Abstract
In response to iron (Fe) deficiency, dicots employ a reduction-based mechanism by inducing ferric-chelate reductase (FCR) at the root plasma membrane to enhance Fe uptake. However, the signal pathway leading to FCR induction is still unclear. Here, we found that the Fe-deficiency-induced increase of auxin and nitric oxide (NO) levels in wild-type Arabidopsis (Arabidopsis thaliana) was accompanied by up-regulation of root FCR activity and the expression of the basic helix-loop-helix transcription factor (FIT) and the ferric reduction oxidase 2 (FRO2) genes. This was further stimulated by application of exogenous auxin (α-naphthaleneacetic acid) or NO donor (S-nitrosoglutathione [GSNO]), but suppressed by either polar auxin transport inhibition with 1-naphthylphthalamic acid or NO scavenging with 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, tungstate, or Nω-nitro-l-arginine methyl ester hydrochloride. On the other hand, the root FCR activity, NO level, and gene expression of FIT and FRO2 were higher in auxin-overproducing mutant yucca under Fe deficiency, which were sharply restrained by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide treatment. The opposite response was observed in a basipetal auxin transport impaired mutant aux1-7, which was slightly rescued by exogenous GSNO application. Furthermore, Fe deficiency or α-naphthaleneacetic acid application failed to induce Fe-deficiency responses in noa1 and nial nia2, two mutants with reduced NO synthesis, but root FCR activities in both mutants could be significantly elevated by GSNO. The inability to induce NO burst and FCR activity was further verified in a double mutant yucca noa1 with elevated auxin production and reduced NO accumulation. Therefore, we presented a novel signaling pathway where NO acts downstream of auxin to activate root FCR activity under Fe deficiency in Arabidopsis.
Iron (Fe) deficiency is one of the major limiting factors affecting crop production in calcareous soils worldwide (Imsande, 1998). Fortunately, many plants have developed various strategies to cope with Fe deficiency in those soils. These strategies are classified as strategy I in nongraminaceous monocots and dicots, and strategy II in graminaceous monocots (Römheld and Marschner, 1981). Strategy I plants employ a range of responses to Fe-deficiency stress to acquire Fe from the soil, including: (1) induction of both a plasmalemma ferric-chelate reductase (FCR; Robinson et al., 1999) and plasmalemma Fe(II) transporter in root cells (Eide et al., 1996; Vert et al., 2002), (2) enhanced release of protons and reductants such as phenolic compounds into the rhizosphere (Curie and Briat, 2003; Jin et al., 2006, 2007), and (3) changes in root architecture, including enhanced root branching (Jin et al., 2008) and subapical root hair development (Römheld and Marschner, 1981; Schmidt, 1999; Santi and Schmidt, 2008). Among these responses, the activation of FCR has been suggested to be a key component (Curie and Briat, 2003) as the strategy I plants must enzymatically reduce Fe(III) before their root cells can take it up in the form of Fe(II) (Chaney et al., 1972). Although FCR induction in Fe-deficient roots of different plant species and its function have been well documented (Robinson et al., 1999; Connolly et al., 2003), the signals involved in the regulatory cascade leading to the activation of FCR are still not well understood.
It has been demonstrated that the regulation of Fe-deficiency responses does not depend solely on root Fe content, but is far more complex and may also involve signals originating from the shoot (Romera et al., 1992; Grusak and Pezeshgi, 1996; Forde, 2002; Vert et al., 2003; Zheng et al., 2003; Enomoto and Goto, 2008). Shoot-derived auxin is a promising candidate as a signal molecule transmitting Fe-deficiency information, since its synthesis is enhanced under Fe starvation (Römheld and Marschner, 1981), and auxin is easily transported from the shoot to the root. Application of exogenous auxin analogs can promote the induction of the root FCR in bean (Phaseolus vulgaris; Li et al., 2000), cucumber (Cucumis sativus; Li and Li, 2004), and Trifolium pratense (Zheng et al., 2003). Hence we have studied here the possible role of auxin as an upstream signal in modulating Fe-deficiency responses in dicots, using Arabidopsis (Arabidopsis thaliana) as a model system.
In addition to auxin, we and others have recently suggested that the gaseous molecule, nitric oxide (NO), could be a general component of root Fe signaling in response to Fe deficiency (Graziano and Lamattina, 2007a; Jin et al., 2009). Under Fe limitation, a rapid and sustained NO accumulation was shown to be triggered in tomato (Solanum lycopersicum) plants. When Fe-deficient roots were treated with the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), the accumulation of mRNA for ferric reduction oxidase 1 (FRO1), IRT1, and the basic helix-loop-helix transcription factor (FIT) homolog, FER, was inhibited. Accordingly, the application of the NO donor, S-nitrosoglutathione (GSNO), induced the expression of these same genes (Graziano and Lamattina, 2007a), which coincided with the alleviation of oxidative damage (Sun et al., 2006) and the enhancement of dinitrosyl-Fe complexes formation (Vanin et al., 2004; Graziano and Lamattina, 2007b), which should improve Fe availability inside the plant (Graziano et al., 2002). However, a down-regulation of FRO1, IRT1, and FER in Fe-deficient roots of the tomato mutant fer could not be reversed by the addition of NO, suggesting that the NO acts upstream of FER to initiate adaptations to Fe deficiency (Graziano and Lamattina, 2007a). Thus it would not be surprising if NO turns out to play a role as a signal reporting shoot Fe-deficient status to the roots.
The synergistic effects of auxin and NO have been well defined in the regulation of a number of physiological processes of plants such as root growth and development (Lanteri et al., 2006) and nitrate reductase (NR) stimulation (Du et al., 2008). Thus, the possibility for cross talk between NO and auxin in modulating the perception and transduction of Fe deficiency, leading to root FCR induction is of interest. The findings reported here are the results of research on this hypothetical link in Arabidopsis, a strategy I plant. From this study we found that both auxin and NO are involved in the induction of the hallmarks of the Fe-deficiency stress response to Fe deprivation in dicots (induction of FRO2 [FCR] and FIT) with NO acting downstream of auxin in this signaling pathway.
RESULTS
Auxin Is Involved in FCR Induction
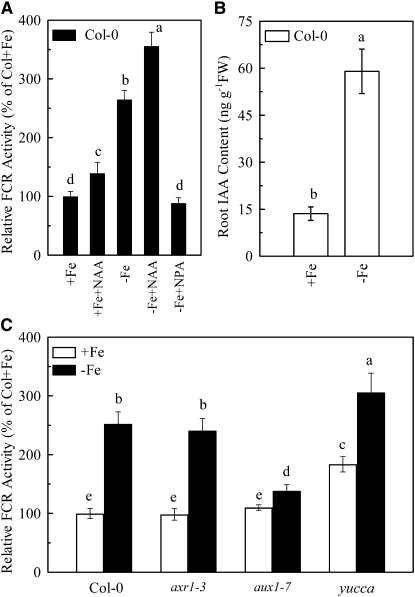
Root FCR activity in Fe-sufficient Arabidopsis plants fluctuated between 10 and 16 μg Fe(II) g−1 fresh weight. To characterize the induction of FCR activity during Fe deficiency, we selected the period of 7 d treatment for further analyses, at which FCR activity could be maximally induced, with an enhancement of more than 150% when compared to that during Fe sufficiency (Fig. 1A; Supplemental Fig. S1). Moreover, this Fe-deficiency-induced reductase activity was further enhanced by incubation with α-naphthaleneacetic acid (NAA), a permeable auxin analog, but was strongly inhibited by incubation with 1-naphthylphthalamic acid (NPA), an auxin transport inhibitor (Fig. 1A). In addition, there was a 4-fold increase in indole-3-acetic acid (IAA) accumulation in the roots of Fe-deficient plants compared with those of Fe-sufficient plants (Fig. 1B).
Figure 1.
Effect of auxin availability on the induction of FCR activity and accumulation of IAA in −Fe roots. A, Relative FCR activity of +Fe or −Fe wild-type plants in the presence or absence of 0.08 μm NAA or 10 μm NPA. B, IAA content in −Fe roots of wild-type plants. FW, Fresh weight. C, Relative FCR activity of three auxin mutant lines in response to −Fe treatment. Means ± sd (n = 7 for FCR activity, n = 3 for auxin content) followed by different letters indicate a statistical difference at P < 0.05.
To further study the role of auxin in FCR activation, the effect of Fe supply on root FCR activity was analyzed in three auxin-related mutants (Fig. 1C). In comparison with that of wild-type plants, root FCR activity induced by Fe deficiency was significantly greater in auxin-overproducing mutant yucca under both Fe-sufficient and -deficient conditions, whereas the opposite response was seen in the basipetal auxin transport impaired mutant aux1-7. Unexpectedly, in auxin-insensitive mutant axr1-3, the Fe-deficiency-induced FCR activity was similar to that in wild-type plants.
NO Stimulates FCR Induction
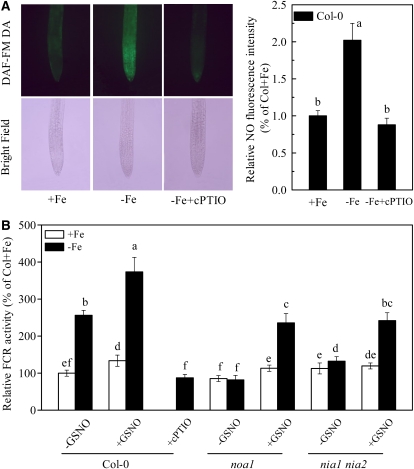
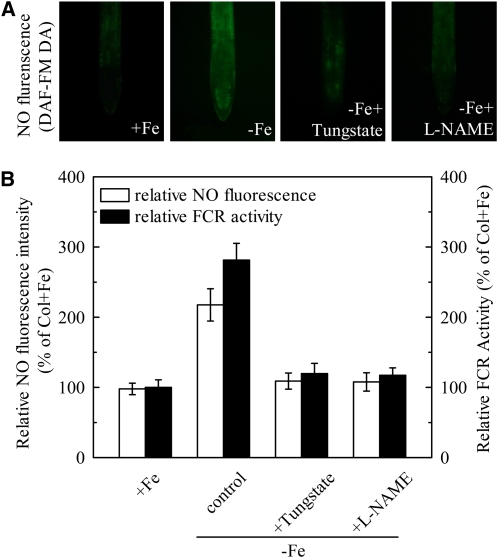
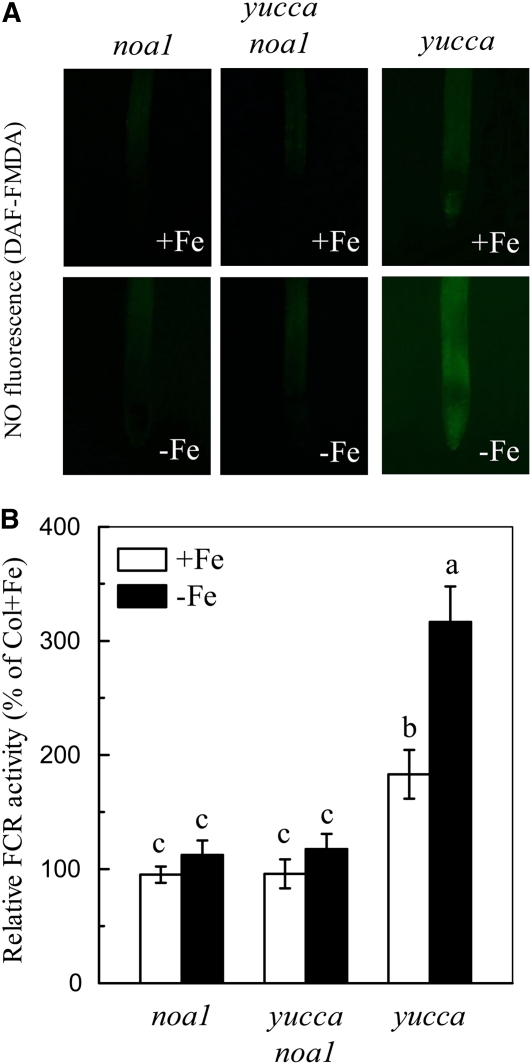
Generally, in strategy I plants, the Fe-deficiency responsive FCR is mainly localized in young lateral roots, with highest activity at the root apex and subapical root regions (Römheld and Marschner, 1981). The same localization pattern was observed in Fe-deficient roots of wild-type Arabidopsis plants (Supplemental Fig. S2). Interestingly, the root NO production determined by 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FMDA) fluorescence was also intensified in the same region of Fe-deficient root, but this Fe-deficiency-induced increase of NO fluorescence could be greatly suppressed by the NO scavenger, cPTIO (Fig. 2A). The coincidence between FCR location and NO production under Fe deficiency led us to verify whether NO is involved in root FCR induction in Arabidopsis. As shown in Figure 2B, application of an exogenous NO source, GSNO, greatly elevated FCR activity in Fe-deficient roots, whereas application of cPTIO resulted in inhibition of activity. The inhibition in inducing root FCR activity and NO accumulation was also observed in Fe-deficient wild-type plants treated with either tungstate, a NR inhibitor, or Nω-nitro-l-Arg methyl ester hydrochloride (l-NAME), a NO synthase-type enzyme (NOA1) inhibitor (Fig. 3). Additionally, Fe deficiency failed to induce NO burst and the reductase activity in noa1 and nial nia2, two mutants with reduced NO synthesis, but root FCR activities in both mutants could be significantly rescued by GSNO (Figs. 2B and 5B). These results indicate that NO is required for the induction of the reductase activity during Fe deficiency, and both NOA1 and NR are involved in this NO generation.
Figure 2.
Effect of Fe-deficiency-induced NO on the induction of root FCR activity. A, NO production shown as green fluorescence and relative fluorescence intensity from DAF-FMDA in roots of wild-type plants under +Fe, −Fe, or −Fe + cPTIO treatment, respectively. B, Relative FCR activity in roots of wild-type and NO-related mutants (noa1 and nia1 nia2) in response to exogenous NO treatments. GSNO (50 μm) was added to both +Fe and −Fe plants, while cPTIO (0.5 mm) was only added to −Fe wild-type plants. Means ± sd (n = 10 for NO production, n = 7 for FCR activity) followed by different letters indicate a statistical difference at P < 0.05.
Figure 3.
Involvement of NR and NO synthase-type enzyme (NOA1) in NO production and FCR induction during Fe deficiency. Wild-type Arabidopsis plants were grown in +Fe media for 6 weeks and then transferred to −Fe media in the presence of the NR inhibitor, tungstate (1 mm), and the NOA inhibitor, l-NAME (1 mm) or without any treatment (control). A, The NO production as DAF-FMDA fluorescence detected in roots of wild type. B, The corresponding fluorescence intensity of NO and FCR activity in roots under treatments as described. Data are expressed as means ± sd (n = 10 for NO production, n = 7 for FCR activity).
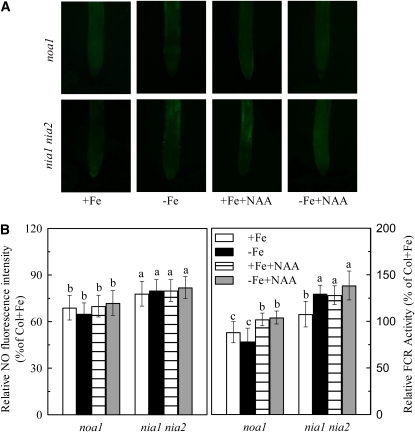
Figure 5.
Effect of auxin on NO accumulation in roots of two NO-related mutants, noa1 and nia1nia2. A, NO green fluorescence, and the corresponding NO fluorescence intensity and relative FCR activity (B) in +Fe or −Fe roots of mutants noa1 and nia1nia2 in response to 0.08 μm NAA treatment. Data are expressed as means ± sd (n = 10 for NO production, n = 7 for FCR activity) with different letters indicating significant differences at P < 0.05.
NO Acts Downstream of Auxin to Activate FCR
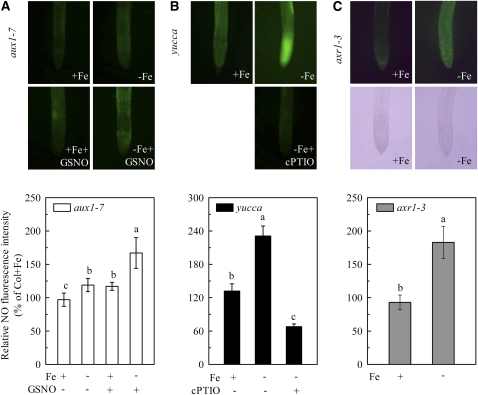
Since both NO and auxin are involved in regulating root FCR induction in response to Fe deficiency, we investigated the possible linkage between these two signals in signal transduction pathway of Fe-deficient conditions. Figure 4 shows that in the aux1-7 mutant, root NO levels were only slightly induced by Fe deficiency, which were significantly increased by treatment of Fe-deficient plants with GNSO, whereas in mutant yucca, root NO levels were higher in both Fe-sufficient and -deficient plants, which were suppressed by treatment of Fe-deficient plants with cPTIO. Interestingly, in the axr1-3 mutant, this Fe-deficiency-induced NO production accumulated to a similar level as that in the wild-type plants (Fig. 4C). However, in mutants noa1 and nia1 nia2, exogenous NAA application failed to induce additional root NO accumulation and FCR activity either under Fe-sufficient or -deficient conditions (Fig. 5). The inability to induce NO burst and FCR activity was also found in a double mutant yucca noa1 with elevated auxin production and reduced NO accumulation (Fig. 6). These data indicate that the effect of NO is more closely linked to FCR induction than the auxin effect.
Figure 4.
Effect of auxin on NO accumulation in roots of three auxin-related mutants during Fe deficiency. A, NO green fluorescence and the corresponding relative fluorescence intensity in +Fe and −Fe roots of aux1-7 plants treated with 50 μm GSNO. B, NO green fluorescence and the corresponding relative fluorescence intensity in roots of yucca plants under treatments of +Fe, −Fe, and −Fe + cPTIO (0.5 mm), respectively. C, NO green fluorescence and the corresponding relative fluorescence intensity in +Fe and −Fe roots of yucca plants. Means ± sd (n = 10) with different letters indicate significant differences at P < 0.05.
Figure 6.
Physiological analysis of NO burst and FCR induction in yucca noa1 double mutant grown under Fe deficiency. A, NO green fluorescence, and the relative FCR activity (B) in roots of yucca noa1 double mutant after exposed to +Fe or −Fe media for 7 d. Means ± sd is shown (n = 9) with different letters suggesting a statistical difference (P < 0.05) between these two treatments.
Role of Auxin and NO on the Expression of Genes Involved in FCR Induction
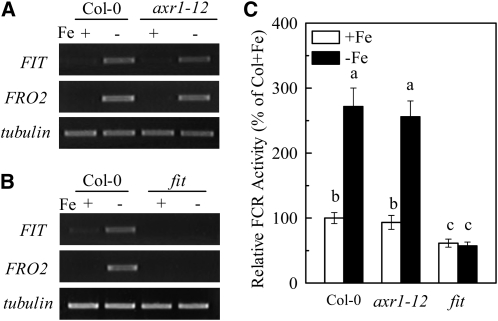
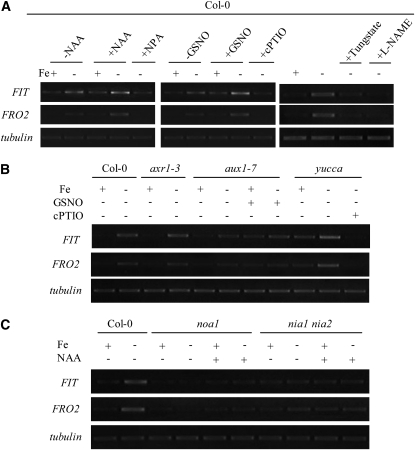
Reduction of Fe(III) to Fe(II) by root FCR [AtFRO2] is a key step in Fe acquisition by strategy I plants. Here, in mutant fit with no accumulation of FIT transcripts, FRO2 transcript abundance is dramatically reduced and root FCR activity is not induced by Fe deficiency (Fig. 7, B and C). This result is in agreement with previous studies documenting the role of FIT, the bHLH protein in the induction of FRO2 expression in Fe-deficient Arabidopsis (Jakoby et al., 2004; Yuan et al., 2005). We also observed that in the roots of wild-type plants, Fe deficiency significantly up-regulated the gene expression of FRO2 and FIT, and this up-regulation was further enhanced when roots were exposed to auxin (NAA) or NO (GSNO) treatments. However the up-regulation is completely blocked when roots were exposed to NPA, cPTIO, or NOA1/NR inhibitor treatments (Fig. 8A), indicating the involvement of both auxin and NO in gene expression of FRO2 and FIT in Fe-deficient roots.
Figure 7.
Role of Fe deficiency in regulating expression levels of FIT and FRO2 genes and FCR activation in roots of axr1-12 and fit mutant lines. Six-week-old plants were transferred to +Fe or −Fe media for a further 7 d, and then the expression of FIT and FRO2 genes (A and B) was analyzed. Total RNA extracted from roots and the transcript level was analyzed by RT-PCR with tubulin β-3 expression as a control. Relative FCR activity (C) was conducted as well. Means ± sd are shown (n = 7). Different letters indicate a significant difference (P < 0.05) among the treatments.
Figure 8.
Role of auxin and NO in regulating expression levels of FIT and FRO2 in −Fe roots of wild-type seedlings and several auxin or NO-related mutant lines. Six-week-old Arabidopsis plants were transferred to −Fe conditions for different treatments. A, Expression of FIT and FRO2 genes in +Fe or −Fe roots of wild-type plants treated with exogenous auxin, NO, or NR/NOA1 inhibitors. B, Expression of FIT and FRO2 genes in +Fe or −Fe roots of three auxin-related mutants treated with exogenous NO. C, Expression of both genes in +Fe or −Fe roots of two NO-related mutants treated with NAA. Total RNA was extracted from roots and the mRNA level was analyzed by RT-PCR. tubulin β-3 expression was used as a control.
To further investigate the interaction of auxin and NO during Fe deficiency, the gene expression of FRO2 and FIT was analyzed in auxin- and NO-related Arabidopsis mutants. Like that seen in wild-type plants, a similar expression of FRO2 and FIT genes was induced in the axr1-12 mutant (Fig. 7A) and the axr1-3 mutants (Fig. 8B). This increased expression correlated with the enhanced activity of FCR (Figs. 1C and 7C). Nevertheless, in aux1-7 mutant, this gene expression was relatively impaired although it could be slightly rescued by GSNO treatment, while in yucca mutant, greater expression of FRO2 and FIT was evident even in Fe-sufficient plants, and was further stimulated in Fe-deficient plants, and completely inhibited in cPTIO-treated plants (Fig. 8B). Moreover, in noa1 and nia1 nia2 mutants, neither Fe deficiency nor NAA application stimulated the gene expression of FRO2 and FIT (Fig. 8C). These results strongly suggest that during Fe deficiency, auxin acts upstream of NO in regulating the gene expression of FRO2 and FIT in roots.
DISCUSSION
Both Endogenous Root Auxin Level and Auxin Basipetal Transport Are Needed for FCR Induction
Auxin has been implicated in regulating plant adaptive responses to Fe-deficiency stress (Landsberg, 1996), including the induction of FCR activity in roots. For example, Fe deficiency increased auxin production in a number of strategy I plant species, such as sunflower (Helianthus annuus; Römheld and Marschner, 1981), bean (Li and Li, 2004), and Arabidopsis in this study (Fig. 1B; Supplemental Fig. S1). Exogenous application of auxin to plants significantly enhanced FCR activity (Fig. 1A; Schmidt, 1994; Li and Li, 2004). Here we also found that higher endogenous auxin levels resulted in greater root FCR activity and FIT and FRO2 expression, but these responses could be markedly repressed by exposure to the auxin transport inhibitor, NPA (Figs. 1A and 8A), implying that a certain endogenous auxin level is necessary for the Fe-deficiency-based induction of root FCR activity.
In this study, although aux1-7 and axr1-3 are both auxin-insensitive mutants, they responded differently to Fe deficiency in terms of FCR induction. The induction was impaired to certain extent in aux1-7, but appeared normal in axr1-3 compared to wild type (Fig. 1C). Zheng et al. (2003) demonstrated that shoot-to-root transmission of an unknown signal, possibly auxin, could be involved in root FCR induction after a prolonged exposure to Fe-deficient treatment in T. pratense. Similar results have also been seen in the pea (Pisum sativum) mutant dg1 (Grusak and Pezeshgi, 1996), as well as cucumber and bean (Li and Li, 2004). As auxin basipetal transport is blocked in aux1-7, it seems reasonable that FCR activity in this mutant was much reduced in comparison to wild type. Moreover, the AUX1 gene is a member of a family of 29 members with strong genetic redundancy (Overvoorde et al., 2005), thus, it also seems plausible that the mutant with a particular mutation in one of these members may only have a modest effect on auxin-induced responses, as auxin may interact with multiple receptors and transduction pathways to regulate different responses (Lau et al., 2008). This can explain partly why Fe deficiency still induced small but not significant increases in FCR activity in aux1-7, and this response does not necessarily negate the involvement of auxin in modulating root FCR induction. In axr1-3, although a component for sensing auxin is impaired, auxin basipetal transport from the shoot to root may still be functioning (Lincoln et al., 1990), thus the signal encoding Fe deficiency from shoot to root may still be transduced, allowing subsequent NO production in roots as well (Fig. 4C), which leads to a normal reductase activation. Therefore, besides maintaining proper endogenous auxin levels, normal auxin basipetal transport is also important in the process of FCR induction under Fe-deficient condition in Arabidopsis.
Auxin-Mediated Induction of FCR Is Dependent on NO
How does auxin mediate the induction of FCR under Fe-deficient conditions? Are other signals downstream of auxin involved in FCR activation? NO has been demonstrated to function as a signal and effector molecule in the adaptive mechanisms of plant to low Fe conditions. For example, NO can improve Fe availability inside plants, mediate Fe-dependent ferritin expression, and alleviate Fe-deficiency-induced chlorosis and oxidative damage (Ramirez et al., 2009). NO can also activate FCR by enhancing FRO1 expression in tomato, which may involve NO-mediated expression of the transcription factors, FER or FIT (Graziano and Lamattina, 2007a; Jin et al., 2009). Moreover, Besson-Bard et al. (2009) showed that NO produced in response to cadmium acts as an Fe-deficiency-related signal and up-regulates Fe uptake genes including FCR. Here we found that Fe-deficiency-induced increase in root NO levels had a similar spatial distribution to that of FCR (Fig. 2A; Supplemental Fig. S2), and they were correlated with the increased expression of FIT and FRO2 (Fig. 8). Exogenous application of the NO donor, GSNO, further enhanced these elevations in roots of Fe-deficient plants, while scavenging of NO reversed these inductions either via application of an NO scavenger (Figs. 2 and 8A) or inhibitors of NO-synthesizing enzymes (Figs. 3 and 8A), confirming the direct involvement of NO in the induction of FCR and Fe acquisition-related genes in response to Fe deficiency.
As both root increases in auxin and NO precede FCR induction, does one event precede the other? Here we provide evidence that NO acts downstream of auxin to trigger root FCR activity under Fe deficiency in Arabidopsis. When auxin production was decreased or increased constitutively in several mutant lines, NO levels and gene expression of FIT and FRO2 were correspondingly changed. For example, in the mutant line aux1-7 with impaired auxin transport, a weaker accumulation of NO was induced by Fe deficiency, and it could be reversed by exogenous application of GSNO (Fig. 4A), as was the response of FIT and FRO2 expression (Fig. 8B). But in mutant line yucca with increased auxin levels, these responses were greater than wild-type plants even under Fe-sufficient conditions, and they could be further enhanced by Fe deficiency, while significantly suppressed by cPTIO treatment (Figs. 4B and 8B). As the above results were obtained from exogenous application of NAA or cPTIO, we are not sure whether the endogenous situation is the same or not. Thus we generated a double mutant yucca noa1 to have elevated endogenous IAA level but reduced endogenous NO level. As shown in Figure 6, the NO burst and the corresponding FCR activation in Fe-deficient roots of yucca noa1 were greatly impaired, similar to those found in noa1 (Fig. 5). On the other hand, the Fe-deficiency-induced increase in NO production of wild-type plants could be further enhanced by exogenously supplied NAA, or repressed by NPA, respectively (data not shown). These results indicate that auxin is necessary for NO production in roots during Fe deficiency. Therefore, we propose that FCR induction under Fe deficiency is initiated by auxin, which in turn activates NO production, leading to the increased FCR expression as well as the associated Fe-deficiency responses.
Regulation of NO Levels in Roots Is Both NOA and NR Dependent
The identification of a variety of NO biosynthetic pathways has advanced our understanding of the functions of NO in plants (Lamotte et al., 2005). However, the mechanism by which auxin modulates NO levels in roots remains unclear. It is likely that plants employ several different enzyme systems to produce NO, even though to date the most intensively studied are based on NR and a putative NOS enzyme (Wilson et al., 2008). Several studies have questioned the role of AtNOS1 in NO synthesis (Zemojtel et al., 2006). Moreau and coworkers (2008) elegantly demonstrated that AtNOS1 is not a NOS, but a functional GTPase, thus it was renamed as an NO-associated enzyme (AtNOA1). Despite the questions regarding the essence of AtNOS, the point is that in the mutant, Atnoa1 (formerly Atnos1), plant NO levels are remarkably reduced (Fig. 5; Guo, 2006). The NOA1-dependent pathway for NO biosynthesis responds to different chemical and biological stimuli, such as the lipopolysaccharide (Guo et al., 2003), abscisic acid (Bright et al., 2006), salicylic acid (Zottini et al., 2007), and auxin (Lombardo et al., 2006). Besides the NOA1-dependent pathway, generation of NO in roots also involves NR activity (Hu et al., 2005). In this study, we clearly demonstrated that either the NR inhibitor, tungstate, or the NOA1-inhibitor, l-NAME, substantially suppressed NO production in Fe-deficient roots nearly to the levels detected in Fe-sufficient roots (Fig. 3). More importantly, Fe-deficiency-induced accumulation of NO was significantly decreased in the mutants noa1 and nia1 nia2, and exogenously applied auxin failed to induce a normal production of NO in these mutants (Fig. 5). Thus we conclude that both NOA1 and NR are involved in auxin-regulated NO generation during Fe deficiency, but the mechanism involved in the process requires further investigation.
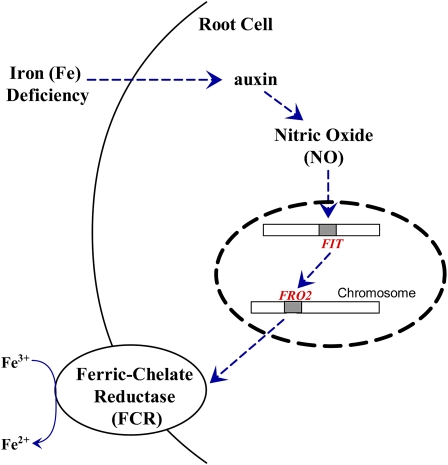
Although previous reports have provided other evidence that auxin or NO are related to root FCR activity induction under Fe deficiency in different plant species, the possible relationship between auxin and NO in regulating Fe-deficiency responses has not previously been examined. Here, using several different auxin and NO-related Arabidopsis mutants, we demonstrate that the NO acts as a signal downstream of the auxin under Fe deficiency that leads to the ultimate induction of FCR via FIT-mediated transcriptional regulation. A model for these signaling pathways is illustrated in Figure 9. To our knowledge, this is the first report to define the signaling pathway for plant FCR induction and also furthers our understanding of the physiological responses of strategy I plants to Fe deficiency.
Figure 9.
Schematic model of hoe NO operates downstream of auxin in the transduction of Fe-deficiency sensing to induce FCR activity in Arabidopsis roots. Fe deficiency could increase auxin levels in roots with the subsequent induction of NO accumulation. This enhanced NO signal then activates FCR activity via FIT-mediated transcriptional regulation of FRO2, which results in the increased accumulation of Fe in response to low plant Fe status. Dashed arrows denote regulatory pathways. [See online article for color version of this figure.]
MATERIALS AND METHODS
Plant Material and Growth Condition
The Columbia ecotype (Columbia-0) of Arabidopsis (Arabidopsis thaliana) and the mutants generated in its background were used here, including the auxin-insensitive mutants, axr1-3 and axr1-12, the auxin-transport mutant aux1-7, the auxin overproducing mutant yucca, the NOA-deficient mutant noa1, the NR-null-deficient double mutant nia1 nia2, and the BHLH029 T-DNA insertion mutant fit were kindly provided by various professors as gifts.
The double mutant line yucca noa1 were obtained by crossing mutant lines yucca with noa1. F2 progeny was obtained through self fertilization of single F1 plant. Homozygotes were confirmed by genomic PCR with the following primers: LBb1 (left border primer of the T-DNA insertion), LP (left genomic primer), and RP (right genomic primer) as described by Guo et al. (2003) for the T-DNA insertion site determination, while Forward (F; 5′-TCCTCGGATTCCATTGCCCAGC-3′) and Reverse (R; 5′-ACGAGAGTGTCGTGCTCCACCA-3′) for YUCCA overexpressed site identification (seen as in Supplemental Fig. S3). Because of the difficulties in identifying homozygous site of YUCCA, some of the double mutants we used maybe heterozygous.
Seeds were surface sterilized and germinated on plates containing full-strength nutrient solution, 0.9% (w/v) agar, and 1.0% (w/v) Suc. On the 7th day, uniform seedlings were transferred to grow on vermiculite supplemented with fresh complete nutrient solutions every other day. After 3 weeks, seedlings were transplanted to 1-L pots (nine holes per holder, and one plant per hole) filled with aerated, complete nutrient solution. The solution was refreshed every 4 d. The nutrient solution had the following composition (in μM): Ca(NO3)2 (300), MgSO4 (50), NaH2PO4 (30), K2SO4 (50), H3BO3 (3), ZnSO4 (0.4), CuSO4 (0.2), MnCl2 (0.5), (NH4)6(Mo7)24 (1), and Fe-EDTA (20), adjusted to pH 6.5 with 1 m KOH. The plants were grown in a controlled-environment room at a temperature of 21°C, a relative humidity of 70%, with a daily cycle of 10-h day/14-h night. The daytime light intensity was 200 to 250 μmol photons m−2 s−1. Two weeks later, plants were transferred to media that contained either 20 μm (+Fe) or 0 μm (−Fe) FeNaEDTA for another 7 d. The solution was renewed daily. For experiments carried out with extrinsic NAA or GSNO supplementation, 0.08 μm NAA or 50 μm GSNO was added to the −Fe or +Fe solution for the time period. For experiments carried out with NPA, tungstate, l-NAME, or cPTIO treatment, 6-week-old plants were subjected to −Fe solution provided with either NPA (10 μm), tungstate (1 mm), or l-NAME (1 mm). A total of 0.5 mm cPTIO was also applied in the same way, except that 50-mL vials were used for the treatment.
Chemicals
GSNO was synthesized as reported previously (Stamler and Loscalzo, 1992). The NPA was purchased from Chemservice (http://www.anal-tech.com/), DAF-FM DA from Beyotime Institute of Biotechnology (http://www.beyotime.com/), cPTIO from Dojin Laboratories (http://www.dojindo.com/), l-NAME and agarose from Sigma (http://www.sigmaaldrich.com/), and Trizol reagent from Invitrogen (http://www. invitrogen.com/). Glutathione, NAA, 4-morpho-lineethanesulfonic acid, sodium tungstate, and ferrozine were purchased from Sangon (http://www.sangon.com/).
Root FCR Activity Determination
FCR activity was determined according to Grusak (1995). Briefly, the whole excised roots (about 0.1 g) were placed in a test tube filled with 5-mL assay solution consisting of 0.5 mm CaSO4, 0.1 mm 4-morpho-lineethanesulfonic acid, 0.1 mm bathophenanthrolinedisulfonic acid disodium salt hydrate (BPDS), and 100 μm Fe-EDTA at pH 5.5 adjusted by 1 m NaOH. The tubes were placed in a dark room at 25°C for 1 h, with periodic hand swirling at 10-min intervals. The absorbance of the assay solutions was recorded by a spectrophotometer at 535 nm, and the concentration of Fe(II)[BPDS]3 was quantified using an extinction coefficient of 22.14 mm−1 cm−1. The data were expressed as the means of relative root FCR activity, which was calculated as percentage of FCR activity of different lines with various treatments to that of wild type with sufficient Fe supply.
To visualize the location of FCR activity along the roots, excised roots were embedded in an agarose (0.75%, w/v) medium containing 0.5 mm CaSO4, 0.5 mm FeNaEDTA, and 0.5 mm ferrozine. Roots were incubated at 23°C ± 2°C for 20 min, and then the color patterns of young roots (5 mm from root tips) were recorded by light microscopy (Nikon Eclipse 80i, Nikon).
IAA Measurement
For analysis of the IAA concentration in roots, the whole root (about 20 mg) under the corresponding treatments was collected for each sample. Four replicates of the samples were purified after addition of 250 pg of 13C6-IAA internal standard and analyzed by gas chromatography-selected reaction monitoring mass spectrometry as described (Ljung et al., 2005).
In Situ Measurement of NO in the Root
NO content was imaged using DAF-FMDA under epifluorescence microscopy. Roots (5 mm from root tip) were loaded with 5 μm DAF-FMDA in 20 mm HEPES/NaOH buffer (pH 7.4) for 20 min, washed three times in fresh buffer, and analyzed microscopically (Nikon Eclipse 80i, Nikon, EX 460–500, DM 505, BA 510–560). The signal intensities of green fluorescence in the images of the young roots were quantified according to the method of Guo and Crawford (2005) by measuring the average pixel intensity with Photoshop software (Adobe Systems). Data is presented as the means of fluorescence intensity relative to that of wild type with sufficient Fe supply
Gene-Expression Analysis by Reverse Transcription-PCR
Root samples were frozen in liquid nitrogen immediately after collection and stored at −80°C. About 100 mg of tissue was ground in liquid nitrogen and total RNA was extracted by Trizol, and then the first-strand cDNA was synthesized with the total RNA by PrimeScript RT reagent kit (Takara). All RNA samples were checked for DNA contamination before cDNA synthesis. The mRNA levels of FIT and FRO2 were amplified by Taq polymerase (Takara) and the following pairs of gene-specific primers: FIT (F: 5′-GTATCAATCCTCCTGCTT-3′, R: 5′-TCTCGGTTACATCATCACT-3′), FRO2 (F: 5′-GTAAACAGGTCCAAAACG-3′, R: 5′-GTAAAGCACACAAAGATAGG-3′). A pair of housekeeping gene of tubulin β-3 was used for a control (F: 5′-AAGTTCTGGGAAGTGGTT-3′, R: 5′-CTCCCAATGAGTGACAAA-3′). The reverse transcription (RT)-PCR analysis was performed with ABI 7300 real-time PCR system (Applied Biosystems) with the following reaction conditions: 3 min at 94°C, 30 s at 95°C, 30 s at 55°C, 30 s at 72°C for cycles. The optimized linear range for FIT amplification was determined as 28 cycles, for FRO2 27 cycles, and for tubulin β-3 28 cycles. Each cDNA sample was run at least twice. Amplification of PCR products were monitored by agarose gel electrophoresis via intercalation of ethidium bromide.
Statistics
Data were analyzed by one-way ANOVA procedure and the means were compared by Duncan’s multiple range test. Different letters on the histograms indicate that the means were statistically different at P < 0.05 level.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_100040.2 and NM_179783.1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. A time course analysis of FCR activity in wild-type plants during Fe deficiency.
Supplemental Figure S2. Location of FCR activity in roots of wild-type plants.
Supplemental Figure S3. Molecular authentication of the yucca noa1 double mutant.
Supplementary Material
Acknowledgments
We thank H.W. Xue (Shanghai Institute for Biological Science, China) for kindly providing mutant strains aux1-7 and axr1-3, Y.D. Zhao (University of California, San Diego, La Jolla, CA) for yucca, N.M. Crawford (University of California, San Diego, La Jolla, CA) for Atnoa1, W.J. Zhang (Beijing Institute for Biological Science, China) for nia1 nia2, H.Q. Ling (Institute of Genetics and Developmental Biology, Beijing) for fit, and H.Q. Yang (Shanghai Jiaotong University, China) for axr1-12. Thanks are given to Dr. Leon Kochian (Plant, Soil and Nutrition Laboratory, U.S. Department of Agriculture, Agricultural Research Service, Cornell University, Ithaca, NY) for his critical reading and revising of the manuscript.
References
- Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D. (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149: 1302–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ. (2006) ABA induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45: 113–122 [DOI] [PubMed] [Google Scholar]
- Chaney RL, Brown JC, Tiffin LO. (1972) Obligatory reduction of ferric chelates in iron uptake by soybeans. Plant Physiol 50: 208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly EL, Campbell NH, Grotz N, Prichard CL, Guerinot ML. (2003) Overexpression of the FRO2 ferric chelate reductase confers tolerance to growth on low iron and uncovers posttranscriptional control. Plant Physiol 133: 1102–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Briat JF. (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Du ST, Zhang YS, Lin XY, Wang Y, Tang CX. (2008) Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L. cv. Baoda). Plant Cell Environ 31: 195–204 [DOI] [PubMed] [Google Scholar]
- Eide D, Broderius M, Fett J, Guerinot ML. (1996) A novel iron-regulated metal transporter from plants identified by functional expression in yeast. Proc Natl Acas Sci USA 93: 5624–5628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto Y, Goto F. (2008) Long-distance signaling of iron deficiency in plants. Plant Signal Behav 3: 396–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde B. (2002) The role of long-distance signalling in plant responses to nitrate and other nutrients. J Exp Bot 53: 39–43 [PubMed] [Google Scholar]
- Graziano M, Beligni MV, Lamattina L. (2002) Nitric oxide improves internal iron availability in plants. Plant Physiol 130: 1852–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. (2007a) Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. Plant J 52: 949–960 [DOI] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. (2007b) Nitric oxide and dinitrosyl iron complexes: roles in plant iron sensing and metabolism. van Fassen E, Vanin AF, , Radicals for Life: The Various Forms of Nitric Oxide. Elsevier, Oxford, pp 161–169 [Google Scholar]
- Grusak MA. (1995) Whole-root iron(III)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae): relevance to the iron nutrition of developing seeds. Planta 197: 111–117 [Google Scholar]
- Grusak MA, Pezeshgi S. (1996) Shoot-to-root signal transmission regulates root Fe(III) reductase activity in the dgl mutant of pea. Plant Physiol 110: 329–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ. (2006) Response to Zemojtel et al.: plant nitric oxide synthase: AtNOS1 is just the beginning. Trends Plant Sci 11: 527–528 [DOI] [PubMed] [Google Scholar]
- Guo FQ, Crawford NM. (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17: 3436–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM. (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302: 100–103 [DOI] [PubMed] [Google Scholar]
- Hu X, Neill S, Tang Z, Cai W. (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137: 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imsande J. (1998) Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Plant Physiol 103: 139–144 [Google Scholar]
- Jakoby M, Wang HY, Reidt W, Weisshaar B, Bauer P. (2004) FRU (BHLH029) is required for induction of iron mobilization genes in Arabidopsis thaliana. FEBS Lett 577: 528–534 [DOI] [PubMed] [Google Scholar]
- Jin CW, Chen WW, Meng ZB, Zheng SJ. (2008) Iron-deficiency-induced increase of root branching contributes to the enhanced root ferric chelate reductase activity. J Integr Plant Biol 50: 1557–1562 [DOI] [PubMed] [Google Scholar]
- Jin CW, Du ST, Chen WW, Li GX, Zhang YS, Zheng SJ. (2009) Elevated carbon dioxide improves plant Fe nutrition through enhancing the Fe-deficiency-induced responses under Fe-limited conditions in tomato (Lycopersicon esculentum M.). Plant Physiol 150: 272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, He XY, Wu P, Zheng SJ. (2006) Mechanisms of microbially enhanced Fe acquisition in red clover (Trifolium pratense L.). Plant Cell Environ 29: 888–897 [DOI] [PubMed] [Google Scholar]
- Jin CW, You GY, He YF, Tang CX, Wu P, Zheng SJ. (2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144: 278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D. (2005) Nitric oxide in plants: the biosynthesis and cell signalling properties of a fascinating molecule. Planta 221: 1–4 [DOI] [PubMed] [Google Scholar]
- Landsberg EC. (1996) Hormonal regulation of iron-stress response in sunflower roots: a morphological and cytological investigation. Protoplasma 194: 69–80 [Google Scholar]
- Lanteri ML, Graziano M, Correa-Aragunde N, Lamattina L. (2006) From cell division to organ shape: Nitric oxide is involved in auxin-mediated root development. Balušca F, Manusco S, Volkmann D, , Communication in Plants: Neuronal Aspects of Plant Life. Springer, Berlin, pp 123–136 [Google Scholar]
- Lau S, Jürgens G, Smet ID. (2008) The evolving complexity of the auxin pathway. Plant Cell 20: 1738–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhu X, Zhang F. (2000) Role of shoot in regulation of iron deficiency responses in cucumber and bean plants. J Plant Nutr 23: 1809–1818 [Google Scholar]
- Li X, Li C. (2004) Is ethylene involved in regulation of root ferric reductase activity of dicotyledonous species under iron deficiency. Plant Soil 261: 147–153 [Google Scholar]
- Lincoln C, Britton JH, Estelle M. (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. (2005) Sites and regulation of auxin biosynthesis in Arabidopsis roots. Plant Cell 17: 1090–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MC, Graziano M, Polacco J, Lamattina L. (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Romheld V, Kissel M. (1986) Different strategies in higher plants in mobilization and uptake of iron. J Plant Nutr 9: 695–713 [Google Scholar]
- Moreau M, Lee GI, Wang YZ, Crane BR, Klessig DF. (2008) AtNOS/A1 is a functional Arabidopsis thaliana cGTPase and not a nitric oxide synthase. J Biol Chem 283: 32957–32967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overvoorde PJ, Okushima Y, Alonso JM, Chan A, Chang C, Ecker JR, Hughes B, Liu A, Onodera C, Quach H, et al. (2005) Functional genomic analysis of the AUXIN/INDOLE-3-ACETIC ACID gene family members in Arabidopsis thaliana. Plant Cell 17: 3282–3300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez L, Zabaleta EJ, Lamattina L. (2009) Nitric oxide and frataxin: two players contributing to keep cellular iron homeostasis. Ann Bot (Lond) 105: 801–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML. (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397: 694–697 [DOI] [PubMed] [Google Scholar]
- Romera FJ, Alcántara E, De la Guardia MD. (1992) Role of roots and shoots in the regulation of the Fe efficiency responses in sunflower and cucumber. Physiol Plant 85: 141–146 [Google Scholar]
- Römheld V, Marschner H. (1981) Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Plant Physiol 53: 354–360 [Google Scholar]
- Santi S, Schmidt W. (2008) Laser microdissection-assisted analysis of the functional fate of iron deficiency-induced root hairs in cucumber. J Exp Bot 59: 697–704 [DOI] [PubMed] [Google Scholar]
- Schmidt W. (1994) Root-mediated ferric reduction-responses to iron deficiency, exogenously induced changes in hormonal balance and inhibition of protein synthesis. J Exp Bot 45: 725–731 [Google Scholar]
- Schmidt W. (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 141: 1–26 [Google Scholar]
- Stamler JS, Loscalzo J. (1992) Capillary zone electrophoretic detection of biological thiols and their S-nitrosated derivatives. Anal Chem 64: 779–785 [DOI] [PubMed] [Google Scholar]
- Sun B, Jing Y, Chen K, Song L, Chen F, Zhang L. (2006) Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays). J Plant Physiol 164: 536–543 [DOI] [PubMed] [Google Scholar]
- Vanin AF, Svistunenko DA, Mikoyan VD, Serezhenkov VA, Fryer MJ, Baker NR, Cooper CE. (2004) Endogenous superoxide production and the nitrite/nitrate ratio control the concentration of bioavailable free nitric oxide in leaves. J Biol Chem 279: 24100–24107 [DOI] [PubMed] [Google Scholar]
- Vert G, Briat JF, Curie C. (2003) Dual regulation of the Arabidopsis high affinity root iron uptake system by local and long-distance signals. Plant Physiol 132: 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C. (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 14: 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ID, Neill SJ, Hancock JT. (2008) Nitric oxide synthesis and signalling in plants. Plant Cell Environ 31: 622–631 [DOI] [PubMed] [Google Scholar]
- Yuan YX, Zhang J, Wang DW, Ling HQ. (2005) AtbHLH29 of Arabidopsis thaliana is a functional ortholog of tomato FER involved in controlling iron acquisition in strategy I plants. Cell Res 15: 613–621 [DOI] [PubMed] [Google Scholar]
- Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, et al. (2006) Plant nitric oxide synthase: a never-ending story? Trends Plant Sci 11: 524–525 [DOI] [PubMed] [Google Scholar]
- Zheng SJ, Tang CX, Arakama Y, Masaoka Y. (2003) The responses of red clover (Trifolium pratense L.) to iron deficiency: a root Fe(III) chelate reductase. Plant Sci 164: 679–687 [Google Scholar]
- Zottini M, Costa A, Michele RD, Ruzzene M, Carimi F, Schiavo FL. (2007) Salicylic acid activates nitric oxide synthesis in Arabidopsis. J Exp Bot 58: 1397–1405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.