (gastrocnemius) muscle examined by electron microscopy. In Type I-rich (soleus) muscle, on the other hand, only a small number of isolated autophagosomes were observed (Figure 1). These results were confirmed by confocal microscopy of isolated single muscle fibers. Labeling fibers with antibodies against the lysosomal-associated membrane protein Type 1 (LAMP-1), revealed expanded lysosomes in both fiber types. Staining for the autophagosomal marker, LC3 (microtubule-associated protein 1 light chain 3), demonstrated accumulation of autophagic vesicles in therapy-resistant Type II fibers, and only pockets of autophagic activity in Type I fibers [Fukuda et al. 2006b].
Figure 1.
Electron micrographs of (a) Type I-rich (soleus) muscle and (b) Type II-rich (gastrocnemius) muscle from a 5-month-old acid α-glucosidase knockout mouse.
Autophagic accumulation starts very early in the Type II muscle fibers of GAA-knockout mice. In fibers taken from 1-month-old mice, centrally located LC3-positive vesicles were already observed, interspersed with smaller LAMP-1-positive vesicles. In older mice, the autophagic area expanded, eventually encompassing more than a third of the fiber diameter in many cases. Accumulation of late endosomes in the autophagic area was also apparent at this stage. As the disease progressed (in 2-year-old mice) the integrity of the vesicles in the autophagic areas was lost and only remnants of vacuolar membranes could be seen [Fukuda et al. 2006a]. The autophagic build-up appeared to interrupt muscle striations, as indicated by myosin staining [Fukuda et al. 2006c]. Massive accumulation of lipofuscin – a presumed product of oxidative damage – was also observed in the autophagic area of Type II fibers from mice as young as 3 months of age [Fukuda et al. 2006a]. Once recognized, the autophagic areas could be easily seen in unstained fixed and live fibers by low resolution transmitted light microscopy (Figure 2). Progressive autophagic build-up is clearly disease-related because large regions of LC3-positive material were not present in muscle fibers from age-matched wild-type mice.
Figure 2.
Differential interference contrast microscopy images of unstained single muscle fibers from (a) 6-month-old wild-type and (b) acid α-glucosidase (GAA)-knockout mice. Centrally located autophagic accumulation is clearly visible throughout the length of the fiber from the GAA-knockout mouse. Bar, 20 μm.
Implications of autophagic accumulation for ERT
It became clear that massive accumulation of autophagic material in muscle would interfere with the delivery of the recombinant enzyme to the lysosomes. The endocytic pathway – the route used by the recombinant enzyme – and the autophagic pathway are part of the same lysosomal degradative system, and the two pathways communicate extensively at several levels. Autophagosomes fuse with endosomes to produce amphisomes, and amphisomes fuse with lysosomes [Berg et al. 1998, Liou et al. 1997]. Thus, rhGAA may become trapped in the autophagic region in Type II fibers. To test this, we isolated live muscle fibers and incubated them with labeled rhGAA. In wild-type fibers, rhGAA was successfully targeted to lysosomes. By contrast, in muscle fibers from GAA-knockout mice, most of the endocytosed recombinant enzyme accumulated in the central autophagic area and very little reached the lysosomes [Fukuda et al. 2006a]. Impaired delivery of rhGAA to lysosomes may thus contribute to the resistance of Type II fibers to ERT.
In adult patients with Pompe disease, we saw many of the same pathological changes as in GAA-knockout mice. In each individual patient with late-onset Pompe disease, muscle fibers are characterized by a continuum of phenotypic severity, allowing us to construct a proposed time course for progressive muscle damage in this condition. In less affected fibers, we observed rows of small LAMP-2-and/or LC3-positive vesicles in the core of the fiber, indicating that a subset of centrally located lysosomes were unable to degrade autophagosomal contents. Other fibers showed an intermediate level of autophagic build-up, where autophagosomes/lysosomes with clear membrane boundaries formed a continuous core in the center of the fiber. Notably, this extensive autophagic region appeared to interrupt the fiber's contractile apparatus far more than the expanded lysosomes in the periphery of the fiber. In the most severely affected fibers, a complete breakdown of muscle structure was observed along with accumulation of lipofuscin. At this stage, the fibers were almost completely devoid of CI-MPR, the protein responsible for delivery of the therapeutic enzyme to the lysosome [Raben et al. 2007].
Our data may help explain the differential responses of Type II and Type I fibers to ERT observed in GAA-knockout mouse model [Raben et al. 2003, 2005]. In Type I fibers, autophagic build-up does not occur. The contractile apparatus is largely preserved and rhGAA is delivered to lysosomes instead of becoming trapped in the autophagic region. By contrast, in Type II fibers autophagic build-up prevents efficient delivery of rhGAA to lysosomes and eventually causes breakdown of myofiber structure. It is unclear why autophagic buildup does not occur in Type I fibers, although the fact that starvation-induced auto-phagy is more robust in Type II fibers may provide a clue [Mizushima et al. 2004].
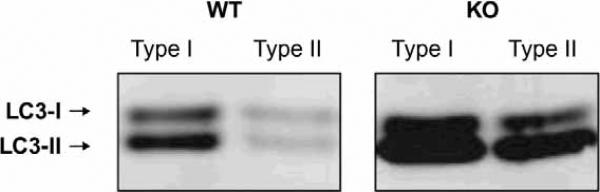
Accumulation of autophagic vacuoles could indicate upregulation of autophagy, and/or defects in autophagosomes-lysosome fusion. Figure 3 presents a western blot for the autophagosome-specific protein LC3. LC3 exists in two forms: LC3-I, a cytoplasmic protein, and LC3-II, a phosphatidylethanolamine-conjugated form that specifically associates with autophagosomes. The level of LC3-II is a reflection of the abundance of autophagosomes [Kabeya et al. 2000]. As shown in Figure 3, the levels of LC3-I and LC3-II were increased in both Type I and Type II-rich muscles of GAA-knockout mice, indicating induction of autophagy. In Type I fibers no build-up of autophagosomes was detected by immunostaining of single fibers. In Type II fibers, however, autophagic buildup was very obvious, suggesting that both increased formation and decreased degradation of autophagosomes occur.
Figure 3.
Western blot analysis of microtubule-associated protein 1 light chain 3 (LC3) in skeletal muscle derived from wild-type (WT) and acid α-glucosidase (GAA)-knockout (KO) mice. In KO mice, both Type I-rich (soleus) muscle and Type II-rich (gastrocnemius) muscle showed higher levels of LC3-I and LC3-II compared with WT. Muscle biopsies were taken from 5-month-old mice.
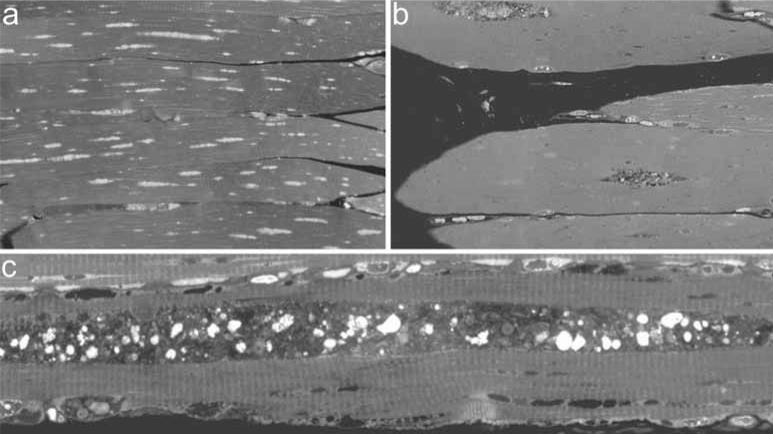
Autophagic pathology in Pompe disease may be one reason why early initiation of ERT is so critical [Thurberg et al. 2006]. Clinical trials have demonstrated that younger patients and/or patients with lower baseline muscle damage tend to respond best to ERT [Kishnani et al. 2006, 2007, Winkel et al. 2003]. We do not know when autophagic build-up begins in humans, but in GAA-knockout mice the process begins between 2 and 4 weeks after birth. No autophagic build-up is detected in biopsies from 0.5-week-old Pompe mice. In biopsies from 1-month-old mice, however, centralized regions of autophagic accumulation are already present, and by 23 months, these areas are dramatically expanded (Figure 4). When GAA-knockout mice were treated with rhGAA, autophagic vacuoles persisted even after clearance of lysosomal glycogen, indicating that vacuolization may cause permanent damage, and highlighting the potential benefit of intervention before autophagic build-up occurs [Raben et al. 2003].
Figure 4.
Toluidin blue-stained biopsies taken from Type-II rich extensor digitorum longus muscle of acid α-glucosidase knockout mice. a: 0.5-month-old mouse, b: 1-month-old mouse, c: 23-month-old mouse.
Conclusion
Observation of dramatic and disruptive autophagic accumulation in both patients with Pompe disease and in a mouse model of the disease calls for a re-evaluation of the lysosomal rupture hypothesis [Griffin 1984]. Our data, both in an animal model and in humans with late-onset disease, strongly indicate that it is not the global expansion and rupture of the lysosomes but rather a profound failure of autophagy that causes skeletal muscle damage (although lysosomal rupture certainly plays a role). One question that remains unanswered, however, is the functional state of the autophagic pathway, namely, whether there is an increase or decrease in the degradation of autophagic substrates. These different scenarios would result in vastly different pathological consequences and approaches to treatment.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health. rhGAA was provided by Genzyme Corporation (Framingham, MA, USA).
Footnotes
LS and NR have no conflicts of interest.
References
- Amalfitano A, Bengur AR, Morse RP, Majure JM, Case LE, Veerling DL, et al. Recombinant human acid α-glucosidase enzyme therapy for infantile glycogen storage disease Type II: results of a Phase I/II clinical trial. Genet Med. 2001;3:132–138. [PubMed] [Google Scholar]
- Berg TO, Fengsrud M, Stromhaug PE, Berg T, Seglen PO. Isolation and characterization of rat liver amphisomes. Evidence for fusion of autophagosomes with both early and late endosomes. J Biol Chem. 1998;273:21883–21892. doi: 10.1074/jbc.273.34.21883. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ahearn M, Roberts A, Mattaliano RJ, Zaal K, Ralston E, et al. Autophagy and mistargeting of therapeutic enzyme in skeletal muscle in Pompe disease. Mol Ther. 2006a;14:831–819. doi: 10.1016/j.ymthe.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda T, Ewan L, Bauer M, Mattaliano RJ, Zaal K, Ralston E, et al. Dysfunction of endocytic and autophagic pathways in a lysosomal storage disease. Ann Neurol. 2006b;59:700–708. doi: 10.1002/ana.20807. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Roberts A, Ahearn M, Zaal K, Ralston E, Plotz PH, et al. Autophagy and lysosomes in Pompe disease. Autophagy. 2006c;2:318–320. doi: 10.4161/auto.2984. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- Griffin JL. Infantile acid maltase deficiency. I. Muscle fiber destruction after lysosomal rupture. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:23–36. doi: 10.1007/BF02889849. [DOI] [PubMed] [Google Scholar]
- Hirschhorn R, Reuser A. Glycogen storage disease Type II: acid α-glucosidase (acid maltase) deficiency. In: Scriver C, Beaudet A, Sly W, Valle D, editors. The metabolic and molecular bases of inherited disease. McGraw-Hill; New York, NY: 2001. pp. 3389–3420. [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalianhomologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Nicolino M, Voit T, Rogers RC, Tsai AC, Waterson J, et al. Chinese hamster ovary cell-derived recombinant human acid α-glucosidase in infantile-onset Pompe disease. J Pediatr. 2006;149:89–97. doi: 10.1016/j.jpeds.2006.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishnani PS, Corzo D, Nicolino M, Byrne B, Mandel H, Hwu WL, et al. Recombinant human acid α-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology. 2007;68:99–109. doi: 10.1212/01.wnl.0000251268.41188.04. [DOI] [PubMed] [Google Scholar]
- Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu Rev Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- Liou W, Geuze HJ, Geelen MJ, Slot JW. The autophagic and endocytic pathways converge at the nascent autophagic vacuoles. J Cell Biol. 1997;136:61–70. doi: 10.1083/jcb.136.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N. The pleiotropic role of autophagy: from protein metabolism to bactericide. Cell Death Differ. 2005;12(Suppl 2):1535–1541. doi: 10.1038/sj.cdd.4401728. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–1111. doi: 10.1091/mbc.E03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland RJ, Jin X, Zhang XK, Decker RW, Albee KL, Lee KL, et al. Lysosomal acid α-glucosidase consists of four different peptides processed from a single chain precursor. J Biol Chem. 2005;280:6780–6791. doi: 10.1074/jbc.M404008200. [DOI] [PubMed] [Google Scholar]
- Raben N, Danon M, Gilbert AL, Dwivedi S, Collins B, Thurberg BL, et al. Enzyme replacement therapy in the mouse model of Pompe disease. Mol Genet Metab. 2003;80:159–169. doi: 10.1016/j.ymgme.2003.08.022. [DOI] [PubMed] [Google Scholar]
- Raben N, Fukuda T, Gilbert AL, de Jong D, Thurberg BL, Mattaliano RJ, et al. Replacing acid α-glucosidase in Pompe disease: recombinant and transgenic enzymes are equipotent, but neither completely clears glycogen from Type II muscle fibers. Mol Ther. 2005;11:48–56. doi: 10.1016/j.ymthe.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Raben N, Takikita S, Pittis MG, Bembi B, Marie SK, Roberts A, et al. Deconstructing Pompe disease by analyzing single muscle fibers: to see a world in a grain of sand. Autophagy. 2007;3:546–552. doi: 10.4161/auto.4591. [DOI] [PubMed] [Google Scholar]
- Thurberg BL, Lynch Maloney C, Vaccaro C, Afonso K, Tsai AC, Bossen E, et al. Characterization of pre- and post-treatment pathology after enzyme replacement therapy for Pompe disease. Lab Invest. 2006;86:1208–1220. doi: 10.1038/labinvest.3700484. [DOI] [PubMed] [Google Scholar]
- Van den Hout H, Reuser AJ, Vulto AG, Loonen MC, Cromme-Dijkhuis A, van der Ploeg AT. Recombinant human α-glucosidase from rabbit milk in Pompe patients. Lancet. 2000;356:397–398. doi: 10.1016/s0140-6736(00)02533-2. [DOI] [PubMed] [Google Scholar]
- Van den Hout JM, Kamphoven JH, Winkel LP, Arts WF, De Klerk JB, Loonen MC, et al. Long-term intravenous treatment of Pompe disease with recombinant human α-glucosidase from milk. Pediatrics. 2004;113:e448–e457. doi: 10.1542/peds.113.5.e448. [DOI] [PubMed] [Google Scholar]
- Winkel LP, Kamphoven JH, van den Hout HJ, Severijnen LA, van Doorn PA, Reuser AJ, et al. Morphological changes in muscle tissue of patients with infantile Pompe's disease receiving enzyme replacement therapy. Muscle Nerve. 2003;27:743–751. doi: 10.1002/mus.10381. [DOI] [PubMed] [Google Scholar]
- Wisselaar HA, Kroos MA, Hermans MM, van Beeumen J, Reuser AJ. Structural and functional changes of lysosomal acid α-glucosidase during intracellular transport and maturation. J Biol Chem. 1993;268:2223–2231. [PubMed] [Google Scholar]