Lignin, a major component of vascular plant cell wall, provides mechanical support for plants to stand upright and enables xylems to withstand the negative pressure generated during water transport. Although important for plant growth, the presence of lignin limits access to cell wall polysaccharides and thereby negatively affects human utilization of biomass such as its use as livestock feed, in paper manufacturing, and in lignocellulosic biofuel production. Because of its significant economic impact, lignin has been one of the most intensively studied subjects in plant biochemistry.
Lignin is a heterogeneous phenolic polymer largely composed of three major types of monomers (monolignols), p-coumaryl, coniferyl, and sinapyl alcohols. During lignin deposition, monolignols are synthesized in the cytoplasm, translocated to the apoplast, and polymerized into lignin. Over the last two decades, the biosynthesis of monolignols has been a major focus of research on lignification. We now believe that the monolignol biosynthetic pathway has been relatively well elucidated, at least in angiosperms. Based upon this knowledge, genetic engineering approaches have been used to successfully modify lignin content and/or composition in a variety of plant species (for review, see Boerjan et al., 2003; Li et al., 2008).
Despite these advances, there remain significant gaps in our knowledge of the lignification process. Little is known about how monolignols are transported through the cell membrane to their polymerization sites. Neither are the catalysts involved in the polymerization process itself well understood. It is also unknown how lignification is restricted to certain regions within the cell wall of a single cell. In addition, it is still unclear how and why perturbation of lignification affects plant growth, an issue that must be addressed if lignin modification is to be successfully applied to the improvement of biofuel crops. Recent reports of the presence of lignin in nonvascular plants and red algae, as well as the identification of alternative routes for lignin monomer biosynthesis in nonangiosperms raise important questions about lignin evolution (Martone et al., 2009; Espiñeira et al., 2010; Weng et al., 2010). In summary, there are still great challenges in lignin research. In this article, we will focus our discussion on important topics that are poised to become the new frontiers in lignin research.
MONOLIGNOL TRANSPORT AND POLYMERIZATION
After their biosynthesis, monolignols must be transported to the cell wall where they undergo oxidation and polymerization to form lignin. In contrast to our detailed knowledge of monolignol biosynthesis, we know little about how these compounds are moved from the cytoplasm to the cell wall. The translocation of small molecules across the cell membrane may occur by at least three different mechanisms: exocytosis, transporter-mediated export, and diffusion. Golgi-derived vesicles are known to be involved in exporting some other cell wall components such as hemicelluloses to cell wall (Cosgrove, 2005). Whereas observations from some early feeding experiments have suggested the involvement of Golgi and associated vesicles in monolignol transport (Pickett-Heaps, 1968), results from a recent autoradiography study aiming to trace the spatial distribution of monolignols during secondary cell wall development in lodgepole pine (Pinus contorta) do not support the hypothesis that monolignols are exported by this mechanism (Kaneda et al., 2008). Considering that ATP-binding cassette transporters are responsible for the translocation of various secondary metabolites in plant cells (Yazaki, 2006; Rea, 2007), current thinking in the field favors the idea that monolignols are exported to the cell wall by membrane-bound transporters (Kaneda et al., 2008), although in vitro partition experiments suggest that monolignols may also be able to pass the cell membrane by diffusion (Boija and Johansson, 2006).
Once transported to the cell wall, monolignols are oxidized to phenolic radicals that undergo polymerization by chemical coupling. Laccases and peroxidases are thought to be the catalysts responsible for the oxidation of monolignols (Boerjan et al., 2003). Indeed, global transcript profiling and coexpression analysis revealed several ATP-binding cassette transporter genes and a set of laccase and peroxidase genes that are coordinately expressed with monolignol biosynthetic genes in Arabidopsis (Arabidopsis thaliana) developing inflorescence stems (Ehlting et al., 2005). The recent identification of MYB58 and MYB63, two Arabidopsis transcription activators specific for lignin biosynthesis (Zhou et al., 2009), highlights an alternative approach to discover candidate genes involved in lignifications such as monolignol transporter and oxidase genes. Overexpression of MYB58 or MYB63 under the control of the 35S promoter in Arabidopsis leads to ectopic lignification of epidermal and mesophyll cells that are normally nonlignified, suggesting that in addition to monolignol biosynthetic genes, monolignol transporter and oxidase genes may also be directly activated by these transcription factors. Indeed, the expression of a laccase gene (LAC4), which was among the 22 candidate oxidase genes identified from the aforementioned gene coexpression study (Ehlting et al., 2005), was found to be induced by these two MYB transcription factors. It would be interesting to test if disruption of LAC4 abolishes the ectopic lignification exhibited in the above MYB58 or MYB63 overexpression lines. In the future, similar analysis may also facilitate the identification of monolignol transporter genes. The candidate genes identified from the above approaches hold great promise to reveal the identity of the transporters and the oxidases involved in monolignol transport and polymerization, although genetic redundancy may hinder the reverse genetic analysis of their functions.
It would also be interesting to determine by what mechanisms lignin is directed to specific sites within the cell wall including cell corners and regions that undergo secondary thickening. Indeed, the regulation of this deposition is so specific that the wall-thickening pattern of tracheary elements can be used to help identify in paleontological and forensic studies the plant species from which tissue remnants are derived (Lane et al., 1990). During secondary wall thickening, microtubule bundles guide the positioning and movement of cellulose synthase on the plasma membrane to deposit cellulose microfibrils at specific sites within the wall where subsequent lignification occurs (Gardiner et al., 2003; Wightman and Turner, 2008; Gutierrez et al., 2009; Pesquet et al., 2010). It is unknown how lignin deposition is directed to these sites. Future identification of monolignol transporters and oxidases may allow us to monitor their localization in live cells using the Arabidopsis in vitro tracheary element differentiation system (Oda et al., 2005).
LIGNIN DEFICIENCY AND PLANT GROWTH
Lignin-deficient mutants or transgenic plants often show reduced growth and, in severe cases, dwarfing (Jones et al., 2001; Franke et al., 2002; Hoffmann et al., 2004; Chen and Dixon, 2007). This growth defect has been assumed to be a direct consequence of the lignin-deficient xylem failing to support water transport and deemed as an inherent limitation of the lignin reduction approach for biomass improvement. A recent physiological study on the transgenic poplar (Populus spp.) trees in which the p-coumaroyl shikimate 3′-hydroxylase gene was silenced showed that their xylem indeed has reduced hydraulic conductivity and is more prone to collapse and cavitation; however, instead of having increased water-use efficiency, which is expected for drought-stressed plants, these low-lignin plants have reduced water-use efficiency, suggesting some other mechanisms may be involved in plant response to lignin perturbation (Coleman et al., 2008).
Recently, it was reported that blocking flavonoid biosynthesis by RNAi silencing of the chalcone synthase gene (CHS) alleviates the dwarf phenotype that results from RNAi silencing of a lignin biosynthetic gene encoding hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase in Arabidopsis, suggesting that flavonoid-mediated inhibition of auxin transport may be responsible for the growth defects exhibited by lignin-deficient plants (Besseau et al., 2007). In contrast, a recent study using the flavonoid-deficient Arabidopsis CHS null mutant instead of CHS-RNAi demonstrated that the growth inhibition of hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase-deficient plants as well as plants having low levels of p-coumaroyl shikimate 3′-hydroxylase activity is independent of flavonoid accumulation (Li et al., 2010). As a result, the mechanism that connects defects in monolignol biosynthesis and plant growth inhibition remains an open question.
One possible explanation for the dwarf phenotypes seen in lignin down-regulated plants may relate to the fact that in addition to being polymerized to form lignin, coniferyl alcohol is also a precursor of dehydrodiconiferyl alcohol glucosides, soluble compounds shown cell-division promoting activity (Lynn et al., 1987; Tamagnone et al., 1998). It is possible that suppression of monolignol biosynthesis results in deficiency of dehydrodiconiferyl alcohol glucosides that in turn affect plant growth. Another interesting hypothesis is that the perturbation of the cell wall in lignin-deficient plants may trigger a cell wall surveillance system and elicit a general growth response. It is well known that in yeast a cell wall integrity signaling pathway orchestrates responses to cell wall changes during growth and development (Levin, 2005). Like lignin biosynthetic mutants, several Arabidopsis irregular xylem mutants that are defective in cellulose or hemicellulose biosynthesis also have collapsed xylem and abnormal growth (Brown et al., 2005). The recent discovery that the growth inhibition exhibited by some Arabidopsis cellulose-deficient mutants can be alleviated by knocking out a receptor-like kinase gene THESEUS1 provides evidence for the involvement of a cell wall integrity sensing and signaling system in mediating growth response to cell wall defects in plants (Hematy et al., 2007; Seifert and Blaukopf, 2010). Perhaps a similar mechanism is responsible for the growth defects of lignin-deficient plants. Elucidating the details of the processes that result in lignin-related dwarfing may open the door to much more extensive modification of lignification in biomass crops than is currently possible and may significantly improve the processing of plants grown for biofuel.
EVOLUTION OF LIGNIFICATION
The emergence of lignified water-conducting cells has been considered as an important adaptation for vascular plants to be able to thrive in terrestrial environments. The recent discovery of lignin in a bryophyte, the liverwort Marchantia polymorpha, expands the distribution of lignification to nonvascular plants (Espiñeira et al., 2010). Even more strikingly, lignin was recently found in the cell wall of the red alga Calliathron cheilosporioides, which shares a common ancestor with vascular plants over 1 billion years ago (Martone et al., 2009). These observations raise new questions about the evolutionary origin and history of lignification.
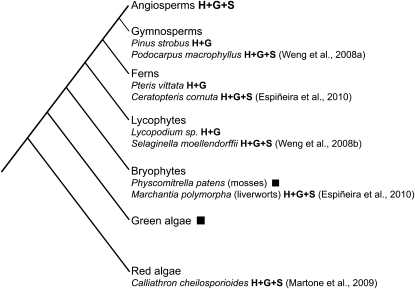
Similarly, recent research on the distribution of lignin monomers in the plant kingdom challenges our perception about the evolution of syringyl (S) lignin monomer biosynthesis (Fig. 1). The incorporation of p-coumaryl, coniferyl, and sinapyl alcohols gives rise to the three major types of lignin units found in nature, p-hydroxyphenyl, guaiacyl, and S lignin, respectively. Despite reports in older literature (Towers and Gibbs, 1953; Erickson and Miksche, 1974; Logan and Thomas, 1985), S lignin has been generally regarded to be characteristic of the angiosperms; however, recent studies using modern diagnostic methods confirmed that S lignin is also present in gymnosperms and some basal vascular plants such as lycophytes and ferns (Weng et al., 2008a, 2008b; Espiñeira et al., 2010). Moreover, S lignin is also detected in liverworts and red algae, which are more distantly related to angiosperms (Martone et al., 2009; Espiñeira et al., 2010).
Figure 1.
Phylogenetic tree showing the distribution of lignification and lignin monomer composition among major plant lineages. It is evident that S lignin is not restricted to angiosperms. The distribution of S lignin within lycophytes, fern, and gymnosperms is not uniform. Black squares indicate no lignification. G, Guaiacyl lignin; H, p-hydroxyphenyl lignin.
The occurrence of lignification in liverworts and red algae suggests that the genes required for lignin deposition evolved in their common ancestor before the divergence of specific lineages (e.g. red algae or liverworts) and were subsequently lost in certain lineages (such as green algae and mosses). Alternatively, red algae and liverworts lineages could have independently evolved the biochemical pathways for lignification. Similar evolutionary scenarios can also be envisaged to explain the phylogenetic distribution pattern of S lignin. Convergent evolution of S lignin biosynthesis between the lycophyte Selaginella and angiosperms has been demonstrated by characterization of an S lignin biosynthetic enzyme, ferulic acid 5-hydroxylase (F5H) from the lycophyte Selaginella moellendorffii. The Selaginella F5H belongs to a phylogenetic clad distinct from the angiosperm F5Hs, indicating independent evolution of these enzymes (Weng et al., 2008b, 2010). Obviously, additional research on the genes, enzymes, and the pathways of monolignol synthesis in other plant lineages is needed to shed more light on the evolution of this important process. The ever-decreasing cost of genome sequencing will soon make possible deep and broad comparisons of the lignification toolkit at nodes of the plant family tree. These studies will provide significant insight into how and when phenylpropanoid metabolism in general and lignification in particular arose and evolved.
CONCLUSION
The potential to reduce lignin’s negative impacts on human uses of biomass has been and will continue to be a major force that propels lignin research. Our current understanding of lignification is mainly limited to the biosynthesis of the building blocks for lignin in angiosperms. Future research on lignin biosynthesis needs to be focused on the identification of the genes involved in monolignol transport and polymerization, the mechanisms that connect lignin biochemistry and plant growth and development, and a broadening of our understanding of evolutionary aspects of lignification. This knowledge will further improve our ability to manipulate lignification in biomass feedstocks for human uses and give us a better understanding of how this important polymer contributed to the dominance of vascular plants in terrestrial environments.
References
- Besseau S, Hoffmann L, Geoffroy P, Lapierre C, Pollet B, Legrand M. (2007) Flavonoid accumulation in Arabidopsis repressed in lignin synthesis affects auxin transport and plant growth. Plant Cell 19: 148–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Ralph J, Baucher M. (2003) Lignin biosynthesis. Annu Rev Plant Biol 54: 519–546 [DOI] [PubMed] [Google Scholar]
- Boija E, Johansson G. (2006) Interactions between model membranes and lignin-related compounds studied by immobilized liposome chromatography. Biochim Biophys Acta 1758: 620–626 [DOI] [PubMed] [Google Scholar]
- Brown DM, Zeef LA, Ellis J, Goodacre R, Turner SR. (2005) Identification of novel genes in Arabidopsis involved in secondary cell wall formation using expression profiling and reverse genetics. Plant Cell 17: 2281–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Dixon RA. (2007) Lignin modification improves fermentable sugar yields for biofuel production. Nat Biotechnol 25: 759–761 [DOI] [PubMed] [Google Scholar]
- Coleman HD, Samuels AL, Guy RD, Mansfield SD. (2008) Perturbed lignification impacts tree growth in hybrid poplar—a function of sink strength, vascular integrity, and photosynthetic assimilation. Plant Physiol 148: 1229–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove DJ. (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6: 850–861 [DOI] [PubMed] [Google Scholar]
- Ehlting J, Mattheus N, Aeschliman DS, Li E, Hamberger B, Cullis IF, Zhuang J, Kaneda M, Mansfield SD, Samuels L, et al. (2005) Global transcript profiling of primary stems from Arabidopsis thaliana identifies candidate genes for missing links in lignin biosynthesis and transcriptional regulators of fiber differentiation. Plant J 42: 618–640 [DOI] [PubMed] [Google Scholar]
- Erickson M, Miksche GE. (1974) Characterization of pteridophyte lignin by oxidative degradation. Holzforschung 28: 157–159 [Google Scholar]
- Espiñeira JM, Uzal EN, Ros LVG, Carrión JS, Merino F, Barceló AR, Pomar F. (2010) Distribution of lignin monomers and the evolution of lignification among lower plants. Plant Biol (in press) [DOI] [PubMed] [Google Scholar]
- Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C. (2002) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30: 47–59 [DOI] [PubMed] [Google Scholar]
- Gardiner JC, Taylor NG, Turner SR. (2003) Control of cellulose synthase complex localization in developing xylem. Plant Cell 15: 1740–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW. (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11: 797–806 [DOI] [PubMed] [Google Scholar]
- Hematy K, Sado PE, Van Tuinen A, Rochange S, Desnos T, Balzergue S, Pelletier S, Renou JP, Hofte H. (2007) A receptor-like kinase mediates the response of Arabidopsis cells to the inhibition of cellulose synthesis. Curr Biol 17: 922–931 [DOI] [PubMed] [Google Scholar]
- Hoffmann L, Besseau S, Geoffroy P, Ritzenthaler C, Meyer D, Lapierre C, Pollet B, Legrand M. (2004) Silencing of hydroxycinnamoyl-coenzyme A shikimate/quinate hydroxycinnamoyltransferase affects phenylpropanoid biosynthesis. Plant Cell 16: 1446–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Ennos AR, Turner SR. (2001) Cloning and characterization of irregular xylem4 (irx4): a severely lignin-deficient mutant of Arabidopsis. Plant J 26: 205–216 [DOI] [PubMed] [Google Scholar]
- Kaneda M, Rensing KH, Wong JC, Banno B, Mansfield SD, Samuels AL. (2008) Tracking monolignols during wood development in lodgepole pine. Plant Physiol 147: 1750–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, Anderson LC, Barkley TM, Bock JH, Gifford EM, Hall DW, Norris DO, Rost TL, Stern WL. (1990) Forensic botany. Bioscience 40: 34–39 [Google Scholar]
- Levin DE. (2005) Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Bonawitz ND, Weng JK, Chapple C. (2010) The growth reduction associated with repressed lignin biosynthesis in Arabidopsis thaliana is independent of flavonoids. Plant Cell 22: 1620–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Weng JK, Chapple C. (2008) Improvement of biomass through lignin modification. Plant J 54: 569–581 [DOI] [PubMed] [Google Scholar]
- Logan KJ, Thomas BA. (1985) Distribution of lignin derivatives in plants. New Phytol 99: 571–585 [DOI] [PubMed] [Google Scholar]
- Lynn DG, Chen RH, Manning KS, Wood HN. (1987) The structural characterization of endogenous factors from Vinca rosea crown gall tumors that promote cell division of tobacco cells. Proc Natl Acad Sci USA 84: 615–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martone PT, Estevez JM, Lu F, Ruel K, Denny MW, Somerville C, Ralph J. (2009) Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr Biol 19: 169–175 [DOI] [PubMed] [Google Scholar]
- Oda Y, Mimura T, Hasezawa S. (2005) Regulation of secondary cell wall development by cortical microtubules during tracheary element differentiation in Arabidopsis cell suspensions. Plant Physiol 137: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesquet E, Korolev AV, Calder G, Lloyd CW. (2010) The microtubule-associated protein AtMAP70-5 regulates secondary wall patterning in Arabidopsis wood cells. Curr Biol 20: 744–749 [DOI] [PubMed] [Google Scholar]
- Pickett-Heaps JD. (1968) Xylem wall deposition. Protoplasma 65: 181–205 [Google Scholar]
- Rea PA. (2007) Plant ATP-binding cassette transporters. Annu Rev Plant Biol 58: 347–375 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Blaukopf C. (2010) Irritable walls: the plant extracellular matrix and signaling. Plant Physiol 153: 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Merida A, Stacey N, Plaskitt K, Parr A, Chang CF, Lynn D, Dow JM, Roberts K, Martin C. (1998) Inhibition of phenolic acid metabolism results in precocious cell death and altered cell morphology in leaves of transgenic tobacco plants. Plant Cell 10: 1801–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towers GH, Gibbs RD. (1953) Lignin chemistry and the taxonomy of higher plants. Nature 172: 25–26 [DOI] [PubMed] [Google Scholar]
- Weng JK, Akiyama T, Bonawitz ND, Li X, Ralph J, Chapple C. (2010) Convergent evolution of syringyl lignin biosynthesis via distinct pathways in the lycophyte Selaginella and flowering plants. Plant Cell 22: 1033–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JK, Banks JA, Chapple C. (2008a) Parallels in lignin biosynthesis: a study in Selaginella moellendorffii reveals convergence across 400 million years of evolution. Commun Integr Biol 1: 20–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng JK, Li X, Stout J, Chapple C. (2008b) Independent origins of syringyl lignin in vascular plants. Proc Natl Acad Sci USA 105: 7887–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Turner SR. (2008) The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J 54: 794–805 [DOI] [PubMed] [Google Scholar]
- Yazaki K. (2006) ABC transporters involved in the transport of plant secondary metabolites. FEBS Lett 580: 1183–1191 [DOI] [PubMed] [Google Scholar]
- Zhou J, Lee C, Zhong R, Ye ZH. (2009) MYB58 and MYB63 are transcriptional activators of the lignin biosynthetic pathway during secondary cell wall formation in Arabidopsis. Plant Cell 21: 248–266 [DOI] [PMC free article] [PubMed] [Google Scholar]