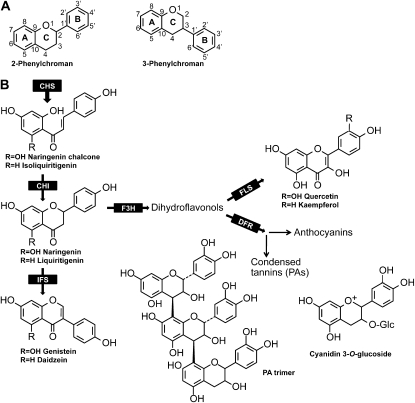
Flavonoids represent one of the largest and most studied classes of phenylpropanoid-derived plant specialized metabolites, with an estimated 10,000 different members. Structurally, they consist of two main groups, the 2-phenylchromans (the flavonoids, including flavanones, flavones, flavonols, flavan-3-ols, and anthocyanidins) and the 3-phenylchromans (the isoflavonoids, including isoflavones, isoflavans, and pterocarpans; Fig. 1A).
Figure 1.
Flavonoid classes and an outline of their biosynthesis. A, The 2- and 3-phenylchroman skeletons. B, Schematic of flavonoid biosynthesis. Enzymes are CHS, chalcone isomerase (CHI), flavanone 3-β-hydroxyalse (F3H), flavonol synthase (FLS), and dihydroflavonol reductase (DFR). Anthocyanins are formed from leucoanthocyanidin (the product of DFR) and condensed tannins; PAs are formed from (epi)catechins, products of leucoanthocyanidin reductase or anthocyanidin reductase. 3-Phenylchromans (isoflavonoids) are formed via IFS. All classes of (iso)flavonoids can be further modified by substitution (e.g. O-methylation, glycosylation, C-prenylation).
Flavonoids act as attractants to pollinators and symbionts, as sunscreens to protect against UV irradiation, as allelochemicals, and as antimicrobial and antiherbivory factors. Their importance in plant biology goes beyond their specific functions within the plant. For example, the early advances in floral genetics were primarily the result of the ease of screening for mutations impacting flavonoid-derived flower colors, and the first demonstration of epigenetic gene silencing in plants was likewise associated with flavonoid biosynthesis (Jorgensen, 1995). Flavonoids have been ascribed positive effects on human and animal health and are central to the current interest in “botanicals” for disease therapy and chemoprevention.
BIOSYNTHESIS, TRANSPORT, AND TURNOVER OF (ISO)FLAVONOIDS
The basic pathways to the core (iso)flavonoid skeletons have been established both enzymatically and genetically. The entry point enzymes are the polyketide synthase chalcone synthase (CHS) and isoflavone synthase (IFS), more correctly termed 2-hydroxyisoflavanone synthase, a cytochrome P450 that catalyzes the aryl migration reaction that converts a 2-phenylchroman to a 3-phenylchroman (Fig. 1B). The structural diversity of (iso)flavonoids is derived by substitution of these basic carbon skeletons through further hydroxylation, glycosylation, methylation, acylation, and prenylation as well as, in the case of the proanthocyanidins (PAs; also known as condensed tannins; Fig. 1B) and phlobaphenes, by polymerization. The enzymes that catalyze the substitution reactions are often encoded by large gene families, which can be recognized in EST and genome data sets through family-specific conserved sequence motifs.
Several areas of flavonoid biosynthesis still require clarification or elucidation. These include in vivo substrate specificity of potentially multifunctional enzymes (e.g. 2-oxoglutarate-dependent dioxygenases such as anthocyanidin synthase or the many family I glycosyltransferases that are active with flavonoids), transport of flavonoids into and out of the central vacuole and other cellular compartments, and the oxidation of flavonoids associated with polymerization to PAs and phlobaphenes (Pourcel et al., 2006).
The distinction between in vitro activity and in vivo function in flavonoid biosynthesis is not always clear. Many flavonoid biosynthetic enzymes, particularly those catalyzing the substitution reactions, are quite promiscuous and are active with multiple flavonoid classes and even nonflavonoid specialized metabolites (e.g. triterpenes or coumarins). For example, the glucosyltransferase UGT78G1 was initially identified as an isoflavone glucosyltransferase in vitro, with anthocyanidins being the kinetically least preferred substrates among the multiple classes of flavonoids tested. However, the UGT78G1 gene is controlled by a MYB transcription factor that is a master regulator of anthocyanin biosynthesis, and gain- and loss-of-function studies confirmed that the enzyme is involved in anthocyanin glycosylation in Medicago truncatula in vivo (Peel et al., 2009). It is possible that the in vivo substrate specificity of flavonoid UGTs is determined in some cases by their association with other pathway enzymes in metabolic complexes rather than by their in vitro kinetic properties alone. Recent advances in live cell imaging in plants (Kwaaitaal et al., 2010) should help to address such possibilities
Much remains to be learned about the intracellular and intercellular transport of (iso)flavonoids. Isoflavone secretion from legume roots, a process critical for the establishment of nodulation, may involve plasma membrane-localized ATP-binding cassette transporters, and vacuolar uptake of anthocyanins and precursors destined for PA biosynthesis can involve multidrug and toxin extrusion family transporters (Zhao et al., 2010). However, alternative mechanisms have been proposed for anthocyanin transport (Grotewold and Davies, 2008), the subcellular localization of some of the components associated with PA precursor uptake is still unclear (Zhao and Dixon, 2010), and little is known about how (iso)flavonoid compounds are mobilized out of the vacuole. It has been known for nearly 20 years that isoflavone conjugates can be remobilized from the vacuole for subsequent conversion to phytoalexins (Mackenbrock and Barz, 1991), but vacuolar efflux carriers for flavonoids have yet to be identified. A broader question to address is how flavonoids are tagged for permanent versus transient storage in the vacuole; early models suggested that acylation helped retain flavonoids in the vacuole, but acylation may also be critical for initial transport into the vacuole (Gomez et al., 2009). Brunfelsia pauciflora rapidly loses its vacuolar anthocyanins over a period of 2 d, such that the flower color changes from purple to white. In spite of such clear evidence for turnover of vacuolar flavonoids, the signals that determine whether such compounds will be remobilized or degraded are currently unknown.
IN PLANTA FUNCTIONS OF (ISO)FLAVONOIDS
We have come a long way from the once-held view that flavonoids are simply waste products of metabolism that are “dumped” into the central vacuole. It is clear that flavonoids function throughout the plant kingdom as UV protectants, pollinator attractants, and antimicrobial compounds. More specific functions have been ascribed to certain flavones and isoflavones as inducers of rhizobial nodulation genes during the establishment of the nitrogen-fixing symbiosis in legumes (Subramanian et al., 2007), as nonessential modulators of auxin transport (Peer and Murphy, 2007), and as factors required for pollen viability. There has been a notable expansion of CHS genes in the legumes. Although scattered throughout the genomes, these genes form clusters of up to five to seven coregulated genes in M. truncatula and soybean (Glycine max) that may reflect ongoing duplication events indicative of selective evolutionary pressure in favor of flavonoids in legume species. It is sometimes assumed that this evolutionary pressure is related to the special roles of flavonoids in the establishment of symbiotic relationships in legumes, but this hypothesis still requires verification.
The property most shared among different (iso)flavonoids is that of antioxidant potential, often cited as the reason for the beneficial effects of flavonoids on human health. However, the relevance of this property for flavonoid function in planta is still a topic of debate (Hernández et al., 2008). The vacuolar localization of many flavonoids allows for their light-screening, photoprotective, and pigmentation functions but probably not their antioxidative functions. Combined genetic, biochemical, and biophysical studies are required to elucidate the functions of the flavonoids that accumulate outside of the vacuole (e.g. in chloroplasts and nuclei).
METABOLIC ENGINEERING OF (ISO)FLAVONOIDS
Metabolic engineering of (iso)flavonoids has been achieved by three strategies: expression of transcription factors, or key metabolic enzymes, to increase flux into endogenous flavonoid pathways; expression of nonendogenous flavonoid-modifying enzymes to direct the formation of novel flavonoids; and reconstitution of natural and unnatural flavonoids in yeast or Escherichia coli. Ectopic expression of specific MYB transcription factors leads to massive accumulation of anthocyanins or PAs (Butelli et al., 2008; Pang et al., 2008). Transcription factor overexpression can lead to pleiotropic off-target effects, but it seems likely that this technology can be developed to provide crop plants with enhanced levels of health-beneficial phytonutrients in the absence of unwanted side products.
The prenylation of flavonoids enhances their antibacterial, antifungal, and other biological activities by increasing their lipophilicity and membrane permeability. (Iso)flavonoid prenyltransferases are among the more recently discovered flavonoid-modifying enzymes, typified by naringenin dimethylallyltransferase from Sophora flavescens (Sasaki et al., 2008). Roots of the legume Desmodium uncinatum produce C-prenylated isoflavones, and these inhibit growth/germination of the parasitic plant Striga, one of Africa’s major limitations to crop production. As a result, intercropping of Desmodium with corn (Zea mays) can significantly increase grain yields (Khan et al., 2006), and transgenic corn, sorghum (Sorghum bicolor), and other crops expressing the necessary genes for the production of prenylated isoflavones could be developed in the future.
Alfalfa (Medicago sativa), the world’s major forage legume, causes pasture bloat due to excessive rumenal fermentation of dietary protein to methane. Modest levels of PAs (Fig. 1B) in forages reduce the occurrence of bloat and at the same time promote increased dietary protein nitrogen utilization in ruminant animals such as cattle and sheep. Classical breeding approaches have failed to introduce PAs into alfalfa foliage, and the biotechnological development of a bloat-safe alfalfa is likely to first require a better understanding of the molecular and cellular biology of PA polymerization, transport, and storage.
Microbial production of flavonoids has depended on the generation of strains expressing the ligase for the hydroxycinnmate CoA ligation step, CHS and chalcone isomerase (Fig. 1B). Specific classes of flavonoids can then be generated via expression of flavone synthase, flavanone 3-β-hydroxylase (for flavonols), or IFS (for isoflavonoids; Katsuyama et al., 2007; Fig. 1B). Such systems probably provide little economic benefit for the formation of commonly occurring (iso)flavonoids but promise much for future combinatorial synthetic biology approaches for the generation of novel compounds. Current approaches involve feeding different organic acids for CoA ligation and subsequent polyketide formation, but it is also easy to envisage the construction of libraries of mutant flavonoid substitution enzymes in microbes engineered to carry out the core pathway reactions.
(ISO)FLAVONOIDS AND PERSONALIZED MEDICINE
An unprecedented consumer demand exists for plant compounds that might help to preserve cognitive function, thereby improving quality of life while maintaining health. A 2005 to 2006 survey estimated that 49% of elderly community-residing U.S. adults, ages 57 through 85, used dietary supplements on a regular basis (Qato et al., 2008). The number of Americans over the age of 85 years (5.8 million) will more than triple to 19.3 million by 2050. Of these 19.3 million, 8 million are predicted to develop dementia (Hebert et al., 2001). This observation, and the fact that nonagerian and centenarian individuals constitute the fastest growing segment of the U.S. population at the highest risk to develop Alzheimer’s disease, makes it increasingly important to better identify methods of preserving and maintaining cognitive functions in the elderly. Recent evidence suggests that flavonoids, particularly fractions rich in PAs, can significantly attenuate cognitive deterioration in animal model systems (Pasinetti et al., 2007; Wang et al., 2008; Ho et al., 2009), and flavonoids may more generally promote improvements in memory acquisition, consolidation, storage, and retrieval under nondegenerative conditions (Spencer, 2009). These studies can now be extended to provide novel mechanisms of action through which certain “phytodrugs” might beneficially influence cognitive function and Alzheimer’s disease dementia.
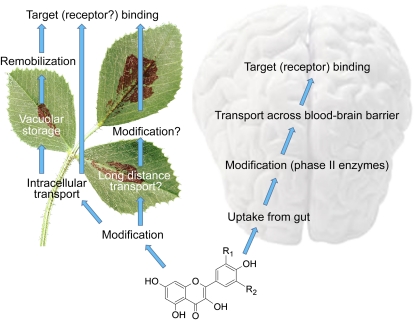
There are several parallels between the metabolic fates of flavonoids within plants and within animals after ingestion (Fig. 2). For example, both plants and animals modify flavonoids by methylation and glycosylation and transport the native and/or modified compounds across membranes. Mechanisms associated with the gut absorption of phytotherapeutic agents may play an important role in their bioavailability and clinical responses. These mechanisms vary widely depending on structural features. In the case of flavonoids, isoflavones, followed by catechins, flavanones, and quercetin glucosides, are best absorbed in humans with varying kinetics. The least absorbable flavonoids are the PAs, galloylated tea catechins, and anthocyanins. This information is highly useful in the design and interpretation of interventional studies investigating the health effects of phytotherapeutic agents. While blood-brain barrier penetration may influence the local bioactivity of compounds, issues related to the absorption of compounds across the gut wall are fundamental and likely better inform the development of flavonoids as therapeutic agents in integrative medicine (Manach et al., 2005; Williamson and Manach, 2005).
Figure 2.
Parallels between flavonoid metabolism, transport, and action in plants and humans.
Flavonoids in herbal drugs must cross the blood-brain barrier to be bioavailable and effective in the central nervous system. The flavonoid must interact with specific brain cells or must be able to flow through the intercellular space in order to manifest its desired effects. More is probably known about flavonoid transport in plants than in animals, and it is possible that similar transport mechanisms are involved, supporting the application of comparative cross-kingdom genomics approaches. Perhaps one of the greatest challenges in determining the efficacy of herbal treatments is to prove the molecular mechanism(s) through which the compounds exert beneficial activity. Flavonoids such as quercetin (Fig. 1B) bind to mammalian actin (Böhl et al., 2007), but it is not clear whether this is related to bioactivity. Knowledge of the potential binding sites of bioactive flavonoids in the brain, for example, might stimulate further studies to determine whether such plant-specialized metabolites have similar target-binding sites within the plant.
Many tend to think of breakthroughs in medicine as the development of a new drug or a high-tech surgical procedure. They often have a hard time believing that the simple choices we make in our lives, like what we eat, can be as powerful as drugs and surgery. Recent studies have suggested that dietary choices may have an effect even at the genome level (Ornish et al., 2008). Specifically, genes associated with heart disease and inflammation can be down-regulated, whereas protective genes can be up-regulated, as a result of intensive nutritional and lifestyle modifications. Emerging large-scale, whole-genome association studies will provide unprecedented understanding of the genetic basis of health and chronic disease. This rapidly evolving genomic science, however, often fails to consider interactions with environmental exposures, for example with “plant derivatives.” Rigorous nutritional science executed in conjunction with genetic evaluations may provide a “personalized medicine” approach, which would allow for the provision of geriatric phytomedicine according to a given individual’s specific needs. Flavonoids are among the phytochemicals most consistently associated with chemoprevention of age-related diseases, and genetically modified high-anthocyanin tomatoes (Solanum lycopersicum) have recently been shown to prolong the life of cancer-susceptible mice, proof of concept for “designer foods” (Butelli et al., 2008). We encourage plant scientists with interests in specialized metabolism to work closely with biomedical researchers and clinicians to develop the science base that will make personalized medicine a reality.
References
- Böhl M, Tietze S, Sokoll A, Madathil S, Pfennig F, Apostolakis J, Fahmy K, Gutzeit HO. (2007) Flavonoids affect actin functions in cytoplasm and nucleus. Biophys J 93: 2767–2780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Schijlen EGWM, Hall RD, Bovy AG, Luo J, et al. (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26: 1301–1308 [DOI] [PubMed] [Google Scholar]
- Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier-Level A, Verries C, Souquet JM, Mazauric JP, Klein M, Cheynier V, et al. (2009) Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150: 402–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E, Davies K. (2008) Trafficking and sequestration of anthocyanins. Nat Prod Commun 3: 1251–1258 [Google Scholar]
- Hebert LE, Beckett LA, Scherr PA, Evans DA. (2001) Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis Assoc Disord 15: 169–173 [DOI] [PubMed] [Google Scholar]
- Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S. (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14: 125–132 [DOI] [PubMed] [Google Scholar]
- Ho L, Chen LH, Wang J, Zhao W, Talcott ST, Ono K, Teplow D, Humala N, Cheng A, Percival SS, et al. (2009) Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J Alzheimers Dis 16: 59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen RA. (1995) Cosuppression, flower color patterns, and metastable gene expression states. Science 268: 686–691 [DOI] [PubMed] [Google Scholar]
- Katsuyama Y, Funa N, Miyahisa I, Horinouchi S. (2007) Synthesis of unnatural flavonoids and stilbenes by exploiting the plant biosynthetic pathway in Escherichia coli. Chem Biol 14: 613–621 [DOI] [PubMed] [Google Scholar]
- Khan ZR, Pickett JA, Wadhams LJ, Hassanali A, Midega CAO. (2006) Combined control of Striga and stemborers in maize-Desmodium spp. intercrops. Crop Prot 25: 989–995 [Google Scholar]
- Kwaaitaal M, Keinath NF, Pajonk S, Biskup C, Panstruga R. (2010) Combined bimolecular fluorescence complementation and Forster resonance energy transfer reveals ternary SNARE complex formation in living plant cells. Plant Physiol 152: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenbrock U, Barz W. (1991) Elicitor-induced formation of pterocarpan phytoalexins in chickpea (Cicer arietinum L.) cell suspension cultures from constitutive isoflavone conjugates upon inhibition of phenylalanine ammonia-lyase. Z Naturforsch 46c: 43–50 [Google Scholar]
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr (Suppl) 81: 230S–242S [DOI] [PubMed] [Google Scholar]
- Ornish D, Magbanua MJ, Weidner G, Weinberg V, Kemp C, Green C, Mattie MD, Marlin R, Simko J, Shinohara K, et al. (2008) Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci USA 105: 8369–8374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Y, Peel GJ, Sharma SB, Tang Y, Dixon RA. (2008) A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc Natl Acad Sci USA 105: 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasinetti GM, Zhao Z, Qin W, Ho L, Shrishailam Y, Macgrogan D, Ressmann W, Humala N, Liu X, Romero C, et al. (2007) Caloric intake and Alzheimer’s disease: experimental approaches and therapeutic implications. Interdiscip Top Gerontol 35: 159–175 [DOI] [PubMed] [Google Scholar]
- Peel GJ, Modolo LV, Pang Y, Dixon RA. (2009) The LAP1 MYB transcription factor orchestrates anthocyanidin biosynthesis and glycosylation in Medicago. Plant J 59: 136–149 [DOI] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12: 556–563 [DOI] [PubMed] [Google Scholar]
- Pourcel L, Routabout JM, Cheynier V, Lepiniec L, Debeaujon I. (2006) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12: 29–36 [DOI] [PubMed] [Google Scholar]
- Qato DM, Alexander GC, Conti RM, Johnson M, Schumm P, Lindau ST. (2008) Use of prescription and over-the-counter medications and dietary supplements among older adults in the United States. JAMA 300: 2867–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Mito K, Ohara K, Yamamoto H, Yazaki K. (2008) Cloning and characterization of naringenin 8-prenyltransferase, a flavonoid-specific prenyltransferase of Sophora flavescens. Plant Physiol 146: 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JP. (2009) The impact of flavonoids on memory: physiological and molecular consideration. Chem Soc Rev 38: 1152–1161 [DOI] [PubMed] [Google Scholar]
- Subramanian S, Stacey G, Yu O. (2007) Distinct, crucial roles for flavonoids during legume nodulation. Trends Plant Sci 12: 282–285 [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao W, Ono K, Rosensweig C, Chen L, Humala N, Teplow DB, Pasinetti GM. (2008) Grape-derived polyphenolics prevent A-beta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J Neurosci 28: 6388–6392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson G, Manach C. (2005) Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am J Clin Nutr (Suppl) 81: 243S–255S [DOI] [PubMed] [Google Scholar]
- Zhao J, Dixon RA. (2010) The “ins” and “outs” of flavonoid transport. Trends Plant Sci 15: 72–80 [DOI] [PubMed] [Google Scholar]
- Zhao J, Pang Y, Dixon RA. (2010) The mysteries of proanthocyanidin transport and polymerization. Plant Physiol 153: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]