Abstract
Chromera velia is a newly cultured photosynthetic marine alveolate. This microalga has a high iron requirement for respiration and photosynthesis, although its natural environment contains less than 1 nm of this metal. We found that this organism uses a novel mechanism of iron uptake, differing from the classic reductive and siderophore-mediated iron uptake systems characterized in the model yeast Saccharomyces cerevisiae and present in most yeasts and terrestrial plants. C. velia has no trans-plasma membrane electron transfer system, and thus cannot reduce extracellular ferric chelates. It is also unable to use hydroxamate siderophores as iron sources. Iron uptake from ferric citrate by C. velia is not inhibited by a ferrous chelator, but the rate of uptake is strongly decreased by increasing the ferric ligand (citrate) concentration. The cell wall contains a large number of iron binding sites, allowing the cells to concentrate iron in the vicinity of the transport sites. We describe a model of iron uptake in which aqueous ferric ions are first concentrated in the cell wall before being taken up by the cells without prior reduction. We discuss our results in relation to the strategies used by the phytoplankton to take up iron in the oceans.
Chromera velia is a newly cultured marine alveolate containing a photosynthetic plastid phylogenetically related to vestigial plastids in apicomplexan (Moore et al., 2008). It represents the closest free-living photosynthetic relative to apicomplexan parasites, thus providing a powerful model to study the evolution of eukaryotic adaptability (Moore et al., 2008). To gain further insight into the biology of this organism, the genome of which remains unsequenced, we investigated its iron metabolism and its mechanisms of iron uptake. We compared the data obtained with other phytoplanktonic organisms sharing the same ecological niche, and with a terrestrial unicellular eukaryote, the yeast Saccharomyces cerevisiae. S. cerevisiae is phylogenetically distant from C. velia, but its mechanisms of iron uptake are well characterized, and thus constitutes a useful model in these studies.
Iron uptake by terrestrial microorganisms and plants is mostly based on the use of two main strategies, both of which have been previously characterized in S. cerevisiae. The first strategy is the reductive mechanism of uptake. Extracellular ferric complexes are first dissociated by reduction, via trans-plasma membrane electron transfer catalyzed by specialized flavohemoproteins (Fre). Free iron is then imported by a high-affinity permease system (Ftr1) coupled to a copper-dependent oxidase (Fet3), allowing iron to be channeled through the plasma membrane. In the second strategy, the siderophore-mediated mechanism, siderophores excreted by the cells or produced by other bacterial or fungal species are taken up without prior dissociation, via specific, copper-independent high-affinity receptors. Iron is then dissociated from the siderophores inside the cells, probably by reduction (for review, see Kosman, 2003; Philpott, 2006). Chlamydomonas reinhardtii is a photosynthetic eukaryotic model organism for the study of iron homeostasis, which shares with yeast the strategy 1 of iron uptake (copper-dependent reductive iron uptake; Merchant et al., 2006).
Much less is known about the strategies used by marine phytoplankton to acquire iron. Some data suggest that these two strategies are used by some marine microalgae (Soria-Dengg and Horstmann, 1995; Allen et al., 2008). However, for most marine unicellular eukaryotes the mechanisms of iron assimilation are completely unknown. The strategies used by these organisms to acquire iron must have evolved to adapt to the very particular conditions that prevail in their surrounding natural environment: The transition metal composition of the ocean differs greatly from that of terrestrial environments (Butler, 1998). In particular, iron levels in surface seawater are extremely low (0.02–1 nm; Turner et al., 2001). Therefore a strategy of iron uptake operating efficiently in a terrestrial environment that contains iron at a micromolar level may be inefficient in a marine environment. No classic iron uptake system with an affinity constant in the nanomolar range has ever been found. Additionally, the marine environment imposes physical limits on the classic strategies of uptake, including the high diffusion rate of the species of interest (siderophores or reduced iron; Völker and Wolf-Gladrow, 1999). It is well known that the low levels of iron limits primary production of phytoplankton and carbon fluxes across vast regions of the world’s oceans (Coale et al., 2004; Pollard et al., 2009). It is thus of particular interest to elucidate the molecular mechanisms underlying acquisition of iron by marine phytoplankton and to determine which iron sources are preferentially assimilated with regards to the yield of carbon fixation.
In this study, we investigated the mechanisms of iron uptake by C. velia, and found that this organism uses a nonreductive uptake system of ferric ions, which are first concentrated in the cell wall. Our findings provide a better understanding of the biology of this organism, and highlights the need for further study on the mechanisms of iron acquisition in marine phytoplankton.
RESULTS
Iron Requirement
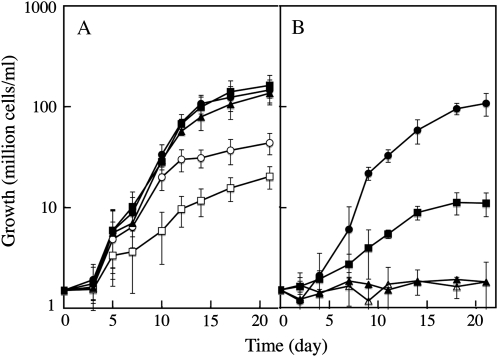
Iron is generally present at extremely low concentrations in ocean surface water (Turner et al., 2001). The typical iron concentration in seawater where C. velia was isolated (South Pacific Ocean, 33°S, 151°E; Moore et al., 2008) is estimated between 0.1 and 0.8 nm (de Baar and de Jong, 2001). We determined the effect of iron on the growth of C. velia by adding various amounts of ferric citrate to the medium, and found that iron strongly enhanced cell growth at concentrations of up to 0.01 μm (Fig. 1A). Concentration of iron greater than 0.01 μm did not significantly increase the growth rate in exponential growth phase, but increased the maximum cell yield (Fig. 1A). Iron supplied in the form of the hydroxamate siderophores ferrioxamine B or ferrichrome did not sustain cell growth (Fig. 1B). Cells grown in the presence of excess iron (1 μm ferric citrate) had surprisingly high total iron content, reaching 30 × 106 atoms/cell. This value is similar to values previously found in organisms with high iron requirements and high storage capacity such as yeast cells (Seguin et al., 2009). This suggests that C. velia is able to store intracellular iron in special proteins/organelles, as do pennate diatoms in ferritin (Marchetti et al., 2009). The distribution of soluble iron-containing proteins analyzed by gel filtration gave no evidence for the presence of ferritin (the highest molecular weight peak associated with iron was 200 kD; Supplemental Fig. S1). The way C. velia stores iron at the intracellular level thus remains to be elucidated.
Figure 1.
Iron-dependent growth of C. velia. Effect of the amount (A) and of the form (B) of iron on growth. C. velia cells precultured without iron (1.5 106 cells/mL) were added to iron-free Mf medium. Iron was then added to the medium, at various concentrations and in various forms. A, Iron added as Fe(III)-citrate (1:20) at 1 nm (white squares), 10 nm (white circles), 100 nm (black triangles), 1 μm (black circles), or 500 μm (black squares). B, No iron added (black squares), 10 nm Fe(III)-citrate (1:20; black circles), 10 nm ferrioxamine B, or 10 nm ferrichrome (black and white triangles, respectively). Means ± sd from six experiments are given.
We examined the effect of iron supply on cell morphology and on various biochemical parameters. Reduction in cell volume in response to iron limitation has previously been observed in several microalgae (Greene et al., 1992; Allen et al., 2008; Marchetti and Cassar, 2009). The change in cell size in response to iron availability may be physiologically relevant, through its potential effects on reductive iron uptake and siderophore-mediated iron uptake: Smaller cells can reach a higher density using a fixed amount of nutrient, leading to improved uptake efficiency (Völker and Wolf-Gladrow, 1999). To determine whether cells grown under different iron concentrations displayed distinct morphological phenotypes, we used light microscopy and calculated the volume of the cells. We did not detect any significant difference in the morphology of cells grown in the presence of 0, 0.01, and 1 μm iron added to the medium. Similarly, cells grown with or without added iron, and evaluated by transmission electron microscopy, did not show any major differences in ultrastructure (Supplemental Table S1; Supplemental Fig. S2). Despite iron limitation having no apparent effect on cell morphology, low iron supply resulted in the limitation of cellular energy, as shown by a decreased rate of respiration and photosynthesis and by a reduced amount of cellular chlorophyll a in iron-deprived conditions (Supplemental Fig. S3). At high iron concentration (1 μm), both respiration and photosynthesis were strongly boosted (Supplemental Fig. S3). Thus, C. velia is able to grow on low iron medium but is able to make use of higher iron concentrations for metabolism when available. This organism must therefore possess a very efficient system to take up iron from its surrounding environment and concentrate it into the cell.
Iron Uptake Assays: Speciation of Iron
In most experiments, we used ferric citrate (1:20) added to concentrated cell suspensions (100 106 cells/mL), and determined iron uptake on short periods of time. The speciation of iron in these conditions was difficult to evaluate. Due to the high concentration of Ca2+ in seawater medium, iron added as ferric citrate (1 μm) is theoretically expected to distribute as follows when the thermodynamic equilibrium is reached: 4% iron complexed with citrate, 96% iron precipitated, and free iron concentration [Fe3+] of 10−15.4 m (estimations based on the use of the GEOCHEM-EZ software; Shaff et al., 2010). This is however not what we observed experimentally: When we added 1 μm ferric citrate (1:20) to cell-free seawater medium, we determined (by ultracentrifugation at 300,000g for 1 h) that 90% of the iron was still soluble after 30 min (data not shown). This discrepancy might result from the fact that thermodynamic equilibrium cannot be reached within this short period of time (30 min). Also, the chemistry of ferric citrate speciation in aqueous solution is complicated and not fully understood yet, and the estimated affinity constants for the monoiron dicitrate complex ranges from (log) 19.1 to 38.7 in the literature (Silva et al., 2009). As we worked with concentrated cell suspensions, predictions on iron speciation should also account for the charges and potential ligands of metals introduced in the system by the cells themselves. Regarding iron speciation, one crucial parameter is the concentration of reduced thiols in the medium. We therefore measured exofacial thiols associated with whole C. velia cells. Our results show that for a suspension of 100 106 cells/mL, the concentration of surface thiol groups is 12 ± 3 μm (mean ± sd, n = 3). A new simulation with GEOCHEM-EZ, taking account for the thiols present in the medium gave the following values: 100% of iron (added as 1 μm ferric citrate) is expected to be soluble (complexed by thiols and citrate), with a free iron concentration [Fe3+] of 10−17.7 m.
Iron Uptake Is an Inducible, Energy-Dependent Two-Step Mechanism
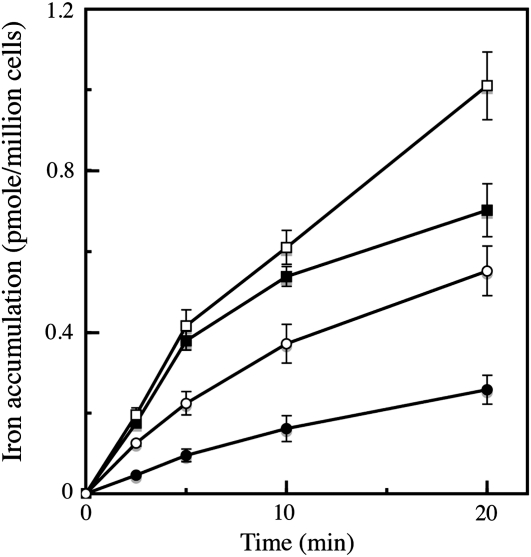
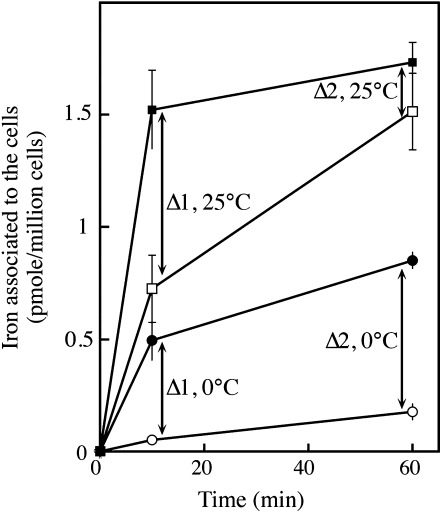
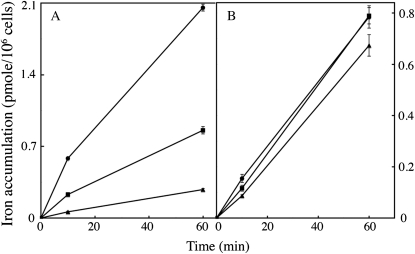
We studied the mechanisms of iron uptake by C. velia grown in iron-limited (0.01 μm iron in the growth medium) and iron-rich (1 μm iron) media, using different ferric and ferrous (55Fe) chelates. For these experiments, we used the yeast S. cerevisiae as a comparative model. As shown in Figure 2, iron was taken up efficiently by C. velia cells using ferric citrate, with a higher level of uptake in iron-deficient cells than in iron-replete cells. However, the kinetics of iron uptake in C. velia differed from those observed for yeasts. Unlike in yeast, the initial rate (the first 5–10 min) of iron uptake in C. velia was unaffected by iron starvation during growth (Fig. 2). Enhanced iron uptake was apparent in iron-deficient cells during a subsequent phase of uptake, suggesting that a step of iron binding occurred before uptake. Thus, the iron uptake process itself, but not the initial binding of iron seems to be regulated by the abundance of iron in the environment. Evidence for a two-step mechanism of uptake was further demonstrated in the following experiment. C. velia cells were incubated with 0.5 μm 55Fe(III)-citrate for various periods of time at 0°C or 25°C, and then washed with Mf medium (see “Materials and Methods”) containing 1 mm citrate buffer (pH 6.5) to remove any nonspecifically bound iron from the cell surface. The cells were then washed with the same Mf/citrate buffer containing strong ferric and ferrous iron chelators (EDTA, bathophenanthroline disulfonic acid [BPS], and desferrichrome, each at 10 mm), to remove all the chelatable iron from the cell surface. The amount of iron associated with the cells (intracellular iron, Fig. 3) and that removed by the chelators (extracellular iron, Fig. 3) were then measured. Extracellular and intracellular levels of bound iron were temperature dependent and increased with time (Fig. 3). However, at 25°C and not at 0°C, the difference between these two pools of iron became smaller with time (Δ1 and Δ2 in Fig. 3), suggesting that the iron specifically bound to the cell surface was progressively taken up by the cells to become nonchelatable (Fig. 3). An alternative explanation of these data would imply that the strength of iron binding to the surface increased with time, as a consequence of iron precipitation in the cell wall, or of iron binding to increasingly stronger ligands. This hypothesis is however improbable, since further treatment of the cells (after washing them with the mix of strong chelators) with a strong reducing agent (dithionite 10 μm) did not result in subsequent iron release (data not shown). It is thus reasonable to assume that the iron bound to the cell wall was progressively internalized, as previously shown by pulse-chase experiments in the coccolithophore Pleurochrysis carterae (Hudson and Morel, 1990). It is worth noting that the dependence on temperature of iron binding to the cell wall was greater than expected from a simple physical process (3-fold more iron bound to the cell wall at 25°C than at 0°C; Fig. 3). This observation suggests that a biological or metabolically driven process is at play not only for iron uptake by the cells, but also for iron binding to the cell surface. As expected from a mechanism involving several components/steps, this iron uptake by C. velia did not display simple Michaelis-Menten kinetics. Plotting initial rates of uptake as a function of iron concentrations resulted in a sigmoid curve indicative of a cooperative process occurring in a two-step reaction (Supplemental Fig. S4). The same kinetics were observed for iron uptake, whether iron was added in the form of Fe(III) (citrate) or Fe(II) (ascorbate; Supplemental Fig. S4).
Figure 2.
Initial rates of iron accumulation by C. velia and S. cerevisiae. C. velia was grown in iron-rich (1 μm; black squares) or iron-limited (10 nm; white squares) Mf medium. S. cerevisiae was grown in iron-rich (1 μm; black circles) or in iron-deficient (white circles) medium. Cells were washed once with iron-free Mf medium containing 1 mm EDTA and twice with iron-free Mf medium (C. velia) or 50 mm citrate buffer (S. cerevisiae), and resuspended in the same final media at 100 × 106 cells/mL. For yeast, 2% Glc was added to the citrate buffer. Cells were incubated in the light at 25°C or 30°C, respectively, in the presence of 1 μm 55Fe(III)-citrate (1:20). Aliquots were taken at intervals and washed three times with Mf medium containing 1 mm EDTA before counting. Means ± sd from six experiments are given.
Figure 3.
Iron binding to C. velia cells; temperature and time dependence. C. velia was grown in iron-free Mf medium, harvested, washed with iron-free Mf medium, and resuspended in the same medium at 0°C (circles) or 25°C (squares) in the presence of 0.5 μm 55Fe(III)-citrate (1:20). At the times indicated, aliquots were taken and washed with Mf medium containing 1 mm citrate buffer. The amount of extracellular (black symbols) and intracellular (white symbols) iron specifically bound to the cells was determined by washing the cells with Mf medium containing EDTA, BPS, and desferrichrome (10 mm each). The difference between extracellular iron (washable by strong chelators) and intracellular iron (nonwashable) decreased with time only at 25°C (represented by Δ values on the figure). Means ± sd from three experiments are given.
Iron Uptake Is Nonreductive and Not Mediated by Siderophores
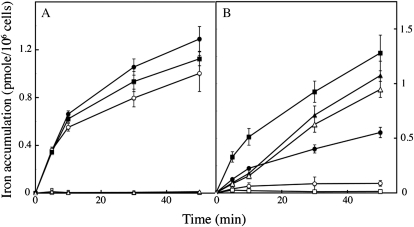
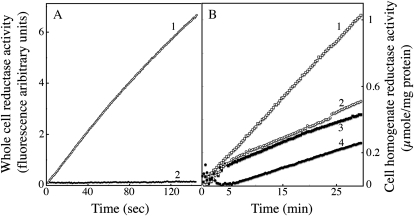
The two main strategies used by plants, bacteria, and fungi to take up iron involve either the use of siderophores, or the reduction of naturally occurring Fe(III) complexes followed by uptake of the reduced iron. Ferrous iron chelators like ferrozine or BPS fully inhibit reductive iron uptake, by trapping the iron after its reduction by the surface reductases, but do not affect siderophore-mediated (nonreductive) iron uptake (Lesuisse and Labbe, 1989). Iron uptake by C. velia was efficient when Fe(III) (ferric citrate) or Fe(II) (ferrous ascorbate) was used as the iron source, but only the uptake of Fe(II) was blocked by the addition of BPS (Fig. 4A). Cells did not take up iron in the form of ferrichrome (Fig. 4A). By contrast, the reductive uptake of Fe(III) by yeast cells was inhibited by BPS, whereas nonreductive uptake of ferrichrome—due to the presence of a specific ferrichrome transporter in S. cerevisiae (Heymann et al., 2000)—was not (Fig. 4B). Another strong chelator of ferrous iron, ferrozine, gave similar results (data not shown). We attribute the small inhibitory effect of these chelators on ferric iron uptake by C. velia cells to photoreduction of iron, which under our experimental conditions could not completely be avoided. The observation that ferrous uptake, but not ferric uptake was blocked by BPS (or ferrozine) in C. velia implies that this organism is able to take up ferric iron without any prior reduction step. We investigated this further by measuring the ferrireductase activity associated with whole cells of C. velia grown in iron-deficient or in iron-rich conditions. Our results show that, unlike yeast, C. velia had no detectable ferrireductase activity associated with intact cells whatever their growth conditions (Supplemental Table S2). We previously showed that the trans-plasma membrane electron transport involved in the reduction of extracellular ferric complexes by yeast cells could be measured with a highly sensitive fluorometric assay using resazurin as the electron acceptor (Lesuisse et al., 1996). This assay was negative for C. velia cells (Fig. 5), demonstrating that these cells cannot transfer intracellular electrons to an extracellular acceptor. Although C. velia lacks the trans-plasma membrane electron transfer system that is required for reductive uptake of iron, we found that this organism does have intracellular reductase(s) able to reduce iron with NAD(P)H as the electron donor. Indeed, whole cell extracts of C. velia showed NADH- and NADPH-dependent reduction of ferric citrate (Fig. 5). The level of NADPH-dependent reductase activity was higher in cells grown in iron-deficient conditions than in cells grown in iron-rich medium (Fig. 5). Thus, after nonreductive uptake of ferric iron by C. velia cells, intracellular reductase(s) may be involved in intracellular iron assimilation by this organism.
Figure 4.
Nonreductive iron uptake in C. velia compared to reductive and siderophore-mediated iron uptake in S. cerevisiae. C. velia (A) and S. cerevisiae (B) cells were grown in iron-deficient medium and treated as described in Figure 2. Iron (1 μm) was added to the cell suspensions, in the form of 55Fe(III)-citrate (1:20; circles), 55Fe(II)-ascorbate (1:10; squares), or 55Fe-ferrichrome (triangles), with (white symbols) or without (black symbols) addition of the ferrous chelator BPS (100 μm). Aliquots were taken at intervals and washed three times with Mf medium containing EDTA, BPS, and desferrichrome (10 mm each) before counting. Means ± sd from three experiments are shown.
Figure 5.
Whole cell and intracellular reductase activity. C. velia and S. cerevisiae cells were grown in iron-deficient medium and treated as described for Figure 2. A, Trans-plasma membrane electron transfer for whole cells was monitored by fluorimetric analysis of the formation of resorufin from resazurin (10 μm). Both C. velia (curve 2) and S. cerevisiae, used as a control (curve 1), were analyzed at a concentration 100 × 106 cells/mL. B, NAD(P)H-dependent ferrireductase activity of soluble whole-cell homogenate of C. velia. Cells were grown in iron-rich (1 μm; black symbols) or iron-limited (10 nm; white symbols) Mf medium, washed, and broken up with glass beads. The soluble fraction (10,000 g supernatant) was tested for ferrireductase activity by measuring absorbency at 535 nm after the addition of 0.5 mm Fe(III)-citrate (1:20), 1 mm BPS, and either 1 mm NAPDH (curves 1 and 3) or NADH (curves 2 and 4) as the electron donor. One representative experiment out of three (A) or two (B) is shown.
Another major difference between the C. velia iron uptake system and the yeast model is related to the properties of the iron permeation step. In yeast (as in other organisms using the reductive pathway of iron uptake), iron reduction at the cell surface is followed by the reoxidation of iron coupled to its transport inside the cell. The yeast iron permease Ftr1 thus transports ferric ions produced by the oxidase Fet3. This mechanism was extensively studied by Kwok et al. (2006a, 2006b), who showed that despite Fe3+ being the substrate of the permease, these ferric ions are not normally available to any Fe3+ chelator present in the extracellular medium, because they are channeled through the Fet3-Ftr1 complex. This would imply that the transfer of Fe3+ from one protein to the other is nondissociative. In this model, an added Fe3+ chelator would only inhibit the uptake by scavenging Fe3+ from the complex, a kinetically rather than thermodynamically controlled process (Kwok et al., 2006a). This mechanism contrasts with a dissociative mechanism, in which the Fe3+ ions bound to the permease equilibrate with the bulk phase, where they can be trapped by a ferric chelator added to the medium (Kwok et al., 2006a). We measured the effect of increasing the concentration of the ferric chelator citrate [increasing the Fe(III):citrate ratio from 1:10 to 1:250] on the uptake of ferric iron by C. velia and yeast cells (Fig. 6). Increasing the concentration of citrate had little effect on the rate of iron uptake by yeast cells (Fig. 6B), but strongly inhibited iron uptake by C. velia cells (Fig. 6A). This suggests that, unlike yeast, which uses a channeling mechanism for uptake, C. velia uses a dissociative mechanism, in which the ferric ions equilibrate with the bulk solution.
Figure 6.
Effect of Fe3+ ligand concentration on iron accumulation. C. velia (A) and S. cerevisiae (B) cells were grown in iron-deficient medium and treated as described for Figure 2. Iron (1 μm) was added to the cell suspensions, in the form of 55Fe(III)-citrate, at different Fe(III):citrate ratios. Circles: 10 μm citrate [Fe(III)-citrate 1:10]; squares: 50 μm citrate [Fe(III)-citrate 1:50]; triangles: 250 μm citrate [Fe(III)-citrate 1:250]. Aliquots were taken at intervals and washed three times with Mf medium containing EDTA, BPS, and desferrichrome (10 mm each) before counting. Means ± sd from three experiments are shown.
Iron Binding at the Cell Surface Strongly Increases the Local Iron Concentration
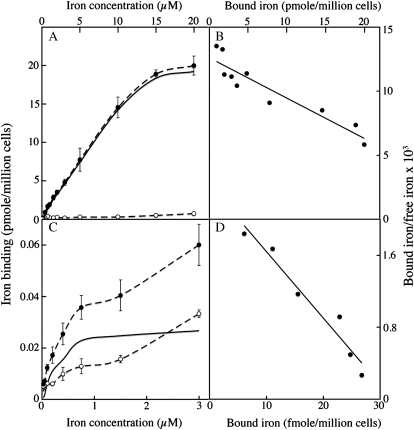
A dissociative mechanism of uptake is likely to be much more efficient if there are many iron-binding sites in the vicinity of the permeation sites, by ensuring a high local concentration of iron available for transport. We evaluated the number of specific iron-binding sites at the surface of C. velia cells and of yeast cells using Scatchard plots (Fig. 7). Cells were incubated at 4°C with increasing concentrations of 55Fe (as the 1:10 citrate complex) with or without a large excess (1,000-fold) of cold ferric citrate to saturate the nonspecific binding sites, and the cells were then washed with water to remove unbound iron before counting. This allowed one to calculate the parameters of specific iron binding at the cell surface (Fig. 7). These analyses showed strong specific binding of Fe3+ at the surface of C. velia cells, with an estimated number of specific binding sites of 45 pmol per million cells and an apparent Kd of 2.5 10−6 m (the number for yeast being 40 fmol per million cells). These binding sites at the cell wall probably serve to increase the local iron concentration near the transport sites, rather than directly participating in the uptake process itself, i.e. the binding of iron to the cell wall is not necessary for subsequent intracellular uptake. We showed this to be the case in experiments using gallium to compete iron for binding. C. velia cells were preincubated on ice for 5 min with Ga3+ (to saturate the binding sites) and then washed (with iron-free Mf medium) before adding iron. The initial iron uptake rates in cells preincubated with Ga3+ were significantly higher than in control cells (preincubated without Ga3+; Supplemental Fig. S5). This finding suggests that the binding of Ga3+ prevented Fe3+ binding to the cell wall, allowing quicker intracellular uptake of newly added iron. Indeed, at the high concentration used in our experiments (1 μm), the added iron could reach the transport sites directly, at a saturating concentration. When Ga3+ was present not only during the preincubation of cells, but also during the incubation of cells with iron, it had an inhibitory effect on iron uptake, suggesting that Ga3+ competed not only for the binding sites at the cell surface but also for the iron transport sites (Supplemental Fig. S5). By contrast, gallium has no effect at all on reductive iron uptake by yeast cells (Lesuisse and Labbe, 1989). The high iron-binding capacity of C. velia cells is probably crucial for efficient iron uptake when the extracellular iron concentration is very low, such as in the ocean water. We showed this in the following experiment. C. velia cells (10 million cells per mL) were incubated for 10 min on ice in Mf medium containing 1 nm 55Fe (as ferric citrate). The iron associated with the cells was measured after centrifugation. We found that 17% ± 3% (mean ± sd, n = 3) of the total iron initially present in solution was associated with the cells. Assuming a mean cell volume of 215 μ3 (see Supplemental Table S1), we estimated the volume of the cell wall to be maximum 8 μ3 per cell (highest estimation) on the basis of electron micrographs (Supplemental Fig. S2). Using this estimation, we calculated a local concentration of iron in the cell wall of about 2 μm, i.e. a concentration of iron 2,000-fold higher than the concentration of iron in the solution. This experimental value fits well with the value that can be calculated from the parameters of the Scatchard plots (Fig. 7). This process of iron concentration is probably crucial for microalgae like C. velia being able to take up iron from an environment where the concentration of iron is generally lower than 1 nm. By itself, concentration of iron in the cell wall does not provide a mechanism for uptake at the plasma membrane, from a thermodynamic point of view. Other mechanism(s) should be at play, which remain to discover, to account for the utility of iron concentration in the cell wall for uptake. Local acidification by proton excretion and complex ligand exchange reactions could be part of this process, by which the iron bound to the cell wall can gain access to the transport sites.
Figure 7.
Iron binding at the cell surface. C. velia (A and B) or S. cerevisiae (C and D) cells were incubated at 100 × 106 cells/mL for 10 min at 4°C with increasing concentrations of 55Fe(III)-citrate (1:10), with (white symbols, nonspecific binding) or without (black symbols, total binding) a 1,000-fold excess of cold iron. Specific binding of iron at the cell surface was calculated (solid lines). A and C, Iron binding as a function of iron concentration. B and D, Scatchard plots. Means ± sd from three experiments are shown.
DISCUSSION
The excretion/utilization of siderophores (nonreductive uptake) and the reduction of naturally occurring ferric complexes at the cell surface (reductive uptake) are common strategies used by terrestrial plants, fungi, and bacteria to take up iron from their environment (Staiger, 2002; Philpott, 2006; Sutak et al., 2008). The yeast S. cerevisiae has become a paradigm to study these mechanisms at the molecular level (Philpott, 2006). We thus used this model organism, in which both strategies of iron uptake have been well characterized, as a standard in comparisons with the characteristics of iron uptake in a newly discovered organism, the photosynthetic alveolate C. velia. Our findings suggest that C. velia displays a nonreductive, siderophore-independent mechanism of iron uptake. Part of this process seems to be based on the specific binding of Fe3+ to the cell wall, leading to a high local concentration of iron near the transport sites, followed by the nonreductive uptake of ferric ions. However, what happens between the step of iron binding to the cell wall and the step of iron transport into the cell remains a black box. Our data suggest that the uptake system is dissociative, whereby ferric ions bound to the transport sites equilibrate with the bulk phase. The efficiency of such a system is likely to require both an iron-enriched local environment (which is made possible by the high number of iron binding sites at the cell surface) and a mechanism to increase the concentration of unbound ferric ions near the transport sites. This mechanism, in which local acidification could play a role, remains to discover. Dissociative mechanisms of iron uptake probably exist in other microalgae. Indeed, it has been reported that, in some diatoms and coccolithophores, the rate of iron uptake does not depend on the concentration of chelated Fe(III) but rather on the equilibrium concentration of unchelated Fe’ (Sunda, 2001; Shi et al., 2010), as would be expected from a dissociative, thermodynamically controlled process of uptake.
The efficiency of the uptake system that we describe here may be tightly related to the aqueous Fe3+ available for binding at the cell surface, and thus to the form of iron present in the extracellular medium. The iron binding sites at the surface of C. velia cells have an affinity that is sufficient for the binding of iron ligated to citrate but not to hydroxamate siderophores (logKD of about 30). The form in which iron exists in ocean water remains unclear. Morel et al. (2008) have suggested that unchelated iron may be an important source of iron for phytoplankton, whereas other authors have suggested that most of the ferric iron in ocean water is complexed to organic ligands, with conditional stability constants ranging from 1011 to 1022 m−1 (Rue and Bruland, 1995; Butler, 1998). Other studies have identified colloidal iron as a major form of iron at the surface of the ocean (Wu et al., 2001).
Very little is known about the mechanisms used by marine phytoplankton to fulfill their iron requirement. Despite this lack of data, most authors postulate that the mechanism of iron uptake by marine phytoplankton must be either reductive or siderophore mediated (Sunda, 2001). Both strategies of uptake are undoubtedly used by several marine microalgae. A yeast-like reductive uptake system has been suggested on the basis of gene sequence homology or transcriptomic analyses in the marine diatoms Thalassiosira pseudonana (Armbrust et al., 2004) and Phaeodactylum tricornutum (Kustka et al., 2007; Allen et al., 2008; Bowler et al., 2008), and direct evidence for copper-dependent reductive uptake of iron has been shown for Thalassiosira oceanica (Maldonado et al., 2006). The presence of marine siderophores has also been well established (Butler, 1998, 2005; Mawji et al., 2008), and their use as an iron source, through reductive or nonreductive mechanisms, has been shown for certain microalgae (Soria-Dengg and Horstmann, 1995; Naito et al., 2008; Hopkinson and Morel, 2009). However, these organisms may need to adapt to particular features of the marine environment, requiring the use of different strategies. Physical limitations affect both strategies (reductive and siderophore mediated) of iron uptake from the marine environment, including the very low concentration of the species of interest and their rapid diffusion (Völker and Wolf-Gladrow, 1999). Moreover, the sequencing of genomes of unicellular free-living eukaryotes now challenges the ubiquity of the traditional reductive iron uptake system characterized in the yeast model. Querying draft genomes of unicellular free-living eukaryotes against components of the yeast reductive iron uptake system showed that ferrireductase homologs are present only in the ciliate Tetrahymena thermophila and the amoeba Naegleria gruberi, with no significant BLAST hits being detected for others (Supplemental Table S3). This may be due to either the reductive iron uptake mechanism not being conserved or the sequences being too divergent to be detected by the homology searches. Use of the diatom annotated homologs as BLAST queries revealed additional significant hits in some but not all draft genomes (Supplemental Table S3). Using the diatom FTR1 homolog (EEC45906.1, P. tricornutum) as a query, we found this permease to be the most conserved component of iron import, with significant hits in all studied draft genomes. These matches were all referred to as ZIP transporters, a family of metal transporters able to transport a variety of cations (Guerinot, 2000). Thus, most of the sequenced unicellular free-living eukaryote genomes have homologs of Ftr/Irt proteins, but no clear Fre and Fet homologs (Supplemental Table S3), suggesting the presence of uptake systems other than the classic reductive system. Analysis of the genomes of two Ostreococcus species suggests a complete lack of reductive iron uptake components. It was further speculated that they possess a novel system of iron acquisition that differs from yeast and diatoms, although experimental evidence for a new nonreductive iron uptake system is missing (Palenik et al., 2007). Similarly, the diversity of iron uptake systems in the organisms most closely related to C. velia, such as the parasitic apicomplexa, remains to be fully understood (Sutak et al., 2008). The next closest group to C. velia is the dinoflagellates and its sister group, which includes Perkinsus marinus. Analysis of the draft genome of P. marinus did not reveal any evidence of classic iron uptake machinery (Supplemental Table S3).
The iron uptake system that we have preliminarily characterized here may not therefore be restricted to C. velia, but may be used by other phylogenetically related or unrelated organisms. Another nonreductive iron uptake system involving binding of iron by a surface transferrin-like protein was described in the halotolerant alga Dunaniella salina (Paz et al., 2007a, 2007b). In this alga, iron deficiency induces primarily iron binding to the cell surface rather than iron internalization (Paz et al., 2007b), which is the opposite of what we observed in C. velia. The kinetic parameters of iron uptake by D. salina and C. velia are also strongly different (Paz et al., 2007b) and it thus seems improbable that these species share the same mechanism of iron uptake. We are currently comparing the iron uptake mechanisms used by various microalgae belonging to different phyla, to determine whether or not the iron uptake mechanism identified in C. velia can be found in other species of the oceanic phytoplankton. Many other questions about iron uptake by C. velia remain unanswered. In particular, it remains unclear whether Fe(II) and Fe(III) can be taken up by the same system or by separate mechanisms. We observed that C. velia was able to take up Fe(III) and Fe(II) at similar rates. The same observation was made for Thalassiosira weissflogii (Anderson and Morel, 1982), and it was suggested that the uptake of Fe(II) may involve autoxidation to Fe(III) following binding to the cell surface, a process known to occur in Fe(II) chelation to strong Fe(III)-binding ligands (Sunda, 2001).
A major step forward will be the identification of the molecular components involved in this nonreductive uptake system.
MATERIALS AND METHODS
Cell Culture
The yeast Saccharomyces cerevisiae YPH499 was grown at 30°C in iron-rich (yeast nitrogen base) or iron-deficient (yeast nitrogen base + 0.1 mm BPS) medium as previously described (Lesuisse et al., 2001). Chromera velia cells were grown at a temperature of 21°C and a 16:8 light (3,000 lux) dark regime in a filtered modified f medium (Guillard and Ryther, 1962) containing per liter (Mf medium): sea salts (Sigma) 40g; MES hydrate 250 mg; NH4NO3 2.66 mg; NaNO3 75 mg; Na2SiO3 5H2O 22.8 mg; NaH2PO4 15 mg; 1 mL of vitamin stock (thiamine HCl 20 mg/L, biotin 1 mg/L, B12 1 mg/L); 1 mL of trace metal stock (MnCl2 4H2O 200 mg/L, ZnSO4 7H2O 40 mg/L, Na2MoO4 2H2O 20 mg/L, CoCl2 6H2O 14 mg/L, Na3VO4 nH2O 10 mg/L, NiCl2 10 mg/L, H2SeO3 10 mg/L); and 1 mL of antibiotic stock (ampicillin sodium and streptomycin sulfate 100 mg/mL); pH 6.8. Iron was added in the form of ferric citrate (1:20). The chemical speciation of iron was estimated using the GEOCHEM-EZ software (http://www.plantmineralnutrition.net/Geochem/Geochem%20Download.htm; Shaff et al., 2010).
Iron Uptake Assays
Iron uptake by S. cerevisiae was measured in microtiter plates as previously described (Lesuisse et al., 2001). Iron uptake by C. velia was measured in microtiter plates, after incubating cells in the light at 25°C for various periods of time in Mf medium. Different amounts of 55Fe (29,600 MBq/mg) were added, in the form of ferrous ascorbate, ferric citrate, ferrioxamine B, or ferrichrome. The cells were collected with a cell harvester (Brandel) and washed on the filter before counting in a Wallac 1450 MicroBeta TriLux scintillaton counter.
Reductase Assays
Whole cell ferrireductase activity of S. cerevisiae and C. velia was measured as described previously (Lesuisse and Labbe, 1989) with either Fe(III)-citrate or Fe(III)-EDTA (0.5 mm) as the iron source. The cells (100 × 106 cells/mL) were incubated at 25°C in the dark in the presence of iron (0.5 mm) and BPS (1.5 mm) for various periods of time, and then centrifuged before measuring absorbency (535 nm) of the supernatant (ε = 19.5 mm−1 cm−1). Intracellular ferrireductase activity was measured from soluble cell extracts (100 μg/mL) as previously described (Lesuisse et al., 1990), with Fe(III)-EDTA (0.5 mm) as the iron source and either NADH or NADPH (1 mm) as the electron donor, in the presence of BPS (1.5 mm) at 30°C with magnetic stirring. The formation of the Fe(II)(BPS)3 complex was continuously monitored at 535 nm with a Varian Cary 4000 spectrophotometer. Trans-plasma membrane electron transfer was assessed for whole cells with resazurin as the electron acceptor. Reductase activity was recorded by the appearance of resorufin at 30°C with a Jobin Yvon JY3D spectrofluorimeter (λex 560 nm, λem 585 nm, slit widths of 2 nm for both excitation and emission). The incubation mixture in 50 mm sodium citrate buffer (pH 6.5; S. cerevisiae) or Mf medium (C. velia) containing 10 mm resazurin was magnetically stirred (Lesuisse et al., 1996).
Oxygen Assay
The metabolic activity of C. velia cells in the light and in the dark was evaluated by an oxypolarographic method. The rate of oxygen consumption/production by the cells (100 × 106 cells/mL Mf medium) was measured with a 1 mL thermostatically controlled oxypolarographic cell equipped with a Clark-type electrode.
Light Microscopy
Cells in an exponential growth phase were observed and measured under a 100× oil objective using an Olympus BX62 microscope equipped with differential interference contrast. We used the nonparametric Mann-Whitney-Wilcoxon test to determine whether the obtained measurements were consistent with the expected distribution.
Electron Microscopy
C. velia cells were centrifuged in culture medium. Supernatants were removed. The remaining concentrated suspensions of cells were placed in flat specimen holders for high-pressure freezing (flat specimen carrier, gold plated, 0.5 mm thick, 1.5 mm in diameter, 200 μm deep; EMpact 2 Leica), frozen, and stored in liquid nitrogen.
Cells were freeze substituted at −90°C in 2% osmium tetroxide in acetone and embedded in Epon-Araldite. Serial sections (60 nm) were cut on an Ultracut E microtome (Leica Microsystems), poststained with uranyl acetate and lead citrate. Staining was visualized using a Tecnai12 electron microscope operating at 80 kV.
FPLC
The cells grown with 0.5 μm 55Fe-citrate as the sole source of iron were washed and disrupted by sonication. Cell extracts were spinned down to remove remnants of the cell wall and organelles. The supernatant was loaded onto a Superdex 200 10/300 GL column (GE Healthcare) and proteins were eluted with 50 mm HEPES and 140 mm NaCl, pH 8.0, using the FPLC system ÄKTApurifier UPC 10 (GE Healthcare). Fractions (0.2 mL) were collected, and radioactivity was measured in a scintillator counter. The column was calibrated with a gel filtration standard (Bio-Rad).
Other Assays
Surface thiols of C. velia cells were measured with 5,5′-dithio-bis(2-nitrobenzoic acid), as previously described (Lesuisse and Labbe, 1992). Chlorophyll from 10 mL of culture was extracted in 90% acetone using 0.45 to 0.45 mm glass beads. The concentration of chlorophyll a was measured with a Varian Cary 4000 spectrophotometer using the equation:
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Gel filtration analysis of soluble whole cell extracts from C. velia.
Supplemental Figure S2. Iron-dependent metabolic activity of C. velia.
Supplemental Figure S3. Effect of iron on C. velia cell morphology.
Supplemental Figure S4. Initial iron uptake rates by C. velia; dependence on iron concentration.
Supplemental Figure S5. The effect of gallium on C. velia iron uptake.
Supplemental Table S1. Effect of iron on C. velia morphology.
Supplemental Table S2. Ferrireductase activity of C. velia and S. cerevisiae cells.
Supplemental Table S3. Sequence homology between different components of iron uptake systems in unicellular eukaryotes.
Supplementary Material
References
- Allen AE, Laroche J, Maheswari U, Lommer M, Schauer N, Lopez PJ, Finazzi G, Fernie AR, Bowler C. (2008) Whole-cell response of the pennate diatom Phaeodactylum tricornutum to iron starvation. Proc Natl Acad Sci USA 105: 10438–10443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MA, Morel FMM. (1982) The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol Oceanogr 27: 789–813 [Google Scholar]
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou S, Allen AE, Apt KE, Bechner M, et al. (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306: 79–86 [DOI] [PubMed] [Google Scholar]
- Bowler C, Allen AE, Badger JH, Grimwood J, Jabbari K, Kuo A, Maheswari U, Martens C, Maumus F, Otillar RP, et al. (2008) The Phaeodactylum genome reveals the evolutionary history of diatom genomes. Nature 456: 239–244 [DOI] [PubMed] [Google Scholar]
- Butler A. (1998) Acquisition and utilization of transition metal ions by marine organisms. Science 281: 207–210 [DOI] [PubMed] [Google Scholar]
- Butler A. (2005) Marine siderophores and microbial iron mobilization. Biometals 18: 369–374 [DOI] [PubMed] [Google Scholar]
- Coale KH, Johnson KS, Chavez FP, Buesseler KO, Barber RT, Brzezinski MA, Cochlan WP, Millero FJ, Falkowski PG, Bauer JE, et al. (2004) Southern Ocean iron enrichment experiment: carbon cycling in high- and low-Si waters. Science 304: 408–414 [DOI] [PubMed] [Google Scholar]
- de Baar HJW, de Jong JTM. (2001) Distributions, sources and sinks of iron in seawater. Turner DR, Hunter KA, , The Biogeochemistry of Iron in Seawater. John Wiley & Sons Ltd, Chichester, UK, pp 123–253 [Google Scholar]
- Greene RM, Geider RJ, Kolber Z, Falkowski PG. (1992) Iron-induced changes in light harvesting and photochemical energy conversion processes in eukaryotic marine algae. Plant Physiol 100: 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML. (2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465: 190–198 [DOI] [PubMed] [Google Scholar]
- Guillard RR, Ryther JH. (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol 8: 229–239 [DOI] [PubMed] [Google Scholar]
- Heymann P, Ernst JF, Winkelmann G. (2000) Identification and substrate specificity of a ferrichrome-type siderophore transporter (Arn1p) in Saccharomyces cerevisiae. FEMS Microbiol Lett 186: 221–227 [DOI] [PubMed] [Google Scholar]
- Hopkinson BM, Morel FM. (2009) The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals 22: 659–669 [DOI] [PubMed] [Google Scholar]
- Hudson RJM, Morel FMM. (1990) Iron transport in marine phytoplankton: kinetics of cellular and medium coordination reactions. Limnol Oceanogr 35: 1002–1020 [Google Scholar]
- Kosman DJ. (2003) Molecular mechanisms of iron uptake in fungi. Mol Microbiol 47: 1185–1197 [DOI] [PubMed] [Google Scholar]
- Kustka AB, Allen AE, Morel FMM. (2007) Sequence analysis and transcriptional regulation of iron acquisition genes in two marine diatoms. J Phycol 43: 715–729 [Google Scholar]
- Kwok EY, Severance S, Kosman DJ. (2006a) Evidence for iron channeling in the Fet3p-Ftr1p high-affinity iron uptake complex in the yeast plasma membrane. Biochemistry 45: 6317–6327 [DOI] [PubMed] [Google Scholar]
- Kwok EY, Stoj CS, Severance S, Kosman DJ. (2006b) An engineered bifunctional high affinity iron uptake protein in the yeast plasma membrane. J Inorg Biochem 100: 1053–1060 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Blaiseau PL, Dancis A, Camadro JM. (2001) Siderophore uptake and use by the yeast Saccharomyces cerevisiae. Microbiology 147: 289–298 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Casteras-Simon M, Labbe P. (1996) Evidence for the Saccharomyces cerevisiae ferrireductase system being a multicomponent electron transport chain. J Biol Chem 271: 13578–13583 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Crichton RR, Labbe P. (1990) Iron-reductases in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta 1038: 253–259 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Labbe P. (1989) Reductive and non-reductive mechanisms of iron assimilation by the yeast Saccharomyces cerevisiae. J Gen Microbiol 135: 257–263 [DOI] [PubMed] [Google Scholar]
- Lesuisse E, Labbe P. (1992) Iron reduction and trans-plasma membrane electron transfer in the yeast Saccharomyces cerevisiae. Plant Physiol 100: 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MT, Allen AE, Chong JS, Lin K, Leus D, Karpenko N, Harris SL. (2006) Copper-dependent iron transport in coastal and oceanic diatoms. Limnol Oceanogr 51: 1729–1743 [Google Scholar]
- Marchetti A, Cassar N. (2009) Diatom elemental and morphological changes in response to iron limitation: a brief review with potential paleoceanographic applications. Geobiology 7: 419–431 [DOI] [PubMed] [Google Scholar]
- Marchetti A, Parker MS, Moccia LP, Lin EO, Arrieta AL, Ribalet F, Murphy ME, Maldonado MT, Armbrust EV. (2009) Ferritin is used for iron storage in bloom-forming marine pennate diatoms. Nature 457: 467–470 [DOI] [PubMed] [Google Scholar]
- Mawji E, Gledhill M, Milton JA, Tarran GA, Ussher S, Thompson A, Wolff GA, Worsfold PJ, Achterberg EP. (2008) Hydroxamate siderophores: occurrence and importance in the Atlantic ocean. Environ Sci Technol 42: 8675–8680 [DOI] [PubMed] [Google Scholar]
- Merchant SS, Allen MD, Kropat J, Moseley JL, Long JC, Tottey S, Terauchi AM. (2006) Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim Biophys Acta 1763: 578–594 [DOI] [PubMed] [Google Scholar]
- Moore RB, Obornik M, Janouskovec J, Chrudimsky T, Vancova M, Green DH, Wright SW, Davies NW, Bolch CJ, Heimann K, et al. (2008) A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451: 959–963 [DOI] [PubMed] [Google Scholar]
- Morel FMM, Kustka AB, Shaked Y. (2008) The role of unchelated Fe in the iron nutrition of phytoplankton. Limnol Oceanogr 53: 400–404 [Google Scholar]
- Naito K, Imai I, Nakahara H. (2008) Complexation of iron by microbial siderophores and effects of iron chelates on the growth of marine microalgae causing red tides. Phycological Res 56: 58–67 [Google Scholar]
- Palenik B, Grimwood J, Aerts A, Rouze P, Salamov A, Putnam N, Dupont C, Jorgensen R, Derelle E, Rombauts S, et al. (2007) The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci USA 104: 7705–7710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Y, Katz A, Pick U. (2007a) A multicopper ferroxidase involved in iron binding to transferrins in Dunaliella salina plasma membranes. J Biol Chem 282: 8658–8666 [DOI] [PubMed] [Google Scholar]
- Paz Y, Shimoni E, Weiss M, Pick U. (2007b) Effects of iron deficiency on iron binding and internalization into acidic vacuoles in Dunaliella salina. Plant Physiol 144: 1407–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott CC. (2006) Iron uptake in fungi: a system for every source. Biochim Biophys Acta 1763: 636–645 [DOI] [PubMed] [Google Scholar]
- Pollard RT, Salter I, Sanders RJ, Lucas MI, Moore CM, Mills RA, Statham PJ, Allen JT, Baker AR, Bakker DC, et al. (2009) Southern ocean deep-water carbon export enhanced by natural iron fertilization. Nature 457: 577–580 [DOI] [PubMed] [Google Scholar]
- Rue EL, Bruland KW. (1995) Complexation of iron(III) by natural organic ligands in the Central North Pacific as determined by a new competitive ligand equilibration/adsorptive cathodic stripping voltammetric method. Mar Chem 50: 117–138 [Google Scholar]
- Seguin A, Bayot A, Dancis A, Rogowska-Wrzesinska A, Auchere F, Camadro JM, Bulteau AL, Lesuisse E. (2009) Overexpression of the yeast frataxin homolog (Yfh1): contrasting effects on iron-sulfur cluster assembly, heme synthesis and resistance to oxidative stress. Mitochondrion 9: 130–138 [DOI] [PubMed] [Google Scholar]
- Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV. (2010) GEOCHEM-EZ:a chemical speciation program with greater power and flexibility. Plant Soil 330: 207–214 [Google Scholar]
- Shi D, Xu Y, Hopkinson BM, Morel FM. (2010) Effect of ocean acidification on iron availability to marine phytoplankton. Science 327: 676–679 [DOI] [PubMed] [Google Scholar]
- Silva AMN, Le Kong X, Parkin MC, Cammack R, Hider RC. (2009) Iron(III) citrate speciation in aqueous solution. Dalton Trans 8616–8625 [DOI] [PubMed] [Google Scholar]
- Soria-Dengg S, Horstmann U. (1995) Ferrioxamines B and E as iron sources for the marine diatom Phaeodactylum tricornutum. Mar Ecol Prog Ser 127: 269–277 [Google Scholar]
- Staiger D. (2002) Chemical strategies for iron acquisition in plants. Angew Chem Int Ed Engl 41: 2259–2264 [DOI] [PubMed] [Google Scholar]
- Sunda WG. (2001) Bioavailability and bioaccumulation of iron in the sea. Turner DR, Hunter KA, , The Biogeochemistry of Iron in Seawater. John Wiley & Sons Ltd, Chichester, UK, pp 41–84 [Google Scholar]
- Sutak R, Lesuisse E, Tachezy J, Richardson DR. (2008) Crusade for iron: iron uptake in unicellular eukaryotes and its significance for virulence. Trends Microbiol 16: 261–268 [DOI] [PubMed] [Google Scholar]
- Turner DR, Hunter KA, de Baar HJW. (2001) Introduction. Turner DR, Hunter KA, , The Biogeochemistry of Iron in Seawater. John Wiley & Sons Ltd, Chichester, UK, pp 1–7 [Google Scholar]
- Völker C, Wolf-Gladrow DA. (1999) Physical limits on iron uptake mediated by siderophores or surface reductases. Mar Chem 65: 227–244 [Google Scholar]
- Wu J, Boyle E, Sunda W, Wen LS. (2001) Soluble and colloidal iron in the oligotrophic North Atlantic and North Pacific. Science 293: 847–849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.