Plants have evolved a complex immune system to perceive microbial pathogens and respond by producing defense compounds preventing infection. Defense hormones such as salicylic acid (SA), jasmonates, and ethylene are key signals regulating the production of antimicrobial defenses. Moreover, other hormone pathways have critical actions by controlling responses to pathogen attack such as distribution of resources, cell death, water stress, or plant architecture. A fine-tuning regulation of these pathways through complex regulatory networks is necessary to achieve resistance against different pathogen classes (López et al., 2008; Grant and Jones, 2009).
Plants activate two forms of immunity by recognition of distinct pathogen molecules. A first and rapid response, known as basal resistance (microbe-associated molecular pattern-triggered immunity or MTI), is triggered after recognition of conserved microbial molecules (microbe-associated molecular patterns) by extracellular plant receptors (pattern recognition receptors; Boller and Felix, 2009). Second, effector-triggered immunity (ETI) is activated by resistance (R)-gene products (largely inside the cell) after recognition of specific effectors molecules (delivered into the plant cell by pathogens) and is commonly accompanied by a hypersensitive reaction (HR) involving localized host cell death at the point of infection (Jones and Dangl, 2006).
Oxidative burst involving production of reactive oxygen species (ROS) is a nearly ubiquitous response of plants to pathogen attack and has a key role in both MTI and ETI signaling (Torres et al., 2006). ROS activation is likely a primary consequence of the damage produced during the course of infection. However, whereas overaccumulation of ROS might enhance plant susceptibility (Govrin and Levine, 2000; Kariola et al., 2005) or cause an uncontrolled defense with spreading cell death lesions that can kill the plant (Lorrain et al., 2003; Moeder and Yoshioka, 2008), a tight regulation over ROS production and elimination through enzymatic and nonenzymatic antioxidants has allowed plants to use these reactive compounds as a critical feature of MTI and ETI (Torres et al., 2006). Reported defense responses associated with the production of ROS include direct killing of pathogens, activation of host cell death (HR), and contribution to cell wall strengthening (Bolwell and Daudi, 2009). Moreover, emerging data highlight the role of ROS as signals in MTI and ETI (Torres et al., 2006; Van Breusegem et al., 2008) as well as their contribution to provide an appropriate redox environment needed to activate defense (Tada et al., 2008). The importance of ROS in plant defense will be discussed here with a focus on the production of distinct types of ROS and on the cellular compartments involved in their production.
ESTABLISHED ROLE FOR SUPEROXIDE IONS AND HYDROGEN PEROXIDE IN RESPONSE TO PATHOGEN ATTACK
Two distinct reactions can convert ground state oxygen into different types of ROS during an oxidative burst (Fig. 1). Thus, dioxygen can be stepwise reduced by electron transfer to superoxide ion (O2−) and hydrogen peroxide (H2O2), and the later can produce the hydroxyl radical (OH•). Alternatively, dioxygen can be converted by energy transfer to singlet oxygen (1O2), a highly reactive short-lived product (half-life, approximately 200 ns) with a strong oxidizing potential (Apel and Hirt, 2004). Production of ROS occurs at different cellular locations in response to distinct environmental cues, and both the scavenging mechanisms and signaling events triggered by O2−H2O2, and 1O2 have been investigated (op den Camp et al., 2003; Mittler et al., 2004; Gadjev et al., 2006). Results from transcriptomic analyses define common and specific responses toward different types of ROS as well as cross talk between distinct ROS signaling pathways, pointing to a complex scenario in which a fine regulation is critical for plant survival (op den Camp et al., 2003; Gadjev et al., 2006; Laloi et al., 2007).
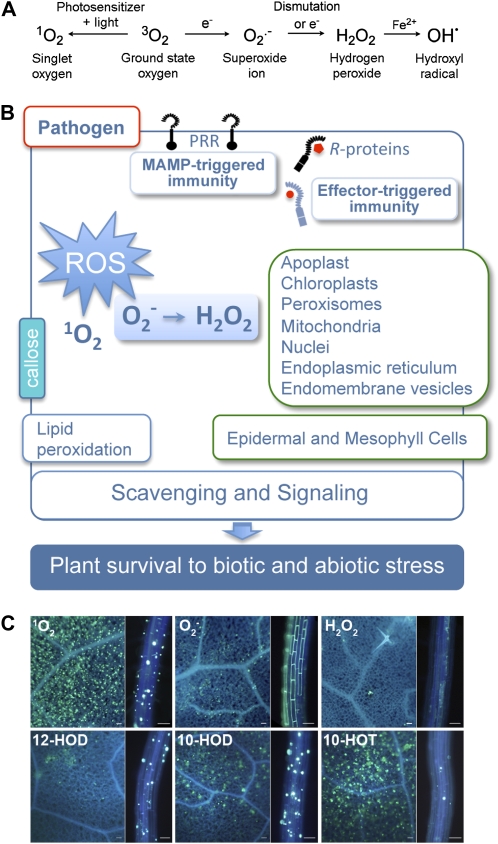
Figure 1.
Production of ROS in plant defense. A, Generation of distinct ROS during oxidative burst. B, Tight regulation of ROS production at different cellular compartments and cell types is critical for plant survival. C, Callose accumulation after production of 1O2, O2−H2O2, and specific 1O2-derived hydroxy fatty acids. Aniline blue staining in Arabidopsis leaves of 4-week-old plants (square-shaped sections) and in roots of in vitro-grown seedlings (rectangular sections). Leaves were infiltrated with Rose Bengal (1 μm as 1O2 producer), xanthine-xanthine oxidase (2 mm-0.1 units per mL as extracellular O2− generator), H2O2 (1 mm), 12-HOD (25 μm), 10-HOD (25 μm), and 10-HOT (25 μm). Roots are from 8-d-old seedlings grown in Murashige and Skoog medium and covered with a solution of the ROS inducers xanthine-xanthine oxidase and H2O2, at the above concentrations, or germinated 4 d in Murashige and Skoog medium and then transferred to a fresh medium containing Rose Bengal (100 nm), or 12-HOD, 10-HOD, and 10-HOT (10 μm). Representative examples of 24 h treated tissues are shown in all cases. Scale bars = 50 μm.
Research examining the role of ROS in plant defense has been focused on the actions of O2−H2O2, whereas other ROS such as OH• and 1O2 have been far less examined (Apel and Hirt, 2004). Different enzymes have been implicated in the generation of apoplastic ROS in plant defense, among which NADPH oxidases (also known as respiratory burst oxidases or Rbohs), similar to those present in mammalian neutrophils, have received most attention. Plant NADPH oxidases catalyze the formation of superoxide by the following reaction: NADPH + 2O2 = NADP+ + H+ + 2O2−. Secondary spontaneous or superoxide dismutase-catalyzed conversion of superoxide provides H2O2, which in turn can afford OH• in the presence of transition metal ions such as Fe2+ or Cu+. Genetic analysis demonstrated that reduction or lack of RbohD and RbohF leads to elimination of extracellular H2O2 (Torres et al., 2002). However, reduced production of O2−of its dismutation product H2O2 exerts different effects in plant pathogen growth and HR cell death, which suggests that apoplastic ROS might interact with distinct signaling pathways to serve different purposes. Thus, the spreading lesion phenotype of the lsd1 mutants (for lesion stimulating disease) is enhanced in the triple mutant lsd1-rbohD-rbohF, which has led to propose the role of RbohD and RbohF in limiting SA-elicited cell death in cells surrounding an infection site (Torres et al., 2005).
In addition to the apoplast, evidence for a role of chloroplast, peroxisomes, or mitochondria in ROS production has been reported (Van Breusegem et al., 2008). Moreover, recent studies identified other cellular sites such as endoplasmic reticulum, endomembranes vesicles, and nuclei as producers of ROS during pathogen responses, although the actions of ROS from these cellular locations remain mostly unknown (Ashtamker et al., 2007). The participation of chloroplasts in pathogen responses is concluded by results showing that light is required to activate defense gene expression and HR (Karpinski et al., 2003) and that the light-growth conditions might affect the formation of infection-like lesions in a number of Arabidopsis (Arabidopsis thaliana) mutants (Lorrain et al., 2003; Moeder and Yoshioka, 2008). In many cases, these phenotypes correlate with a failure in the photosynthetic machinery or in the mechanisms protecting cells against oxidative damage, including the process of photorespiration that mitigates photooxidative damage and requires the participation of peroxisomes and mitochondria (Moreno et al., 2005; Queval et al., 2007).
The role of Enhanced Disease Susceptibility1 (EDS1) as a master regulatory protein that coordinates defense by processing chloroplastic ROS-derived signals has been shown (Straus et al., 2010). However, chloroplastic ROS production and plant defense can be uncoupled. A recent example is the demonstration that chloroplast-derived ROS are essential for the formation of HR cell death but not for the activation of other basal defense responses in tobacco (Nicotiana tabacum) transgenic plants (Zurbriggen et al., 2009). Also, mutation of the chloroplastic Resistance to Phytopthora1 protein in Arabidopsis led to reduced H2O2 and enhanced susceptibility to Phytopthora brassicae, but caused a rapid run away cell death that originated at the point of infection (Belhaj et al., 2009).
Like chloroplasts, the mitochondria can also be an important source of ROS during physiological or pathological conditions that possess an efficient antioxidant machinery to control their toxic effects (Apel and Hirt, 2004). Nevertheless, the role of these organelles in plant cell death and pathogen responses has received little attention. Several studies revealed that treatments with cell death inducers, such as bacterial elicitors or virulence effectors might disrupt the functionality of the mitochondria and increase basal levels of ROS (Balandin and Castresana, 2002; Yao et al., 2004; Block et al., 2010). These results suggest that mitochondrial disturbance is a broadly employed strategy by pathogens to suppress host immunity and that increased ROS may contribute to the protection of plants against pathogen damage.
Results described above indicate that plants have evolved sophisticated mechanisms to use the compartmentalized production of ROS in the modulation of the defense responses against pathogen attack.
EMERGING ROLES FOR 1O2 AND LIPID PEROXIDATION IN PLANT DEFENSE
Information on the role of 1O2 in plant defense is still very limited. However, direct and indirect evidence discussed below, is starting to disclose a signaling role of 1O2 and its participation in the response to pathogens, an area that can be expected to receive much attention in the near future. 1O2 is a highly reactive unstable molecule produced in plants under basal and light stress conditions (Triantaphylidès and Havaux, 2009). In the chloroplast, excited chlorophyll can act as a photosensitizer to produce 1O2 from ground state oxygen. In addition, secondary metabolites such as phenaleno-like phytoalexins and phytoanticipins might act as photosensitizers to generate 1O2 following absorption of light energy (Flors and Nonell, 2006). Increased levels of these metabolites after pathogen attack could thus contribute to generate 1O2 as a product of the plant defense machinery.
1O2 has a crucial role during acclimation of plants to high light intensity and photooxidative stress (Triantaphylidès et al., 2008), a response that shows strong similarities to plant defense, including the functional integration of SA and of defense regulatory proteins such as LSD1, EDS1, and Phytoalexin Deficient4 (Mühlenbock et al., 2008). Of great interest, studies with the conditional flu mutant that generates 1O2 upon light illumination (op den Camp et al., 2003) allow to distinguish two modes of 1O2 activity. Thus, whereas high 1O2 production leads to photooxidative damage, decreased levels mediate a signaling activity, two responses that could be executed by 1O2 or by more stable 1O2-dependent products (Przybyla et al., 2008).
A universal response of plants to pathogen attack is the generation of a host of active lipid derivatives, collectively known as oxylipins (Andreou et al., 2009; Mosblech et al., 2009). Such compounds can be formed either by enzymatic or nonenzymatic peroxidation of fatty acids, however, certain of the hydroxy oxylipins, i.e. linoleic acid-derived 10-hydroxy-octadecadienoic acid (10-HOD) and 12-HOD and linolenic acid-derived 10-hydroxy-octadecatrienoic acid (10-HOT) and 15-HOT, can only be formed by 1O2-dependent nonenzymatic oxygenation and can therefore be used as in vivo markers of 1O2 generation (Przybyla et al., 2008). Whereas oxylipins formed by both specific enzymatical pathways (Hamberg et al., 2005; Kachroo and Kachroo, 2009) and by nonenzymatical free-radical reactions (Loeffler et al., 2005) play important roles in plant defense, no function has yet been assigned to the 1O2-derived hydroxy fatty acids. Of interest, these latter compounds accumulate in etiolated flu seedlings following illumination (Przybyla et al., 2008) and in leaves of Arabidopsis responding to Pseudomonas syringae pv tomato inoculation (Grun et al., 2007), reflecting the generation of 1O2 during stress responses, photooxidation, and pathogen attack.
Deposition of callose is a frequent response of cells to pathogen assault (Hématy et al., 2009). Importantly, production of 1O2 (triggered by Rose Bengal) and application of 1O2-formed hydroxy acids, induce a strong accumulation of callose in leaves (1O2 and 12-HOT) and roots (1O2, 10-HOD, and 12-HOD) of Arabidopsis (Fig. 1). Callose deposition was also observed (preferentially in roots) after application of O2− (generated by xanthine-xanthine oxidase) and was only weakly detected in roots of seedlings responding to H2O2 (Fig. 1). These results suggest that ROS such as 1O2 and O2− might contribute to the accumulation of callose during the response of plants to pathogen attack. Of interest, the differences in the pattern of callose deposition observed after generation of 1O2 and O2− or application of distinct 1O2-derived hydroxy fatty acids point to tissue-specific variations in the mode of action to these compounds. Further support of the participation of 1O2 in plant defense comes from results showing an overrepresentation of biotic stress-related genes during the transcriptomic reprogramming activated after generation of 1O2 (M. Martínez and C. Castresana, unpublished data).
Although these new observations deserve further investigation, these results are indicative of an active response of plants toward specific 1O2-derived hydroxy fatty acids. Related to this, we note that the nonenzymatic oxidation of linolenic acid contributes to limit pathogen infection and spreading cell death and that the action of linolenic acid as a sink for ROS has been suggested (Mène-Saffrané et al., 2009). Also, in line with our discussion, we speculate that the 1O2-derived hydroxy fatty acids could play a role in oxidative stress signaling and actively contribute to protect plant tissues against pathogen attack.
CONCLUDING REMARKS
Results described above reveal that plants have evolved unique defense responses that depend on ROS production and redox signals generated by different mechanisms at specific cellular locations. Tight control over production and accumulation of ROS is likely to be crucial to plants grown in natural environments where survival to pathogen attack and acclimation to prevailing abiotic stress factors (like high light) have to be integrated. However, key aspect of ROS production and signaling remain still poorly understood. Plants generate chemically distinct oxygen derivatives, which may be selectively produced at specific cellular locations in response to different environmental stresses. Studies to investigate the actions of ROS have revealed common and specific responses toward different types of ROS as well as cross talk between distinct ROS signaling pathways, thus showing a complex scenario hampering the investigation of selective actions by a given ROS. Major efforts in examining the actions of O2−H2O2 might have oversimplified the analyses of ROS in plant defense. Compared to other ROS, 1O2 has received little attention, and recent studies indicating its participation in plant defense are emerging. Polyunsaturated fatty acids are a preferred target of 1O2 attack and several of its oxidation products could act as secondary messengers to trigger defense responses. Studies on the actions of ROS will benefit from newly developed tools helping to monitor in a noninvasive manner, the generation and signaling events of distinct types of ROS in the activation of resistance after pathogen attack.
Acknowledgments
We thank G. Bannenberg and J. Paz-Ares for constructive comments.
References
- Andreou A, Brodhun F, Feussner I. (2009) Biosynthesis of oxylipins in non-mammals. Prog Lipid Res 48: 148–170 [DOI] [PubMed] [Google Scholar]
- Apel K, Hirt H. (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 373–399 [DOI] [PubMed] [Google Scholar]
- Ashtamker C, Kiss V, Sagi M, Davydov O, Fluhr R. (2007) Diverse subcellular locations of cryptogein-induced reactive oxygen species production in tobacco Bright Yellow-2 cells. Plant Physiol 143: 1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balandin T, Castresana C. (2002) AtCOX17, an Arabidopsis homolog of the yeast copper chaperone COX17. Plant Physiol 129: 1852–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhaj K, Lin B, Mauch F. (2009) The chloroplast protein RPH1 plays a role in the immune response of Arabidopsis to Phytophthora brassicae. Plant J 58: 287–298 [DOI] [PubMed] [Google Scholar]
- Block A, Guo M, Li G, Elowsky C, Clemente TE, Alfano JR. (2010) The Pseudomonas syringae type III effector HopG1 targets mitochondria, alters plant development and suppresses plant innate immunity. Cell Microbiol 12: 318–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Daudi A. (2009) Reactive oxygen species in plant-pathogen interactions. del Rio LA, Puppo A, , Reactive Oxygen Species in Plant Signalling. Springer, Berlin, pp 113–133 [Google Scholar]
- Flors C, Nonell S. (2006) Light and singlet oxygen in plant defense against pathogens: phototoxic phenalenone phytoalexins. Acc Chem Res 39: 293–300 [DOI] [PubMed] [Google Scholar]
- Gadjev I, Vanderauwera S, Gechev TS, Laloi C, Minkov IN, Shulaev V, Apel K, Inze D, Mittler R, Van Breusegem F. (2006) Transcriptomic footprints disclose specificity of reactive oxygen species signaling in Arabidopsis. Plant Physiol 141: 436–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govrin EM, Levine A. (2000) The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr Biol 10: 751–757 [DOI] [PubMed] [Google Scholar]
- Grant MR, Jones JD. (2009) Hormone (dis)harmony moulds plant health and disease. Science 324: 750–752 [DOI] [PubMed] [Google Scholar]
- Grun C, Berger S, Matthes D, Mueller MJ. (2007) Early accumulation of non-enzymatically synthesised oxylipins in Arabidopsis thaliana after infection with Pseudomonas syringae. Funct Plant Biol 34: 65–71 [DOI] [PubMed] [Google Scholar]
- Hamberg M, Ponce de León I, Rodríguez MJ, Castresana C. (2005) Alpha-dioxygenases. Biochem Biophys Res Commun 338: 169–174 [DOI] [PubMed] [Google Scholar]
- Hématy K, Cherk C, Somerville S. (2009) Host-pathogen warfare at the plant cell wall. Curr Opin Plant Biol 12: 406–413 [DOI] [PubMed] [Google Scholar]
- Jones JD, Dangl JL. (2006) The plant immune system. Nature 444: 323–329 [DOI] [PubMed] [Google Scholar]
- Kachroo A, Kachroo P. (2009) Fatty acid-derived signals in plant defense. Annu Rev Phytopathol 47: 153–176 [DOI] [PubMed] [Google Scholar]
- Kariola T, Brader G, Li J, Palva ET. (2005) Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17: 282–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski S, Gabrys H, Mateo A, Karpinska B, Mullineaux PM. (2003) Light perception in plant disease defence signalling. Curr Opin Plant Biol 6: 390–396 [DOI] [PubMed] [Google Scholar]
- Laloi C, Stachowiak M, Pers-Kamczyc E, Warzych E, Murgia I, Apel K. (2007) Cross-talk between singlet oxygen- and hydrogen peroxide-dependent signaling of stress responses in Arabidopsis thaliana. Proc Natl Acad Sci USA 104: 672–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler C, Berger S, Guy A, Durand T, Bringmann G, Dreyer M, von Rad U, Durner J, Mueller MJ. (2005) B1-phytoprostanes trigger plant defense and detoxification responses. Plant Physiol 137: 328–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López MA, Bannenberg G, Castresana C. (2008) Controlling hormone signaling is a plant and pathogen challenge for growth and survival. Curr Opin Plant Biol 11: 420–427 [DOI] [PubMed] [Google Scholar]
- Lorrain S, Vailleau F, Balague C, Roby D. (2003) Lesion mimic mutants: keys for deciphering cell death and defense pathways in plants? Trends Plant Sci 8: 263–271 [DOI] [PubMed] [Google Scholar]
- Mène-Saffrané L, Dubugnon L, Chetelat A, Stolz S, Gouhier-Darimont C, Farmer EE. (2009) Nonenzymatic oxidation of trienoic fatty acids contributes to reactive oxygen species management in Arabidopsis. J Biol Chem 284: 1702–1708 [DOI] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9: 490–498 [DOI] [PubMed] [Google Scholar]
- Moeder W, Yoshioka K. (2008) Lesion mimic mutants: a classical, yet still fundamental approach to study programmed cell death. Plant Signal Behav 3: 764–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JI, Martín R, Castresana C. (2005) Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. Plant J 41: 451–463 [DOI] [PubMed] [Google Scholar]
- Mosblech A, Feussner I, Heilmann I. (2009) Oxylipins: structurally diverse metabolites from fatty acid oxidation. Plant Physiol Biochem 47: 511–517 [DOI] [PubMed] [Google Scholar]
- Mühlenbock P, Szechynska-Hebda M, Plaszczyca M, Baudo M, Mateo A, Mullineaux PM, Parker JE, Karpinska B, Karpinski S. (2008) Chloroplast signaling and LESION SIMULATING DISEASE1 regulate crosstalk between light acclimation and immunity in Arabidopsis. Plant Cell 20: 2339–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- op den Camp RG, Przybyla D, Ochsenbein C, Laloi C, Kim C, Danon A, Wagner D, Hideg E, Gobel C, Feussner I, (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15: 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przybyla D, Gobel C, Imboden A, Hamberg M, Feussner I, Apel K. (2008) Enzymatic, but not non-enzymatic, 1O2-mediated peroxidation of polyunsaturated fatty acids forms part of the EXECUTER1-dependent stress response program in the flu mutant of Arabidopsis thaliana. Plant J 54: 236–248 [DOI] [PubMed] [Google Scholar]
- Queval G, Issakidis-Bourguet E, Hoeberichts FA, Vandorpe M, Gakière B, Vanacker H, Miginiac-Maslow M, Van Breusegem F, Noctor G. (2007) Conditional oxidative stress responses in the Arabidopsis photorespiratory mutant cat2 demonstrate that redox state is a key modulator of daylength-dependent gene expression, and define photoperiod as a crucial factor in the regulation of H2O2-induced cell death. Plant J 52: 640–657 [DOI] [PubMed] [Google Scholar]
- Straus MR, Rietz S, van Themaat EV, Bartsch M, Parker JE. (2010) Salicylic acid antagonism of EDS1-driven cell death is important for immune and oxidative stress responses in Arabidopsis. Plant J 62: 628–640 [DOI] [PubMed] [Google Scholar]
- Tada Y, Spoel SH, Pajerowska-Mukhtar K, Mou Z, Song J, Wang C, Zuo J, Dong X. (2008) Plant immunity requires conformational changes [corrected] of NPR1 via S-nitrosylation and thioredoxins. Science 321: 952–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. (2005) Pathogen-induced, NADPH oxidase-derived reactive oxygen intermediates suppress spread of cell death in Arabidopsis thaliana. Nat Genet 37: 1130–1134 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JD, Dangl JL. (2006) Reactive oxygen species signaling in response to pathogens. Plant Physiol 141: 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantaphylidès C, Havaux M. (2009) Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci 14: 219–228 [DOI] [PubMed] [Google Scholar]
- Triantaphylidès C, Krischke M, Hoeberichts FA, Ksas B, Gresser G, Havaux M, Van Breusegem F, Mueller MJ. (2008) Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol 148: 960–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breusegem F, Bailey-Serres J, Mittler R. (2008) Unraveling the tapestry of networks involving reactive oxygen species in plants. Plant Physiol 147: 978–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao N, Eisfelder BJ, Marvin J, Greenberg JT. (2004) The mitochondrion—an organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J 40: 596–610 [DOI] [PubMed] [Google Scholar]
- Zurbriggen MD, Carrillo N, Tognetti VB, Melzer M, Peisker M, Hause B, Hajirezaei MR. (2009) Chloroplast-generated reactive oxygen species play a major role in localized cell death during the non-host interaction between tobacco and Xanthomonas campestris pv. vesicatoria. Plant J 60: 962–973 [DOI] [PubMed] [Google Scholar]