Cell walls are important features of plant cells that perform a number of essential functions, including providing shape to the many different cell types needed to form the tissues and organs of a plant. Forming the interface between adjacent cells, plant cell walls often play important roles in intercellular communication. Because of their surface location, plant cell walls play an important role in plant-microbe interactions, including defense responses against potential pathogens. The desire to understand these and other plant functions help explain the strong interest in wall structure and biosynthesis.
Plant cell walls are usually divided in textbooks into two categories: primary walls that surround growing cells or cells capable of growth and secondary walls that are thickened structures containing lignin and surrounding specialized cells such as vessel elements or fiber cells. In reality, all differentiated cells contain walls with distinct compositions, resulting in a spectrum of specialized cell walls with primary and secondary walls as two extremes. This brief prospective overview focuses mainly on issues that must be resolved if we are to understand the role of cell walls in plant physiology. Many outstanding reviews cover recent progress, including a series of excellent updates in a recent special issue of Plant Physiology focused on this topic (see McCann and Rose, 2010). In addition, the lignin component of secondary cell walls is covered elsewhere in this issue (Li and Chapple, 2010) as is the uses of cell walls as a source of energy (Somerville et al., 2010). The author apologizes to the many colleagues whose work could not be cited because of space limitations.
STRUCTURAL ISSUES
The polysaccharide and glycoprotein components found in plant cell walls have been well characterized structurally. We need now to understand how these components are organized into the three-dimensional matrix needed for plant cell walls to perform their functions.
The most characteristic component found in all plant cell walls is cellulose. It consists of a collection of β-1,4-linked glucan chains that interact with each other via hydrogen bonds to form a crystalline microfibril (Somerville, 2006). In addition to cellulose, plant cell walls contain several matrix polysaccharides that are grouped into two general categories: (1) the pectic polysaccharides include homogalacturonan, and rhamnogalacturonan I and II (Harholt et al., 2010) and (2) the hemicellulosic polysaccharides include xyloglucans, glucomannans, xylans, and mixed-linkage glucans (Scheller and Ulvskov, 2010). Plant cell walls also contain many proteins and glycoproteins, including various enzymes and structural proteins (Rose and Lee, 2010). For example, arabinogalactan proteins are structurally complex molecules found on the plasma membrane and in the cell wall; they are thought to play important roles in recognition and signaling events at the cell surface (Ellis et al., 2010). Despite tantalizing evidence for their involvement in many important recognition and signaling events, few details are available regarding how they are recognized or how recognition leads to signal transmission.
How are wall components organized into a functional matrix? Over the years, several models have been proposed to explain the organization of wall components (Keegstra et al., 1973; Carpita and Gibeaut, 1993; Somerville et al., 2004). Most of the models have focused on understanding the organization of components in primary cell walls that would allow regulated reorganization of wall components during cell growth and differentiation. Hemicellulosic polysaccharides are known to bind tightly to cellulose microfibrils via hydrogen bonds and most wall models have incorporated this interaction as one important feature of cell wall architecture. Less is known about how the pectic polysaccharides interact with other components in plant cell walls, but there is increasing awareness of their importance in primary cell walls, where they are most abundant.
The dynamic nature of plant cell walls is an important feature that is lacking from most models. As cells grow and differentiate, new wall material is laid down near the plasma membrane and older wall material is pushed outward. This process has the potential to create a wall where the composition and architecture are not uniform across the wall. For example, pectic polysaccharides are thought to be deposited early after cell division, leading to a middle lamella that is rich in pectins; other components are deposited later. This differentiation of the wall may be especially important for protein and glycoprotein components, such as AGPs that may change as cells mature and differentiate. Information about such heterogeneity is lost when tissues are ground and subjected to biochemical analysis. Thus, to fully understand the dynamic nature of plant cell walls at the molecular level, new visualization techniques are needed that reveal the three-dimensional complexity of the walls on individual cells as well as the ability to monitor any changes as a function of developmental time and space. One important tool that will help in such studies is an array of antibodies and carbohydrate-binding proteins that can be used to visualize specific epitopes within plant cell walls (Pattathil et al., 2010). Preliminary analysis supports the hypothesis that every cell type has a distinct array of wall components, but much more work and even greater resolution will be needed to gain the desired information about the three-dimensional organization of cell wall components.
BIOSYNTHETIC ISSUES
Probably the biggest gap in our knowledge about cell walls relates to biosynthesis of the various wall components. It has been estimated that more than 2000 genes are required for the synthesis and metabolism of cell wall components (McCann and Rose, 2010). Identification of the genes responsible for wall biosynthesis and characterization of the biochemical and biological functions of the gene products that mediate wall biosynthesis are important areas of current research activity. Finally, as the process of wall biosynthesis is revealed, it will be important to understand how these processes are regulated, at both the biochemical and the transcriptional level.
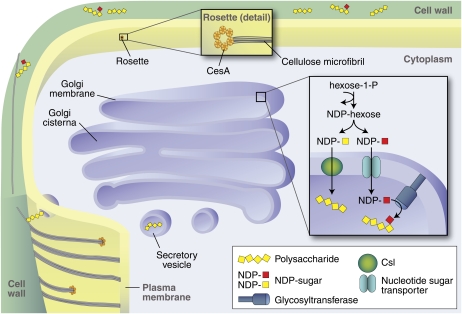
One important feature of plant cell wall biosynthesis is that it involves multiple cellular compartments (Fig. 1). Specifically, cellulose is synthesized at the plasma membrane with the insoluble cellulose microfibrils being deposited directly into the extracellular matrix. On the other hand, matrix polysaccharides and various glycoproteins are synthesized in the endomembrane system, with the polymers being delivered to the wall via secretory vesicles (Fig. 1). Components synthesized in different locations must be assembled into a functional wall matrix. Although very little is known about this assembly process, it seems likely that it is a mediated event, most probably requiring proteins of various kinds.
Figure 1.
Schematic representation of the key events in cell wall biosynthesis. Cellulose biosynthesis occurs at the plasma membrane in large complexes visualized as rosettes. The synthesis of matrix polysaccharides and glycoproteins occurs in the Golgi where the products accumulate in the lumen before transport to the cell wall via vesicles. The regulation of these biosynthetic events is an important issue that needs more study. Abbreviations used in the figure: CesA, cellulose synthase proteins that form the rosette; NDP-sugar, nucleotide sugars that act as donors for the sugars that go into polysaccharides; Csl, cellulose synthase-like proteins that are known to be involved in hemicellulose biosynthesis.
Cellulose biosynthesis involves a large multisubunit complex containing at least three different cellulose synthase enzymes and probably other proteins (Guerriero et al., 2010). These proteins form a complex that appears in the plasma membrane as a rosette structure that is thought to transfer Glc from cytosolic UDP-Glc to produce multiple extracellular glucan chains that eventually coalesce into a cellulose microfibril (Fig. 1). While much has been learned about cellulose biosynthesis in the past two decades (Somerville, 2006; Guerriero et al., 2010), many questions remain. For example, at the biochemical level, how is glucan chain polymerization initiated? How are the individual sugar molecules, or the growing chains, transported across the plasma membrane while still maintaining the membrane potential characteristic of living cells? At a cell biological level, how are the cellulose microfibrils properly oriented? It is known that cortical microtubules are important in determining cellulose microfibril orientation (Wightman and Turner, 2010), but the molecular details of how is this accomplished remain unclear.
The biosynthesis of matrix polysaccharides and glycosylation of various cell wall glycoproteins occur in the Golgi membranes (Fig. 1). Although recent advances have enhanced our understanding of the synthesis of these molecules (Ellis et al., 2010; Harholt et al., 2010; Scheller and Ulvskov, 2010), many important questions remain. At a biochemical level, we must identify and characterize the enzymes needed to synthesize the diverse array of matrix components. For example, it has been estimated that more than 65 different enzymes are required to synthesize the pectic polysaccharides known to exist in plant cells (Harholt et al., 2010). Yet only a few of them have been identified and characterized, partly because of the inherent difficulty of the problem.
Two basic strategies are available for identifying the biochemical and biological functions associated with the many gene sequences that have been identified as candidates for involvement in wall biosynthesis. The first is expression of a cloned gene followed by measurement of the biochemical activity of the resulting protein. Expression of the gene is relatively easy, but measuring the resulting biochemical activity is difficult, largely because of the extreme specificity of the glycosyl transferase enzymes. Many of the substrates that donate sugar molecules are commercially available, but few of the acceptor molecules are. The latter are often complex carbohydrates that are difficult to produce in the laboratory. Finally, there is growing evidence that many wall biosynthetic enzymes act in multienzyme complexes so that in vitro assays may require the action of multiple enzymes. A second strategy for exploring gene function is reverse genetics using the power of model systems such as Arabidopsis (Arabidopsis thaliana; Liepman et al., 2010). However, this approach is often complicated by the presence of multiple genes encoding a particular enzyme, so that double, triple, or even higher-order mutants are needed. Once mutants are obtained, some mutant plants have no visible phenotype, but even when mutants have morphological changes, detailed analysis is needed to define the biochemical changes in wall components and to connect them to the morphological changes.
Although progress is being made in identifying and characterizing the genes required for the synthesis of wall matrix components (Ellis et al., 2010; Guerriero et al., 2010; Harholt et al., 2010; Scheller and Ulvskov, 2010), little is known about how the production and accumulation of wall components are regulated. It is clear that synthesis of wall components is regulated in very specific ways to produce the diversity of cell shapes and functions that characterize a living plant. But understanding how this regulated deposition of wall components is accomplished is a major challenge.
One important aspect of controlling this overall process is regulation of carbon flow to the nucleotide sugars that are the sugar donors for cell wall polymers (Reiter, 2008). How this flow of carbon is regulated, i.e. biochemical controls, transcriptional controls, or both, and how much this regulation contributes to overall regulation of wall deposition are yet unknown.
Another likely point of regulation is the activities of the glycan synthases and glycosyltransferases that assemble wall polysaccharides from the nucleotide sugars. One attractive hypothesis posits that the quantity of these enzymes is regulated by controlling gene expression, probably in a coordinated manner, so that all of the enzymes needed for the production of a particular wall component are coordinately regulated. In addition, it seems likely that the activities of many enzymes may be controlled by phosphorylation or other mechanisms. The quantities and rates of cellulose deposition may be controlled in part by the location and cycling of the rosettes that mediate cellulose synthesis, whereas the orientation of cellulose microfibrils is determined by interactions with the cytoskeleton (Wightman and Turner, 2010; see Fig. 1).
Other potential regulatory points are the delivery and assembly steps. For example, the delivery of matrix components from the Golgi to the cell surface may be regulated by controlling the activity of the secretory system. It has been suggested that cells have feedback mechanisms that sense the status of the cell wall and control wall deposition events in response to need (see Seifert and Blaukopf, 2010, for a recent update). However, many important questions remain regarding how these feedback mechanisms operate.
CONCLUDING REMARKS
One final issue relevant to both the structure of plant cell walls and the biosynthesis of wall components is the evolutionary relationships of cell walls from the many plant species and their algal progenitors. While most work on structure and biosynthesis has focused on angiosperms, especially model systems such as Arabidopsis (Liepman et al., 2010) and crop plants, recent work on cell walls from algae and primitive plants have begun to yield interesting insight into the evolution of cell walls and their components (Popper and Tuohy, 2010; Sørenson et al., 2010). Such studies may lead to important insights into the functional relationships among the various wall components.
A major conclusion from this brief summary is that the plant community faces many challenges in understanding cell wall structure, function, and biosynthesis. New biophysical and visualization methods will be needed to understand the organization of components in the wall of a single cell. With respect to the challenges of understanding cell wall biosynthesis and its regulation, molecular biology, molecular genetics, and genomics has already provided many powerful new tools so that rapid progress can be expected.
References
- Carpita NC, Gibeaut DM. (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Ellis M, Egelund J, Schultz C, Bacic A. (2010) Arabinogalactan-proteins: key regulators at the cell surface. Plant Physiol 153: 403–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerriero G, Fugelstad J, Bulone V. (2010) What do we really know about cellulose biosynthesis in higher plants? J Integr Plant Biol 52: 161–175 [DOI] [PubMed] [Google Scholar]
- Harholt J, Suttangkakul A, Scheller HV. (2010) Biosynthesis of pectins. Plant Physiol 153: 384–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegstra K, Talmadge KW, Bauer WD, Albersheim P. (1973) The structure of plant cell walls: III. A model of the walls of suspension-cultured sycamore cells based on the interconnections of the macromolecular components. Plant Physiol 51: 188–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chapple C. (2010) Understanding lignification: challenges beyond monolignol biosynthesis. Plant Physiol 154: 449–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepman AH, Wightman R, Geshi N, Turner SR, Scheller HV. (2010) Arabidopsis—a powerful model system for plant cell wall research. Plant J 61: 1107–1121 [DOI] [PubMed] [Google Scholar]
- McCann M, Rose J. (2010) Blueprint for building plant cell walls. Plant Physiol 153: 365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popper ZA, Tuohy MG. (2010) Beyond the green: understanding the evolutionary puzzle of plant and algal cell walls. Plant Physiol 153: 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter WD. (2008) Biochemical genetics of nucleotide sugar interconversion reactions. Curr Opin Plant Biol 11: 236–243 [DOI] [PubMed] [Google Scholar]
- Rose JKC, Lee SJ. (2010) Straying off the highway: trafficking of secreted plant proteins and complexity in the plant cell wall proteome. Plant Physiol 153: 433–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P. (2010) Hemicelluloses. Annu Rev Plant Biol 61: 263–289 [DOI] [PubMed] [Google Scholar]
- Seifert GJ, Blaukopf C. (2010) Irritable walls: the plant extracellular matrix and signaling. Plant Physiol 153: 467–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C. (2006) Cellulose synthesis in higher plants. Annu Rev Cell Dev Biol 22: 53–78 [DOI] [PubMed] [Google Scholar]
- Somerville C, Bauer S, Brininstool G, Facette M, Hamann T, Milne J, Osborne E, Paredez A, Persson S, Raab T, et al. (2004) Toward a systems approach to understanding plant cell walls. Science 306: 2206–2211 [DOI] [PubMed] [Google Scholar]
- Somerville C, Youngs H, Taylor C, Davis SC, Long SP. (2010) Feedstocks for lignocellulosic biofuels. Science 329: 790–792 [DOI] [PubMed] [Google Scholar]
- Sørenson I, Domozych D, Willats WGT. (2010) How have plant cell walls evolved? Plant Physiol 153: 366–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Turner S. (2010) Trafficking of the plant cellulose synthase complex. Plant Physiol 153: 427–432 [DOI] [PMC free article] [PubMed] [Google Scholar]