Photosynthesis is the only significant solar energy storage process on Earth and is the source of all of our food and most of our energy resources. An understanding of the origin and evolution of photosynthesis is therefore of substantial interest, as it may help to explain inefficiencies in the process and point the way to attempts to improve various aspects for agricultural and energy applications.
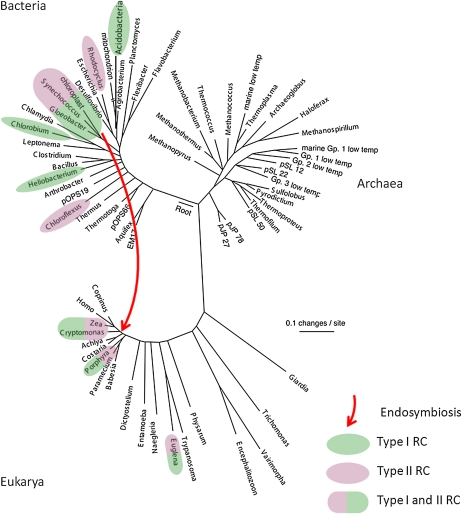
A wealth of evidence indicates that photosynthesis is an ancient process that originated not long after the origin of life and has evolved via a complex path to produce the distribution of types of photosynthetic organisms and metabolisms that are found today (Blankenship, 2002; Björn and Govindjee, 2009). Figure 1 shows an evolutionary tree of life based on small-subunit rRNA analysis. Of the three domains of life, Bacteria, Archaea, and Eukarya, chlorophyll-based photosynthesis has only been found in the bacterial and eukaryotic domains. The ability to do photosynthesis is widely distributed throughout the bacterial domain in six different phyla, with no apparent pattern of evolution. Photosynthetic phyla include the cyanobacteria, proteobacteria (purple bacteria), green sulfur bacteria (GSB), firmicutes (heliobacteria), filamentous anoxygenic phototrophs (FAPs, also often called the green nonsulfur bacteria), and acidobacteria (Raymond, 2008). In some cases (cyanobacteria and GSB), essentially all members of the phylum are phototrop2hic, while in the others, in particular the proteobacteria, the vast majority of species are not phototrophic.
Figure 1.
Small subunit rRNA evolutionary tree of life. Taxa that contain photosynthetic representatives are highlighted in color, with green highlighting indicating a type I RC, while purple highlighting indicates a type II RC. The red arrow indicates the endosymbiotic event that formed eukaryotic chloroplasts. Tree adapted from Pace (1997).
Overwhelming evidence indicates that eukaryotic photosynthesis originated from endosymbiosis of cyanobacterial-like organisms, which ultimately became chloroplasts (Margulis, 1992). So the evolutionary origin of photosynthesis is to be found in the bacterial domain. Significant evidence indicates that the current distribution of photosynthesis in bacteria is the result of substantial amounts of horizontal gene transfer, which has shuffled the genetic information that codes for various parts of the photosynthetic apparatus, so that no one simple branching diagram can accurately represent the evolution of photosynthesis (Raymond et al., 2002). However, there are some patterns that can be discerned from detailed analysis of the various parts of the photosynthetic apparatus, so some conclusions can be drawn. In addition, the recent explosive growth of available genomic data on all types of photosynthetic organisms promises to permit substantially more progress in unraveling this complex evolutionary process.
While we often talk about the evolution of photosynthesis as if it were a concerted process, it is more useful to consider the evolution of various photosynthetic subsystems, which have clearly had distinct evolutionary trajectories. In this brief review we will discuss the evolution of photosynthetic pigments, reaction centers (RCs), light-harvesting (LH) antenna systems, electron transport pathways, and carbon fixation pathways. These subsystems clearly interact with each other, for example both the RCs and antenna systems utilize pigments, and the electron transport chains interact with both the RCs and the carbon fixation pathways. However, to a significant degree they can be considered as modules that can be analyzed individually.
ORIGINS OF PHOTOSYNTHESIS
We know very little about the earliest origins of photosynthesis. There have been numerous suggestions as to where and how the process originated, but there is no direct evidence to support any of the possible origins (Olson and Blankenship, 2004). There is suggestive evidence that photosynthetic organisms were present approximately 3.2 to 3.5 billion years ago, in the form of stromatolites, layered structures similar to forms that are produced by some modern cyanobacteria, as well as numerous microfossils that have been interpreted as arising from phototrophs (Des Marais, 2000). In all these cases, phototrophs are not certain to have been the source of the fossils, but are inferred from the morphology or geological context. There is also isotopic evidence for autotrophic carbon fixation at 3.7 to 3.8 billion years ago, although there is nothing that indicates that these organisms were photosynthetic. All of these claims for early photosynthesis are highly controversial and have engendered a great deal of spirited discussion in the literature (Buick, 2008). Evidence for the timing of the origin of oxygenic photosynthesis and the rise of oxygen in the atmosphere is discussed below. The accumulated evidence suggests that photosynthesis began early in Earth’s history, but was probably not one of the earliest metabolisms and that the earliest forms of photosynthesis were anoxygenic, with oxygenic forms arising significantly later.
PHOTOSYNTHETIC PIGMENTS
Chlorophylls are essential pigments for all phototrophic organisms. Chlorophylls are themselves the product of a long evolutionary development, and can possibly be used to help understand the evolution of other aspects of photosynthesis. Chlorophyll biosynthesis is a complex pathway with 17 or more steps (Beale, 1999). The early part of the pathway is identical to heme biosynthesis in almost all steps and has clearly been recruited from that older pathway. The later steps include the insertion of magnesium and the elaboration of the ring system and its substituents. The earliest version of the pathway (and that used by most modern anoxygenic photosynthetic organisms) almost certainly was anaerobic, both not requiring and not tolerating the presence of O2. However, all modern oxygenic photosynthetic organisms now require O2 as an oxidant at several steps in the pathway. This has been explained in terms of gene replacement of the genes coding for the enzymes at these steps, with the result that the overall pathway is unchanged but the enzymes at key steps are completely different in different groups of phototrophs (Raymond and Blankenship, 2004).
A key concept in using chlorophyll biosynthesis pathways to infer the evolution of photosynthesis is the Granick hypothesis, which states that the biosynthetic pathway of chlorophyll recapitulates the evolutionary sequence (Granick, 1965). This is an appealing idea and probably at least partly true. However, in some cases, in particular the situation of chlorophyll and bacteriochlorophyll, it has been argued that the strict version of the Granick hypothesis is misleading and other interpretations are more likely (Blankenship, 2002; Blankenship et al., 2007).
All photosynthetic organisms contain carotenoids, which are essential for photoprotection, usually also function as accessory pigments, and in many cases serve as key regulatory molecules. Carotenoids, unlike chlorophylls, are also found in many other types of organisms, so their evolutionary history may reflect many other functions in addition to photosynthesis (Sandman, 2009).
REACTION CENTERS
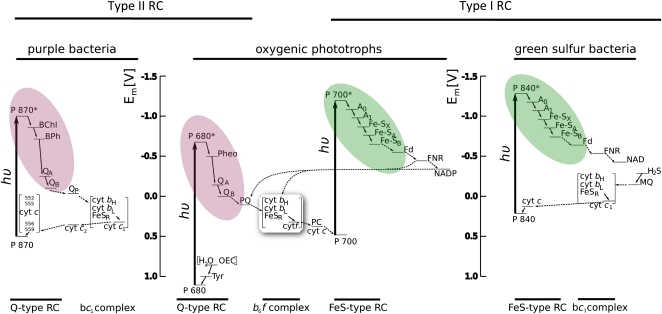
The RC complex is at the heart of photosynthesis; so much attention has been paid to understand the evolution of RCs. A wealth of evidence, including structural, spectroscopic, thermodynamic, and molecular sequence analysis, clearly segregates all known RCs into two types of complexes, called type I and type II (Blankenship, 2002). Anoxygenic phototrophs have just one type, either type I or II, while all oxygenic phototrophs have one of each type. The primary distinguishing feature of the two types of RCs are the early electron acceptor cofactors, which are FeS centers in type I RCs and pheophytin/quinone complexes in type II RCs. The distribution of RC types on the tree of life is shown in Figure 1 and a comparative electron transport diagram that compares the different RCs in different types of organisms is shown in Figure 2, with type I RCs color coded green and type II RCs color coded purple.
Figure 2.
Electron transport diagram indicating the types or RCs and electron transport pathways found in different groups of photosynthetic organisms. The color coding is the same as for Figure 1 and highlights the electron acceptor portion of the RC. Figure courtesy of Martin Hohmann-Marriott.
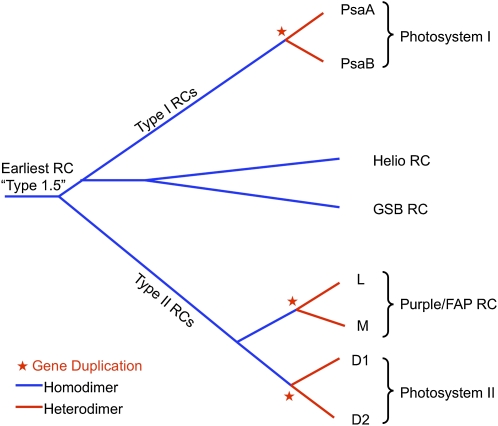
Further analysis strongly suggests that all RCs have evolved from a single common ancestor and have a similar protein and cofactor structure. This is clearly seen when structural overlays of both type I and II RCs are made, showing a remarkably conserved three-dimensional protein and cofactor structure, despite only minimal residual sequence identity (Sadekar et al., 2006). These comparisons have been used to derive structure-based evolutionary trees that do not rely on sequence alignments. Figure 3 shows a schematic evolutionary tree of RCs that is derived from this sort of analysis. It proposes that the earliest RC was intermediate between type I and II (type 1.5) and that multiple gene duplications have given rise to the heterodimeric (two related yet distinct proteins that form the core of the RC) complexes that are found in most modern RCs.
Figure 3.
Schematic evolutionary tree showing the development of the different types of RC complexes in different types of photosynthetic organisms. This tree is based on structural comparisons of RCs by Sadekar et al. (2006). Blue color coding indicates protein homodimer, while red indicates protein heterodimer complexes. Red stars indicate gene duplication events that led to heterodimeric RCs. Helio, Heliobacteria; GSB, green sulfur bacteria; FAP, filamentous anoxygenic phototroph.
A second important issue that relates to RC evolution is the question of how both type I and II RCs came to be in cyanobacteria, while all other photosynthetic prokaryotes have only a single RC. The various proposals that have been made to explain this fact can all be divided into either fusion or selective loss scenarios or variants thereof (Blankenship et al., 2007). In the fusion hypothesis, the two types of RCs develop separately in anoxygenic photosynthetic bacteria and are then brought together by a fusion of two organisms, which subsequently developed the ability to oxidize water. In the selective loss hypothesis, the two types of RCs both evolved in an ancestral organism and then loss of one or the other RC gave rise to the organisms with just one RC, while the ability to oxidize water was added later. Both scenarios have proponents, and it is not yet possible to choose between them.
ELECTRON TRANSPORT CHAINS
The primary photochemistry and several of the early secondary electron transfer reactions take place within the RC complex. However, additional electron transfer processes are necessary before the process of energy storage is complete. These include the cytochrome bc1 and b6f complexes. These complexes oxidize quinols produced by photochemistry in type II RCs or via cyclic processes in type I RCs and pumps protons across the membrane that in turn contribute to the proton motive force that is used to make ATP. All phototrophic organisms have a cytochrome bc1 or b6f complex of generally similar architecture, with the exception of the FAP phylum of anoxygenic phototrophs (Yanyushin et al., 2005). This group contains instead a completely different type of complex that is called alternative complex III. The evolutionary origin of this complex is not yet clear. While the cytochrome bc1 and b6f complexes are similar in many ways, the cytochrome c1 and f subunits are very different and are almost certainly of distinct evolutionary origin (Baniulis et al., 2008).
ANTENNA SYSTEMS
All photosynthetic organisms contain a light-gathering antenna system, which functions to collect excitations and transfer them to the RC where the excited state energy is used to drive photochemistry (Green and Parson, 2003). While the presence of an antenna is universal, the structure of the antenna complexes and even the types of pigments used in them is remarkably varied in different types of photosynthetic organisms. This very strongly suggests that the antenna complexes have been invented multiple times during the course of evolution to adapt organisms to particular photic environments. So while evolutionary relationships are clear among some categories of antennas, such as the LH1 and LH2 complexes of purple bacteria and the LHCI and LHCII complexes of eukaryotic chloroplasts, it is not possible to relate these broad categories of antennas to each other in any meaningful way. This is in contrast to the RCs, where all available evidence clearly points to a single origin that has subsequently undergone a complex evolutionary development.
CARBON FIXATION PATHWAYS
Most phototrophic organisms are capable of photoautotrophic metabolism, in which inorganic substrates such as water, H2S, CO2, or HCO3− are utilized along with light energy to produce organic carbon compounds and oxidized donor species. However, there are some groups of phototrophs that cannot carry out photoautotrophic metabolism and there are at least three entirely separate autotrophic carbon fixation pathways that are found in different types of organisms (Thauer, 2007). By far the dominant carbon fixation pathway is the Calvin-Benson cycle, which is found in all oxygenic photosynthetic organisms, and also in most purple bacteria. The GSB use the reverse tricarboxylic acid cycle, and many of the FAPs use the 3-hydroxypropionate cycle (Zarzycki et al., 2009). The Gram-positive heliobacteria lack any known autotrophic carbon fixation pathway and usually grow photoheterotrophically (Asao and Madigan, 2010). Similarly, the aerobic anoxygenic phototrophs, which are closely related to the purple bacteria, lack any apparent ability to fix inorganic carbon. In the latter case, it seems most likely that the ancestor of this group contained the Calvin-Benson cycle but lost the genes because of their obligate aerobic lifestyle (Swingley et al., 2007).
The carbon fixation machinery is thus similar to the antennas, in that several entirely separate solutions have been adopted by different classes of phototrophic organisms. This would be consistent with the idea that the earliest phototrophs were photoheterotrophic, using light to assimilate organic carbon, instead of being photoautotrophic. The ability to fix inorganic carbon was then added to the metabolism somewhat later during the course of evolution, possibly borrowing carbon fixation pathways that had developed earlier in autotrophic nonphotosynthetic organisms.
TRANSITION TO OXYGENIC PHOTOSYNTHESIS
Perhaps the most widely discussed yet poorly understood event in the evolution of photosynthesis is the invention of the ability to use water as an electron donor, producing O2 as a waste product and giving rise to what is now called oxygenic photosynthesis. The production of O2 and its subsequent accumulation in the atmosphere forever changed the Earth and permitted the development of advanced life that utilized the O2 during aerobic respiration. Several lines of geochemical evidence indicate that free O2 began to accumulate in the atmosphere by 2.4 billion years ago, although the ability to do oxygenic photosynthesis probably began somewhat earlier (Buick, 2008). In order for O2 to accumulate, it is necessary that both the biological machinery needed to produce it has evolved, but also the reduced carbon produced must be buried by geological processes, which are controlled by geological processes such as plate tectonics and the buildup of continents. So the buildup of O2 in the atmosphere represents a coming together of the biology that gives rise to O2 production and the geology that permits O2 to accumulate.
Oxygen is produced by PSII in the oxygen evolving center, which contains a tetranuclear manganese complex. The evolutionary origin of the oxygen evolving center has long been a mystery. Several sources have been suggested, but so far no convincing evidence has been found to resolve this issue (Raymond and Blankenship, 2008). The possibility that functional intermediate stages existed that connect the anoxygenic type II RCs to PSII seems likely (Blankenship and Hartman, 1998).
CONCLUSION
The process of photosynthesis originated early in Earth’s history, and has evolved to its current mechanistic diversity and phylogenetic distribution by a complex, nonlinear process. Current evidence suggests that the earliest photosynthetic organisms were anoxygenic, that all photosynthetic RCs have been derived from a single source, and that antenna systems and carbon fixation pathways have been invented multiple times.
References
- Asao M, Madigan MT. (2010) Taxonomy, phylogeny, and ecology of the heliobacteria. Photosynth Res 104: 103–111 [DOI] [PubMed] [Google Scholar]
- Baniulis D, Yamashita E, Zhang H, Hasan SS, Cramer WA. (2008) Structure-function of the cytochrome b6f complex. Photochem Photobiol 84: 1349–1358 [DOI] [PubMed] [Google Scholar]
- Beale S. (1999) Enzymes of chlorophyll biosynthesis. Photosynth Res 60: 43–73 [Google Scholar]
- Björn LO, Govindjee (2009) The evolution of photosynthesis and chloroplasts. Curr Sci 96: 1466–1474 [Google Scholar]
- Blankenship RE. (2002) Molecular Mechanisms of Photosynthesis. Blackwell Science, Oxford [Google Scholar]
- Blankenship RE, Hartman H. (1998) The origin and evolution of oxygenic photosynthesis. Trends Biochem Sci 23: 94–97 [DOI] [PubMed] [Google Scholar]
- Blankenship RE, Sadekar S, Raymond J. (2007) The evolutionary transition from anoxygenic to oxygenic photosynthesis. Falkowski P, Knoll AN, , Evolution of Aquatic Photoautotrophs. Academic Press, New York, pp 21–35 [Google Scholar]
- Buick R. (2008) When did oxygenic photosynthesis evolve? Philos Trans R Soc Lond B Biol Sci 363: 2731–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Marais DJ. (2000) Evolution: When did photosynthesis emerge on Earth? Science 289: 1703–1705 [PubMed] [Google Scholar]
- Granick S. (1965) Evolution of heme and chlorophyll. Bryson G, Vogel HJ, , Evolving Genes and Proteins. Academic Press, New York, pp 67–88 [DOI] [PubMed] [Google Scholar]
- Green BR, Parson WW. (2003) Light-Harvesting Antennas. Kluwer, Dordrecht, The Netherlands [Google Scholar]
- Margulis L. (1992) Symbiosis in Cell Evolution. WH Freeman, San Francisco [Google Scholar]
- Olson JM, Blankenship RE. (2004) Thinking about the evolution of photosynthesis. Photosynth Res 80: 373–386 [DOI] [PubMed] [Google Scholar]
- Pace NR. (1997) A molecular view of microbial diversity and the biosphere. Science 276: 734–740 [DOI] [PubMed] [Google Scholar]
- Raymond J. (2008) Coloring in the tree of life. Trends Microbiol 16: 41–43 [DOI] [PubMed] [Google Scholar]
- Raymond J, Blankenship RE. (2004) Biosynthetic pathways, gene replacement and the antiquity of life. Geobiology 2: 199–203 [Google Scholar]
- Raymond J, Blankenship RE. (2008) The origin of the oxygen-evolving complex. Coord Chem Rev 252: 377–383 [Google Scholar]
- Raymond J, Zhaxybayeva O, Gerdes S, Gogarten JP, Blankenship RE. (2002) Whole genome analysis of photosynthetic prokaryotes. Science 298: 1616–1620 [DOI] [PubMed] [Google Scholar]
- Sadekar S, Raymond J, Blankenship RE. (2006) Conservation of distantly related membrane proteins: photosynthetic reaction centers share a common structural core. Mol Biol Evol 23: 2001–2007 [DOI] [PubMed] [Google Scholar]
- Sandman G. (2009) Evolution of carotenoid desaturation: the complication of a simple pathway. Arch Biochem Biophys 483: 169–174 [DOI] [PubMed] [Google Scholar]
- Swingley WD, Gholba S, Mastrian SD, Matthies HJ, Hao J, Ramos H, Acharya CR, Conrad AL, Taylor HL, Dejesa LC, et al. (2007) The complete genome sequence of Roseobacter denitrificans reveals a mixotrophic rather than photosynthetic metabolism. J Bacteriol 189: 683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer RK. (2007) A fifth pathway of carbon metabolism. Science 318: 1732–1733 [DOI] [PubMed] [Google Scholar]
- Yanyushin MF, del Rosario M, Brune DC, Blankenship RE. (2005) A new class of bacterial membrane oxidoreductases. Biochemistry 44: 10037–10045 [DOI] [PubMed] [Google Scholar]
- Zarzycki J, Brecht V, Muller M, Fuchs G. (2009) Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci USA 106: 21317–21322 [DOI] [PMC free article] [PubMed] [Google Scholar]