Abstract
The plant-specific SR45 belongs to the highly conserved family of serine/arginine-rich (SR) proteins, which play key roles in precursor-mRNA splicing and other aspects of RNA metabolism. An Arabidopsis (Arabidopsis thaliana) loss-of-function mutant, sr45-1, displays pleiotropic phenotypes, such as defects in flower and leaf morphology, root growth, and flowering time. Here, we show that the sr45-1 mutation confers hypersensitivity to glucose (Glc) during early seedling growth in Arabidopsis. Unlike wild-type plants, the sr45-1 mutant displays impaired cotyledon greening and expansion as well as reduced hypocotyl elongation of dark-grown seedlings when grown in the presence of low (3%) Glc concentrations. In addition, SR45 is involved in the control of Glc-responsive gene expression, as the mutant displays enhanced repression of photosynthetic and nitrogen metabolism genes and overinduction of starch and anthocyanin biosynthesis genes. Like many other sugar response mutants, sr45-1 also shows hypersensitivity to abscisic acid (ABA) but appears to be unaffected in ethylene signaling. Importantly, the sr45-1 mutant shows enhanced ability to accumulate ABA in response to Glc, and the ABA biosynthesis inhibitor fluridone partially rescues the sugar-mediated growth arrest. Moreover, three ABA biosynthesis genes and two key ABA signaling genes, ABI3 and ABI5, are markedly overinduced by Glc in sr45-1. These results provide evidence that the SR45 protein defines a novel player in plant sugar response that negatively regulates Glc signaling during early seedling development by down-regulating both Glc-specific ABA accumulation and ABA biosynthesis and signaling gene expression.
To adapt to environmental and metabolic changes, higher plants can sense sugar levels and adjust growth and development accordingly, modulating various important processes, including germination, early seedling development, leaf and root morphogenesis, flowering, defense responses and senescence, as well as gene expression (Koch, 1996; Gibson, 2005; Rolland et al., 2006). While Suc is the major sugar translocated in plants, Glc plays a preponderant regulatory role, being recognized as a central signaling molecule in addition to a universal carbon and energy source. The first enzyme in glycolysis, hexokinase (HXK), which phosphorylates Glc to Glc-6-P, is the evolutionarily conserved Glc sensor in a wide range of organisms, from yeast to mammals, including plants (Rolland et al., 2006), where both HXK-dependent and HXK-independent Glc signal transduction pathways appear to coexist (Xiao et al., 2000).
In Arabidopsis (Arabidopsis thaliana), cotyledon greening and expansion as well as initiation of true leaf development are suppressed during growth in the presence of 6% Glc, with the majority of wild-type seedlings exhibiting a dramatic developmental arrest (Jang et al., 1997). This severe inhibition of early seedling growth by elevated sugar levels has been broadly used to monitor the response to sugars and screen for sugar signaling mutants. Characterization of these mutants has revealed that several of them are also impaired in phytohormone metabolism or response, disclosing extensive interactions between Glc and hormone signaling pathways that modulate plant growth and metabolism.
Among the plant hormones, abscisic acid (ABA) and ethylene are of major importance in the interaction with sugar signals. ABA mediates a developmental checkpoint that arrests early growth under adverse environmental conditions (Lopez-Molina et al., 2001), and high levels of exogenous Glc similarly repress cotyledon development and hypocotyl elongation. Glc has also been shown to induce the expression of ABA synthesis and signaling genes and to increase endogenous ABA levels, while the ABA-deficient aba2 mutant fails to arrest in 6% Glc (Cheng et al., 2002). These data suggest that Glc-specific ABA accumulation is needed for sugar signaling during early seedling growth. Most ABA biosynthesis and ABA-insensitive mutants described so far are insensitive to high Glc concentrations. Conversely, ethylene-insensitive mutants, such as etr1, ein2, and ein3, are Glc hypersensitive, whereas the ethylene overproduction mutant eto1 and the constitutive ethylene triple response mutant ctr1 display Glc insensitivity. Furthermore, the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) prevents inhibition of cotyledon greening and expansion at high concentrations of Glc (Zhou et al., 1998). Ethylene may antagonize the Glc response partially through repression of ABA biosynthesis, as suggested by the increased ABA levels present in the ein2 mutant (Ghassemian et al., 2000; Cheng et al., 2002).
Ser/Arg-rich (SR) proteins constitute an important class of essential splicing factors that is highly conserved in higher eukaryotes. The splicing of introns from the precursor-mRNA (pre-mRNA) is carried out by the spliceosome, which consists of five small nuclear ribonucleoproteins and many additional proteins. Members of the SR protein family are non-small nuclear ribonucleoprotein spliceosomal factors that have been shown in animal systems to play vital roles in the most crucial steps of spliceosome assembly (Wu and Maniatis, 1993; Kohtz et al., 1994; Shen and Green, 2004). They share a multidomain structure typically characterized by the presence of one or two N-terminal RNA recognition motifs (RRMs) and a C-terminal reversibly phosphorylated Arg/Ser-rich (RS) domain. Binding of SR proteins to the pre-mRNA is mediated by the RRM, while the RS domain is involved in protein-protein interactions that facilitate recruitment of core splicing machinery components to nearby splice sites (Wu and Maniatis, 1993). Therefore, SR proteins influence splice site selection in a concentration-dependent manner (Graveley, 2000), being pivotal in alternative splicing. Some SR proteins that shuttle between the nucleus and the cytoplasm play additional roles in RNA metabolism, including mRNA export (Huang and Steitz, 2001), stability and quality control (Zhang and Krainer, 2004), as well as translation (Sanford et al., 2004).
Plants possess about twice as many SR proteins as animals. Owing to large interchromosomal duplications, the Arabidopsis genome encodes 19 SR proteins (Kalyna and Barta, 2004; Reddy, 2004; Barta et al., 2008), 11 of which display a unique domain organization not found in any metazoan organism (Barta et al., 2008). Functional studies of three individual Arabidopsis SR protein genes have been reported, ectopic expression of SRp30 (Lopato et al., 1999) and RSZ33 (Kalyna et al., 2003) as well as loss of function of the SR45 protein (Ali et al., 2007; Zhang and Mount, 2009), all revealing pleiotropic morphological and developmental effects as well as altered splicing patterns of other SR protein genes.
SR45 is the sole member of a plant-specific Arabidopsis SR protein subfamily that is highly conserved in the plant kingdom and seems to have appeared later in evolution in flowering plants (Ali et al., 2007). It displays a highly atypical SR protein structure, with a single RRM flanked by two RS domains and has been shown to function as an essential splicing factor in an in vitro heterologous complementation assay (Ali et al., 2007). Its nuclear distribution in speckles is regulated by phosphorylation and transcription (Ali et al., 2003; Ali and Reddy, 2006), and it interacts in vitro with the Arabidopsis SR33, AFC2 kinase, and U1-70 K (Golovkin and Reddy, 1999). The latter protein has been found to interact in vivo with both RS domains of SR45 within nuclear speckles (Ali et al., 2008). Activity of the SR45 promoter appears to be largely confined to actively growing and dividing cells (Zhang and Mount, 2009), and the only Arabidopsis SR protein loss-of-function mutant reported so far, sr45-1, displays general delayed development, including late flowering and slower root growth as well as altered leaf and flower morphology (Ali et al., 2007). One of two alternatively spliced SR45 isoforms was found to complement exclusively the mutant’s flower phenotype, while the other rescues only the root defect (Zhang and Mount, 2009), thus ascribing functional significance to alternative splicing of this Arabidopsis gene.
This study reports phenotypic and molecular analyses of the sr45-1 mutant showing that it is defective in Glc control of cotyledon development, hypocotyl elongation in the dark, and gene expression. Our results indicate that the SR45 protein defines a negative regulator of sugar signaling during early seedling development in Arabidopsis involved in the repression of Glc-induced ABA accumulation and down-regulation of ABA biosynthesis and signaling gene expression.
RESULTS
The sr45-1 Mutant Is Hypersensitive to Glc Inhibition of Early Seedling Development
An SR45 loss-of-function mutant, sr45-1, has been previously reported to display pleiotropic developmental phenotypes, such as delayed flowering and root growth, as well as changes in leaf and flower morphology (Ali et al., 2007). In this study, seed germination and early seedling development of the same T-DNA insertion mutant (SALK_004132) were evaluated.
When sr45-1 mutant seeds were plated on Murashige and Skoog (MS) medium under standard growth conditions, radicle emergence, cotyledon greening, and cotyledon expansion occurred slightly later than with Columbia-0 (Col-0) wild-type seeds; about two additional days were necessary for the mutant to attain full germination and cotyledon development rates, but no differences between mutant and wild-type seedlings were apparent by day 4 after stratification (data not shown). This slight delay in germination and early seedling development was not significantly altered under different abiotic stress conditions, such as high salinity, drought, or cold or heat stress (data not shown).
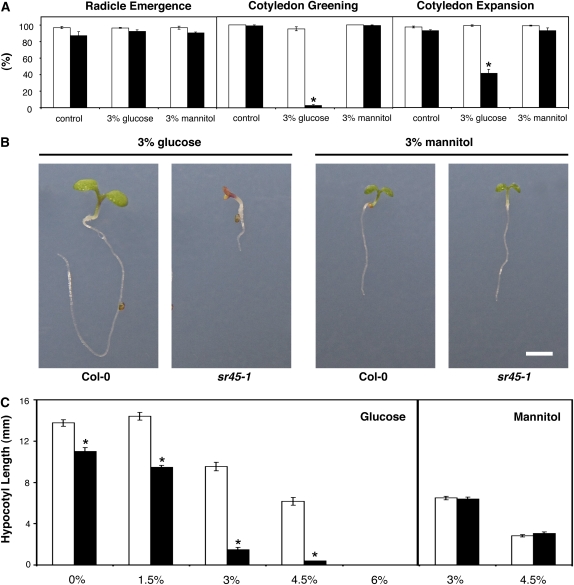
As seen in Figure 1A, when grown on control media for 7 d, Col-0 and sr45-1 seedlings behaved similarly, reaching full seed germination (radicle emergence) and cotyledon development rates. However, although the exogenous supply of 3% Glc had no effect on Col-0 plants or on radicle emergence of sr45-1 seeds, both the greening and expansion of mutant cotyledons were severely affected by the presence of the sugar, with sr45-1 seedlings only being able to green about 5% and expand around 40% of their cotyledons. In contrast to Glc, the effect of mannitol was similar in the wild type and mutant for the three parameters (Fig. 1A). Thus, in the presence of Glc concentrations as low as 3%, unlike Col-0 seedlings, sr45-1 exhibited an arrest in seedling development, displaying small, purple cotyledons that show little expansion (Fig. 1B). Very similar results were obtained in the presence of equimolar concentrations of Suc (data not shown). However, the developmental difference between wild-type and mutant plants was not observed when both genotypes were grown in the presence of equimolar concentrations of mannitol (Fig. 1B), indicating that the growth arrest is sugar specific and not solely due to osmotic stress.
Figure 1.
Glc phenotypes of the sr45-1 mutant. A, Germination (radicle emergence) and early seedling development (cotyledon greening and expansion) rates, scored 7 d after stratification, of Col-0 (white bars) and sr45-1 (black bars) seedlings grown in control conditions or in the presence of 3% Glc or 3% mannitol (means ± se, n = 3). B, Representative images of wild-type (Col-0) and mutant (sr45-1) seedlings grown in the presence of 3% Glc or 3% mannitol for 7 d. Bar = 2 mm. C, Hypocotyl length, measured 6 d after stratification, of Col-0 (white bars) and sr45-1 (black bars) seedlings grown in the dark under different Glc or mannitol concentrations (means ± se, n = 30–50). Asterisks indicate significantly different values (P < 0.01) from the corresponding wild type according to Student’s t test.
The sr45-1 mutation also increases the sensitivity to the Glc inhibitory effects on hypocotyl elongation of dark-grown Arabidopsis seedlings. Consistent with a general delay in development (Ali et al., 2007), sr45-1 seedlings displayed slightly shorter hypocotyls under control conditions, but the Glc-dependent reduction in hypocotyl elongation was strongly enhanced in sr45-1 seedlings, with mutant hypocotyl length being about 20% of that of the wild type in the presence of 3% Glc (Fig. 1C). The sr45-1 mutant exhibited similar hypocotyl length to the wild type in the presence of 3% and 4.5% mannitol (Fig. 1C), again showing that the mutation specifically affects responses to Glc but not to the osmotic stress it induces.
Expression of the SR45 Gene in the sr45-1 Mutant Background Rescues the Glc Phenotype
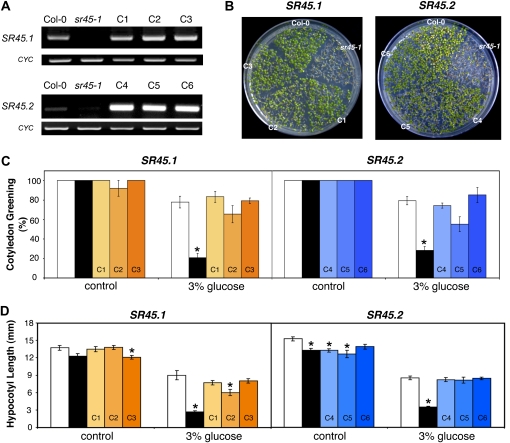
To ensure that the sr45-1 Glc phenotypes are a consequence of a single gene mutation in SR45, the sr45-1 mutant was transformed with the wild-type gene. The coding sequences of both isoform 1 (SR45.1), the longest and most expressed splice variant of SR45, and isoform 2 (SR45.2), which could barely be detected in young Arabidopsis seedlings, were cloned under the control of the 35S promoter and infiltrated into mutant plants. Three independent lines expressing either SR45.1 or SR45.2 in the sr45-1 mutant background (Fig. 2A) were isolated and characterized. Importantly, both the slight delay in early development observed under control conditions (data not shown) and the inhibition of early plant growth in the presence of 3% Glc were reverted in all six transgenic lines (Fig. 2B). Both SR45.1 and SR45.2 complementation lines showed complete restoration of wild-type cotyledon greening (Fig. 2C). Also regarding the Glc-dependent shortening of the hypocotyl, the three SR45.2 and two SR45.1 (C1 and C3) complementation lines allowed total phenotype restoration, while line C2 displayed approximately 70% rescue of the hypocotyl elongation phenotype (Fig. 2D).
Figure 2.
Complementation of the sr45-1 mutant Glc phenotype. A, RT-PCR analysis of the transcript levels of two SR45 isoforms (SR45.1 and SR45.2) in seedlings of the wild type (Col-0), mutant (sr45-1), and six independent complementation lines carrying either the SR45.1 (C1, C2, and C3) or the SR45.2 (C4, C5, and C6) wild-type coding sequence under the control of the 35S promoter (cyclophilin [CYC] was used as a loading control). B, Representative images of Col-0, sr45-1, and C1, C2, and C3 or C4, C5, and C6 seedlings grown in the presence of 3% Glc for 7 d. C, Cotyledon greening rates, scored 7 d after stratification, of Col-0 (white bars), sr45-1 (black bars), and C1, C2, and C3 (orange bars) or C4, C5, and C6 (blue bars) seedlings grown in control conditions or in the presence of 3% Glc (means ± se, n = 3). D, Hypocotyl length, measured 6 d after stratification, of Col-0 (white bars), sr45-1 (black bars), and C1, C2, and C3 (orange bars) or C4, C5, and C6 (blue bars) seedlings grown in the dark in control conditions or in the presence of 3% Glc (means ± se, n = 20–40). Asterisks indicate significantly different values (P < 0.01) from the corresponding wild type according to Student’s t test.
Glc-Responsive Gene Expression Is Altered in the sr45-1 Mutant
In addition to modulating a number of developmental processes, sugars are well known to regulate transcriptionally a wide variety of genes, including those involved in photosynthesis and carbohydrate, nitrogen, and secondary metabolism. Elevated sugar levels have long been known to down-regulate the transcription of both photosynthetic genes and genes associated with nitrogen metabolism, while up-regulating the expression of genes involved in the synthesis of polysaccharides and pigments (Koch, 1996). In order to investigate whether the sr45-1 mutant also displays a Glc hypersensitivity phenotype at the molecular level, expression of several genes regulated by the Glc signaling pathway was analyzed by reverse transcription (RT)-PCR in wild-type and mutant seedlings grown in control conditions or in the presence of 3% Glc or 3% mannitol.
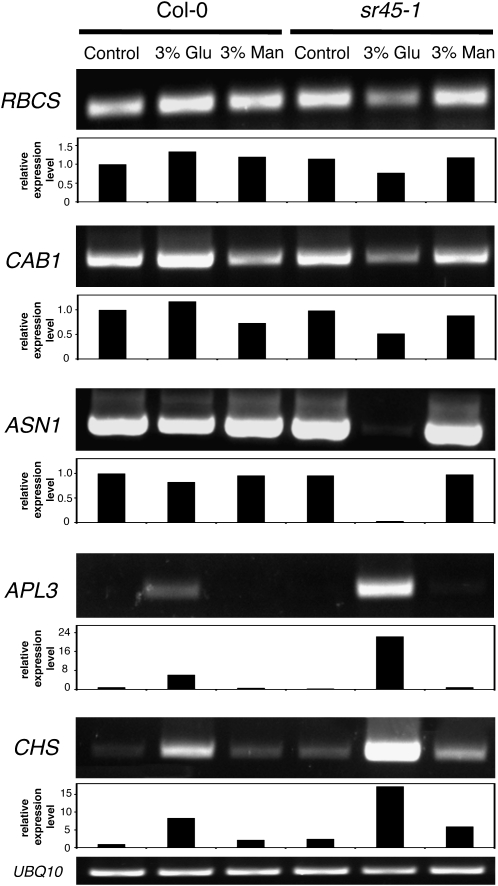
Figure 3 shows that although in Col-0 seedlings 3% Glc was insufficient to repress two photosynthetic genes, RBCS, encoding the small subunit of ribulose-1,5-bisP carboxylase, and CAB1, encoding a chlorophyll a/b-binding protein, both were clearly down-regulated by the same Glc concentration in sr45-1 seedlings. Similarly, 3% Glc exerted only a slight repression on the nitrogen metabolism gene asparagine synthetase1 (ASN1) in wild-type seedlings but strongly repressed its expression in the mutant. Furthermore, APL3, encoding the large subunit of ADP-Glc pyrophosphorylase (AGPase), a key enzyme in starch synthesis, and the anthocyanin biosynthesis gene chalcone synthase (CHS) were clearly overinduced by 3% Glc in sr45-1 (Fig. 3). Exposure to 3% mannitol, used as an osmotic control, did not affect appreciably the expression of any of the analyzed genes (Fig. 3), indicating that the observed alterations in transcript levels are Glc specific.
Figure 3.
Regulation of Glc-responsive gene expression in the sr45-1 mutant. RT-PCR analysis of the transcript levels of RBCS (ribulose-1,5-bisphosphate carboxylase, small subunit), CAB1 (chlorophyll a/b-binding protein1), two ASN1 splice variants, APL3 (ADP-Glc pyrophosphorylase, large subunit), and CHS in wild-type (Col-0) and mutant (sr45-1) seedlings grown in control conditions or in the presence of 3% Glc (Glu) or 3% mannitol (Man). Ubiquitin10 (UBQ10) was used as a loading control. Amplified bands were quantified and relative expression levels determined using UBQ10 as a reference, with the expression of each gene in wild-type Col-0 in control conditions set to one. Results are representative of at least three independent experiments.
The sr45-1 Mutant Is Unaffected in Ethylene Signaling But Displays ABA Hypersensitivity and Enhanced Glc-Induced ABA Accumulation
Previous studies have demonstrated central roles for the phytohormones ethylene and ABA in plant Glc responses (Zhou et al., 1998; Arenas-Huertero et al., 2000; Ghassemian et al., 2000; Cheng et al., 2002). It was therefore of major importance to investigate the effect of the sr45-1 mutation on ethylene and ABA signaling.
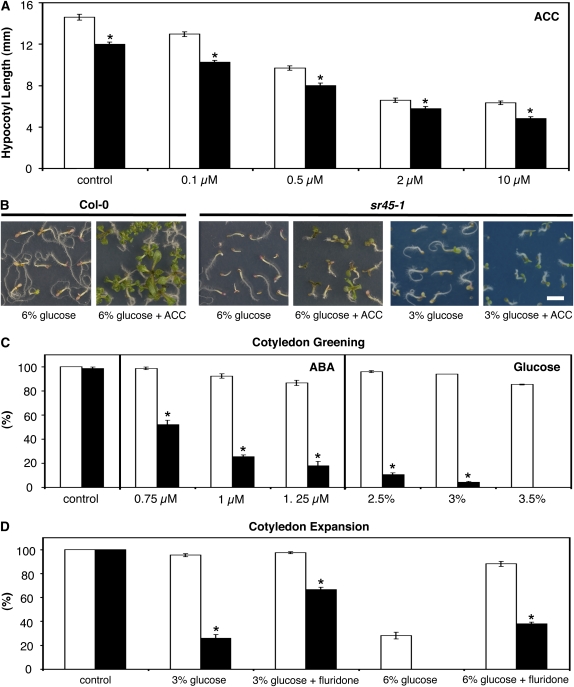
In the presence of ethylene, dark-grown seedlings undergo morphological modifications, referred to as the ethylene triple response, which include shortening of the hypocotyls. In order to determine the response of the sr45-1 mutant to this phytohormone, hypocotyl lengths of seedlings grown in the dark in the presence of increasing concentrations of the ethylene precursor ACC and in the absence of exogenous sugar were measured. As shown in Figure 4A, the sr45-1 mutation did not alter sensitivity to ACC, with hypocotyl elongation of wild-type and mutant seedlings decreasing to a similar extent with the increase in ethylene production. Ethylene is also known to antagonize the Glc response, and the developmental arrest induced by 6% Glc in Col-0 seedlings was prevented by addition of 50 μm ACC to the growth medium (Fig. 4B), as described previously (Zhou et al., 1998). Similarly, although 6% Glc exerted a stronger effect on the development of the hypersensitive sr45-1 mutant, addition of ACC to this high Glc concentration allowed rescue of both cotyledon greening and expansion in sr45-1. This rescue was also observed in 3% Glc, where the mutant’s early development is affected to a similar extent as that of the wild type in 6% Glc (Fig. 4B). Taken together, these results indicate that sr45-1 is unaffected in ethylene signaling.
Figure 4.
Ethylene and ABA phenotypes of the sr45-1 mutant. A, Hypocotyl length, measured 6 d after stratification, of Col-0 (white bars) and sr45-1 (black bars) seedlings grown in the dark in control conditions or in the presence of different concentrations of the ethylene precursor ACC (means ± se, n = 60–80). B, Representative images of Col-0 and sr45-1 seedlings grown in the presence of Glc supplemented or not with 50 μm ACC for 15 d. Bar = 3.3 mm. C, Comparison of cotyledon greening rates, scored 7 d after stratification, of Col-0 (white bars) and sr45-1 (black bars) seedlings grown in control conditions or in the presence of different concentrations of Glc or ABA (means ± se, n = 3). D, Cotyledon expansion rates, scored 7 d after stratification, of Col-0 (white bars) and sr45-1 (black bars) seedlings grown in control conditions or in the presence of 3% or 6% Glc supplemented or not with 1 μm fluridone (means ± se, n = 3). Asterisks indicate significantly different values (P < 0.01) from the corresponding wild type according to Student’s t test.
An ABA sensitivity assay in the absence of exogenous sugar was conducted to investigate whether the Glc phenotype conferred by the sr45-1 mutation is accompanied by altered sensitivity to ABA, as appears to be the case for the vast majority of the identified sugar response mutants. The exogenous application of ABA delayed germination of Col-0 and sr45-1 seeds to a similar extent, with both genotypes reaching full germination rates 7 d after stratification (data not shown). However, exogenous application of ABA affected cotyledon greening to a higher extent in the sr45-1 mutant than in the wild type (Fig. 4C). In 0.75 μm ABA, Col-0 seedlings attained full cotyledon greening rates, whereas the mutant was only able to green about 50% of its cotyledons. Application of higher ABA concentrations (1 and 1.25 μm) resulted in a more pronounced decrease of cotyledon greening rates in sr45-1 (to 20%–25%) when compared with Col-0 seedlings (to approximately 90%). These results show that the sr45-1 mutant is also hypersensitive to the stress hormone ABA. Nonetheless, exogenous application of Glc concentrations exerting a comparable effect to ABA on wild-type seedlings led to a more drastic arrest in the mutant’s early seedling development (Fig. 4C), indicating that the sr45-1 mutation confers higher sensitivity to Glc than to the stress hormone.
Several lines of evidence indicate that Glc-specific ABA accumulation is required for sugar signaling during early seedling growth (Cheng et al., 2002). In agreement with this, wild-type seedlings grown in 6% Glc fail to arrest development in the presence of fluridone, an inhibitor of ABA biosynthesis (Ullah et al., 2002; Lin et al., 2007). We examined the behavior of the sr45-1 mutant in the presence of this inhibitor to investigate whether ABA accumulation is required for the growth arrest in Glc. As fluridone blocks carotenoid synthesis, which leads to an albino-like seedling phenotype (Henson, 1984), only cotyledon expansion rates could be scored. The presence of 3% Glc had no effect on the early development of Col-0 seedlings, and, as expected, the growth arrest observed in 6% Glc was reverted upon the addition of fluridone (Fig. 4D). On the other hand, sr45-1 seedlings expanded 25% to 40% of their cotyledons in the presence of 3% Glc alone (Figs. 1B, 2C, and 4D), but when this medium was supplemented with fluridone, cotyledon expansion rates increased to about 65%. This partial rescue of the sr45-1 phenotype was also seen in 6% Glc, where mutant seedlings were unable to expand their cotyledons altogether, but around 35% expansion rates were scored after supplementation with fluridone (Fig. 4D).
The fact that treatment with fluridone partially rescued the Glc inhibition of cotyledon expansion in the mutant suggests that the growth arrest is operating, at least to some extent, via Glc-induced ABA biosynthesis. To test this hypothesis, we determined ABA endogenous levels in Col-0 and sr45-1 seedlings grown in control media or in the presence of 3% Glc and included the aba2 (Leon-Kloosterziel et al., 1996) and abi4 (Finkelstein, 1994) mutants as controls. Table I shows that there was no significant difference in ABA content between the four genotypes in the absence of sugar. As expected, the ABA-deficient mutant aba2 was unable to respond to the presence of 3% Glc, which was also insufficient to induce a significant increase of ABA endogenous levels in both Col-0 wild-type seedlings and the ABA-insensitive abi4 mutant. However, sr45-1 seedlings responded to the presence of 3% Glc by increasing their ABA content nearly 3-fold, suggesting that the hypersensitivity of the mutant to Glc is due to enhanced endogenous accumulation of ABA.
Table I. Effect of Glc treatment on endogenous ABA levels of wild-type and mutant seedlings.
Quantification of ABA levels (expressed in ng ABA/g fresh weight) in Col-0 and sr45-1 seedlings grown in control conditions or in the presence of 3% Glc (means ± se, n = 6). The ABA-deficient aba2-1 mutant and the ABA-insensitive abi4-101 mutant are shown as controls. Different superscript letters indicate significantly different values (P < 0.05, Student’s t test).
| Control | 3% Glc | |
| Col-0 | 7.28 ± 1.07a | 10.74 ± 1.74a |
| sr45-1 | 6.67 ± 1.75a | 18.18 ± 1.68b |
| aba2-1 | 10.54 ± 1.42a | 9.86 ± 1.48a |
| abi4-101 | 6.94 ± 0.46a | 8.33 ± 0.61a |
Transcription of ABA Biosynthesis and Signaling Genes Is Overinduced by Glc in the sr45-1 Mutant
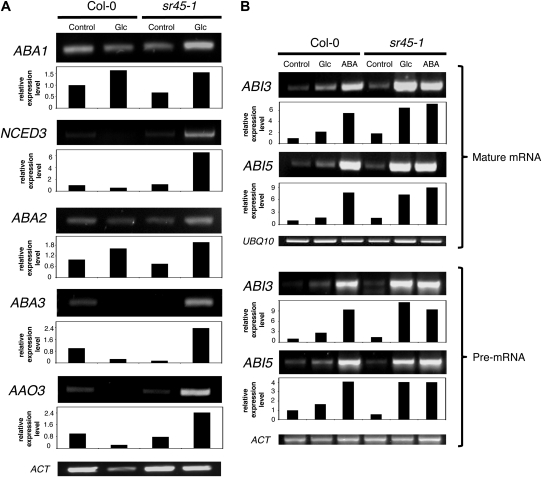
The enhanced ability of the sr45-1 mutant to accumulate ABA in response to Glc prompted us to examine Glc regulation of ABA biosynthesis gene expression in wild-type and mutant seedlings. RT-PCR analysis of the five key genes in the ABA biosynthetic pathway, ABA1, NCED3, ABA2, ABA3, and AAO3, is shown in Figure 5A. The NCED3 transcript was barely detectable in the wild type, and exposure of these seedlings to 3% Glc only resulted in induced expression of ABA1 and ABA2. By contrast, expression of all five genes was clearly induced by Glc in sr45-1, with NCED3, ABA3, and AAO3 being markedly activated in the mutant but not at all in the wild type. This is consistent with a 3-fold increase in the mutant’s ABA content, indicating that the SR45 protein is involved in Glc-mediated repression of ABA biosynthesis gene expression. It has recently been reported that the Glc-induced delay of rice (Oryza sativa) seed germination is mediated by suppression of ABA catabolism rather than enhancement of ABA biosynthesis (Zhu et al., 2009). We therefore also analyzed the expression levels of the four Arabidopsis CYP707A genes, but no significant differences were observed between wild-type and mutant seedlings both under control conditions and in the presence of Glc (data not shown).
Figure 5.
Regulation of ABA biosynthesis and signaling gene expression in the sr45-1 mutant. A, RT-PCR analysis of the transcript levels of ABA biosynthesis genes, ABA1, NCED3, ABA2, ABA3, and AAO3, in wild-type (Col-0) and mutant (sr45-1) seedlings grown in control conditions or in the presence of 3% Glc (Glu). B, RT-PCR analysis of ABI3 and ABI5 transcript levels in Col-0 and sr45-1 seedlings grown in control conditions or in the presence of 3% Glc (Glu) or 1 μm ABA. To detect pre-mRNA, after reverse transcription using random primers, PCR amplification was performed using one primer annealing in an intronic sequence. Ubiquitin10 (UBQ10) and actin (ACT) were used as loading controls. Amplified bands were quantified and relative expression levels determined using UBQ10 or ACT as a reference, with the expression of each gene in wild-type Col-0 in control conditions set to one. Results are representative of at least three independent experiments.
The ABA-induced postgermination arrest depends on functional ABI3 and ABI5 genes, with ABI5 acting downstream of ABI3 to execute this developmental checkpoint (Lopez-Molina et al., 2002). These two key ABA signaling genes encode transcription factors that are activated by both ABA and Glc (Lopez-Molina et al., 2001; Cheng et al., 2002) and also appear to mediate the Glc-induced arrest during early seedling growth (Rolland et al., 2006). We therefore investigated whether transcription of ABI3 and ABI5 would be misregulated in sr45-1 seedlings, which are hypersensitive to Glc and to ABA during early seedling development.
As expected, RT-PCR analysis showed that ABI3 and ABI5 transcript levels are induced by ABA and Glc in wild-type Col-0 seedlings (Fig. 5B). This induction is striking in the presence of 1 μm ABA but also present at the relatively low concentration of 3% Glc. In the sr45-1 mutant, by contrast, induction of the expression of these genes by 3% Glc was much more pronounced, with both transcripts being highly overinduced by Glc but not by ABA. This is consistent with the mutant’s higher sensitivity to Glc and points to involvement of SR45 in transcriptional down-regulation of ABI3 and ABI5 in the presence of the sugar. When pre-mRNA levels were analyzed instead of the mature ABI3 and ABI5 mRNA, very similar results were obtained (Fig. 5B). This strongly suggests that the processing of these transcripts is unaffected and that the changes in steady state levels of the spliced transcripts observed in sr45-1 reflect changes in transcription rates.
The alternative splicing pattern of other SR protein genes has been found to be altered in transgenic lines overexpressing Arabidopsis SRp30 (Lopato et al., 1999) and RSZ33 (Kalyna et al., 2003) as well as in the sr45-1 mutant (Ali et al., 2007). In an attempt to identify additional putative direct target transcripts of the SR45 protein, a candidate gene strategy was employed. About 25 genes predicted to undergo alternative splicing, including those coding for Glc-responsive transcription factors and ABA, ethylene, or stress signaling components (Supplemental Table S1), were selected from the Arabidopsis Information Resource database (http://www.arabidopsis.org) and their splicing patterns analyzed by RT-PCR. Although in a few cases the predicted splice variants were not all detectable by RT-PCR at the early development stage under study (Supplemental Table S1), comparisons between wild-type and mutant seedlings grown under control conditions or in the presence of Glc revealed no apparent changes in alternative splicing for any of the analyzed genes (data not shown).
DISCUSSION
The sr45-1 Arabidopsis mutant displays pleiotropic phenotypes, including late flowering, altered leaf and flower morphology, delayed root growth, and smaller overall plant size when compared to the wild type (Ali et al., 2007). In this report, we show that the sr45-1 mutation also enhances Glc sensitivity during early seedling growth both under light and dark conditions. In the presence of exogenous Glc, the sr45-1 mutant shows a drastic delay in the greening and expansion of cotyledons under long-day photoperiod as well as severe inhibition of hypocotyl elongation in dark-grown seedlings. These altered Glc responses are not due to osmotic stress hypersensitivity and result from loss of function of the SR45 gene, showing that the SR45 protein is a negative regulator of sugar signaling during early seedling development in Arabidopsis.
Alternative splicing generates two SR45 splice variants (Palusa et al., 2007; Zhang and Mount, 2009), with SR45.1 containing a 21-nucleotide sequence that is absent from SR45.2. Zhang and Mount (2009) recently showed that SR45.1 complements the floral but not the root phenotype reported for the sr45-1 mutant (Ali et al., 2007), while SR45.2 is only able to complement the defect in root growth. In the young Arabidopsis seedlings analyzed in our study, SR45.1 was largely the most expressed splice variant, with SR45.2 being barely detectable. However, ectopic expression of either isoform was able to complement the sr45-1 Glc phenotypes, rescuing both the arrest in cotyledon development and the inhibition of hypocotyl elongation in the dark. These results indicate that the alternatively spliced region of SR45 does not play a role in Glc signaling during early seedling growth.
The sr45-1 mutant also displays a Glc phenotype at the molecular level. Consistent with the growth arrest and hypocotyl shortening observed at low Glc concentrations, the sr45-1 mutation confers enhanced Glc-responsive gene expression. High sugar levels typically promote the expression of genes associated with carbohydrate consumption and storage, whereas the expression of genes associated with carbohydrate production, mobilization, and conservation is reduced (Koch, 1996). In the sr45-1 mutant, 3% Glc was sufficient to strongly repress photosynthetic (RBCS and CAB1) and nitrogen metabolism (ASN1) genes, while causing no or little effect in the wild type. Furthermore, starch synthesis (APL3) and anthocyanin biosynthesis (CHS) genes were clearly overinduced in the mutant. Thus, our analyses show that the SR45 protein is involved in the control of sugar-mediated gene activation and repression. While the reduction in photosynthesis-related gene expression is correlated with the HXK1-mediated signaling function (Jang and Sheen, 1994), the effects of Glc on the expression of ASN1, AGPase, and CHS are independent of the HXK1 sensor (Xiao et al., 2000), suggesting that SR45 could be involved in both the HXK1-dependent and the HXK1-independent Glc signaling pathways.
The identification of ABA- and ethylene-related mutants in sugar response screens demonstrates the close interaction between sugar and hormonal control of developmental processes. In particular, a mutually antagonistic relationship has been uncovered between Glc and ethylene signaling during early seedling development (Zhou et al., 1998). However, the enhanced sugar sensitivity of the sr45-1 mutant is not due to ethylene insensitivity, as hypocotyl elongation is equally sensitive to ethylene inhibition in mutant and wild-type seedlings. Moreover, the antagonistic effect of ethylene on the Glc response is still observed in the sr45-1 mutant. Hence, the mode of action of SR45 in sugar signaling appears to be independent of the ethylene signaling pathway.
Nevertheless, the sr45-1 mutation leads to altered ABA signaling, providing further support to extensive crosstalk between the Glc and ABA signal transduction pathways. Although not as striking as the Glc response, mutant seedlings show enhanced sensitivity to the hormone during cotyledon development. Previously characterized ABA hypersensitive mutants, such as enhanced response to ABA1 (era1; Dekkers et al., 2008), era3 (Cheng et al., 2002), and ABA-hypersensitive germination1 (Nishimura et al., 2007), also display Glc oversensitivity. Furthermore, many mutations that lead to altered sugar signaling have previously been found to be allelic to those that affect ABA response or synthesis (Arenas-Huertero et al., 2000; Laby et al., 2000; Rook et al., 2001), suggesting that sugar responses are directly mediated by ABA via the induction of its biosynthesis and activation of ABA signaling genes. Indeed, we have shown that the sr45-1 mutant displays enhanced ABA accumulation in response to Glc, which correlates with a marked induction of ABA biosynthesis gene expression. In addition, rescue of Glc inhibition of cotyledon expansion in 3% Glc with the ABA biosynthesis inhibitor fluridone indicates that the Glc-induced rise in ABA levels is required for the growth arrest observed in the mutant. It is therefore clear that SR45-mediated sugar signaling is operating, at least to a certain extent, via regulation of Glc-specific ABA accumulation.
On the other hand, transcript levels of two key ABA signaling genes, ABI3 and ABI5, are markedly overinduced by Glc in the sr45-1 mutant, suggesting that the growth arrest in 3% Glc is mediated by these two transcription factors whose concerted action leads to an ABA-dependent postgermination arrest (Lopez-Molina et al., 2002). The SR45 protein is therefore involved in the transcriptional repression of ABI3 and ABI5 in the presence of Glc, probably via down-regulation of the levels of ABA, which is known to activate transcription of these genes. Hence, down-regulation of ABI3 and ABI5 expression may underlie the effect of SR45 in counteracting the sugar-mediated growth arrest.
Thus, the role of SR45 in sugar signaling involves down-regulation of the ABA pathway, via both reduction of the sensitivity to the phytohormone and a decrease in its endogenous accumulation. Nonetheless, it appears that ABA only partially accounts for the behavior of sr45-1 in the presence of Glc. In fact, the sugar induced a stronger phenotypical response and higher activation of ABI3 and ABI5 transcription than ABA in the sr45-1 mutant. Moreover, the ABA biosynthesis inhibitor fluridone was unable to fully rescue the mutant’s sugar phenotype, suggesting that Glc-specific ABA biosynthesis may not be the sole factor mediating the observed sr45-1 Glc responses.
The fact that the mutant’s overinduction of ABI3 and ABI5 by Glc is also seen at the pre-mRNA level strongly suggests that the observed variations in gene expression are due to an increase in transcription rates rather than in mRNA stability. As the evolutionarily conserved SR proteins have been mainly implicated in pre-mRNA splicing and postsplicing activities (such as mRNA stability, export, and translation) in various organisms and not in transcriptional control, it is unlikely that ABI3 and ABI5 represent direct molecular targets of the SR45 protein. In fact, as pointed out above, it is probable that these two ABA signaling genes are indirectly activated upon Glc-induced ABA accumulation.
In an attempt to gain further insight into the molecular mechanisms of SR45, we compared the splicing patterns of numerous candidate genes in the mutant and Col-0 backgrounds. However, no appreciable changes in alternative splicing were observed either under control conditions or in the presence of Glc for any of the analyzed genes. On one hand, our candidate gene strategy may have been insufficient in the coverage of putative splicing target genes. On the other hand, it should be noted that the RT-PCR approach employed may fail to detect subtle changes in alternative splicing that could still account for the observed phenotypes. The fact remains, however, that SR protein splicing targets apart from other members of the SR gene family (Lopato et al., 1999; Kalyna et al., 2003; Ali et al., 2007) are still to be identified in plants despite considerable efforts by several groups in recent years. In particular, microarray experiments combined with verification by RT-PCR revealed up-regulation of the flowering repressor FLC in sr45-1, but no distinguishable alteration in the alternative splicing pattern of any flowering gene (Ali et al., 2007). Although SR45 has been shown to possess splicing activity in vitro and affect alternative splicing of five other SR genes (Ali et al., 2007), the possibility that it is also involved in postsplicing activities cannot be excluded. In fact, SR45 is likely to be an ortholog of the human RNPS1 (Zhang and Mount, 2009), a component of the exon-exon junction complex (Le Hir et al., 2001), which has major influence in localization, export, surveillance, and translation of spliced mRNA (Tange et al., 2004). Further molecular and biochemical characterization of the SR45 splicing factor should reveal whether it plays additional roles in posttranscriptional regulation and will help pinpoint the molecular targets underlying its mode of action.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds from Arabidopsis (Arabidopsis thaliana), ecotype Col-0, were surface sterilized and sown in petri dishes containing MS salts (Duchefa Biochemie), 2.5 mm MES (pH 5.7), 0.5 mm myoinositol, and 0.8% agar. After stratification for 3 d in the dark at 4°C (to break dormancy), the seeds were transferred to a growth chamber under 16-h photoperiod (80 μmol m−2 s−1 white light) at 22°C and 60% relative humidity. After 2 to 3 weeks, plants were transferred to soil in individual pots.
Seeds containing a T-DNA insertion in the SR45 (At1g16610) gene (insertion line Salk_004132, derived from the Col-0 ecotype) were obtained from the SALK collection (http://signal.salk.edu) and grown as described above. The exact location of the insertion (exon 7, position 1967 bp) was verified with SR45-specific primers (Supplemental Table S2) and primers annealing at the left border of the T-DNA. Genotyping by PCR allowed the identification of homozygous lines for the insertion. This mutant line was previously isolated by Ali et al. (2007) and named sr45-1.
Growth Assays
Plants of different genotypes were sown and grown simultaneously under identical conditions. Seeds from fully matured siliques of dehydrated plants of the same age were collected and stored in the dark at room temperature. All assays were performed with seeds from comparable lots stored for 2 to 18 months.
For germination assays, seeds were surface sterilized and water imbibed in the dark for 3 d at 4°C. After stratification, 80 to 100 seeds of each genotype were sown in triplicate in petri dishes containing MS medium (MS salts, 2.5 mm MES, pH 5.7, 0.5 mm myoinositol, and 0.8% agar), supplemented or not with the appropriate concentrations of d-Glc, mannitol, ABA (mixed isomers, A1049; Sigma-Aldrich), ACC (MP-Biomedicals), or fluridone (Fluka), before transfer to the growth chamber (16-h photoperiod). Germination (defined as the protrusion of the radicle through the seed coat), cotyledon greening, and cotyledon expansion were scored every day after transfer to the growth chamber, and cotyledon greening and expansion rates were calculated over the total of germinated seeds. Average percentages were calculated with standard errors of the triplicates.
For assessment of hypocotyl elongation, 20 to 50 seeds of each genotype were surface sterilized and stratified as described above, plated in MS plates supplemented or not with the appropriate concentrations of Glc, mannitol, or ACC, and grown vertically in complete darkness at 22°C for 6 d. Etiolated seedlings were illuminated for 12 h before hypocotyl length measurements using ImageJ software (http://rsbweb.nih.gov/ij).
All assays were repeated at least three times with similar results.
Isolation of SR45 cDNA and Complementation Analysis
First-strand SR45 cDNA was obtained from total RNA extracted from 1- to 3-week-old wild-type seedlings using M-MLV reverse transcriptase (Promega) and a poly-T primer. Primers 5′-TGGTTGGCGCGCCATGGCGAAACCAAGTCGTGG-3′ and 5′-ACCCATTAATTAATTAAGTTTTACGAGGTGGAGGTGG-3′, which introduced an AscI and PacI restriction site, respectively, were used for PCR amplification, and the DNA fragments corresponding to the coding sequences of the two SR45 isoforms were cloned into the binary pBA002 vector under the control of the 35S promoter and sequenced. Each of these constructs was then introduced into Agrobacterium tumefaciens for floral dip transformation (Clough and Bent, 1998) of homozygous sr45-1 plants. T1 transformants were selected on BASTA-containing medium, grown to maturity, and selfed. At least two independent transgenic lines were selected from each transformation. All phenotypic analysis was carried out in T3 plants.
RNA Extraction and RT-PCR Analysis
Col-0 and sr45-1 seedlings were grown in MS plates supplemented or not with 3% Glc, 3% mannitol, or 1 μm ABA. Plant material was harvested at day 7 after stratification for Glc-responsive gene expression analysis, at the same developmental stage (approximately 50% cotyledon expansion) for analysis of ABA biosynthesis genes, and 4 d after stratification for analysis of ABI3 and ABI5 expression. For SR45 expression analysis in transgenic complementation lines, 2-week-old plants grown in MS medium were used. Total RNA was extracted from whole seedlings using the innuPREP Plant RNA kit (Analytik Jena BioSolutions) following the protocol provided. All RNA samples were digested with DNAse I (Promega) and phenol-chloroform purified before reverse transcription with M-MLV reverse transcriptase according to the manufacturer’s protocol. SR45, Glc-responsive genes, ABA biosynthesis and signaling genes, and the housekeeping cyclophilin (CYC), ubiquitin10 (UBQ10), and actin (ACT) genes were amplified by PCR with the gene-specific primers shown in Supplemental Table S2. Preliminary PCRs were carried out with different cycles to determine the linear range of amplification. Based on these analyses, 20 to 35 cycles were chosen for DNA detection, depending on the gene (Supplemental Table S2). Amplified DNA fragments were separated in 1% agarose gels. Band intensities were quantified using the NIH IMAGEJ program (http://rsb.info.nih.gov/ij/), normalizing transcript levels to CYC, UBQ10, or ACT and setting the expression of each gene in the wild type under control conditions to one.
To detect pre-mRNA, reverse transcription was carried out using Random Primers (Invitrogen) instead of a poly-T primer, and PCR amplification was performed with one primer annealing at an intronic sequence.
ABA Content Determination
For quantification of ABA content, whole seedlings grown in MS media with or without 3% Glc were collected at the same developmental stage (approximately 50% cotyledon expansion). The plant material (30–60 mg) was grounded in liquid nitrogen and then homogenized in 1 mL of ABA extraction buffer (10 mg L−1 butylated hydroxytoluene, 20 mL L−1 acetic acid, and 90% methanol). Extraction was carried out overnight with constant shaking at 4°C and the supernatant subsequently collected and evaporated to dryness using a speed vac. ABA levels were then quantified with the Phytodetek-ABA kit (AGDIA) using the protocol provided. Mixed ABA isomers (A1049; Sigma-Aldrich) were used as a standard.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. List of genes for which the splicing pattern was compared in Col-0 and sr45-1 seedlings grown in the absence or presence of Glc.
Supplemental Table S2. Sequences of the gene-specific primers used in RT-PCR analyses.
Acknowledgments
We thank Elena Baena-González for many helpful discussions.
References
- Ali GS, Golovkin M, Reddy AS. (2003) Nuclear localization and in vivo dynamics of a plant-specific serine/arginine-rich protein. Plant J 36: 883–893 [DOI] [PubMed] [Google Scholar]
- Ali GS, Palusa SG, Golovkin M, Prasad J, Manley JL, Reddy AS. (2007) Regulation of plant developmental processes by a novel splicing factor. PLoS ONE 2: e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali GS, Prasad KV, Hanumappa M, Reddy AS. (2008) Analyses of in vivo interaction and mobility of two spliceosomal proteins using FRAP and BiFC. PLoS ONE 3: e1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali GS, Reddy AS. (2006) ATP, phosphorylation and transcription regulate the mobility of plant splicing factors. J Cell Sci 119: 3527–3538 [DOI] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P. (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14: 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Barta A, Kalyna M, Lorkovic ZJ. (2008) Plant SR proteins and their functions. Curr Top Microbiol Immunol 326: 83–102 [DOI] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al. (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dekkers BJ, Schuurmans JA, Smeekens SC. (2008) Interaction between sugar and abscisic acid signalling during early seedling development in Arabidopsis. Plant Mol Biol 67: 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein RR. (1994) Mutations at 2 new Arabidopsis ABA response loci are similar to the abi3 mutations. Plant J 5: 765–771 [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12: 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI. (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8: 93–102 [DOI] [PubMed] [Google Scholar]
- Golovkin M, Reddy AS. (1999) An SC35-like protein and a novel serine/arginine-rich protein interact with Arabidopsis U1-70K protein. J Biol Chem 274: 36428–36438 [DOI] [PubMed] [Google Scholar]
- Graveley BR. (2000) Sorting out the complexity of SR protein functions. RNA 6: 1197–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson EA. (1984) Inhibition of abscisic acid accumulation in seedling shoots of pearl millet (Pennisetum americanum (L.) Leeke) following induction of chlorosis by Norflurazon. Z Pflanzenphysiol 114: 35–43 [Google Scholar]
- Huang Y, Steitz JA. (2001) Splicing factors SRp20 and 9G8 promote the nucleocytoplasmic export of mRNA. Mol Cell 7: 899–905 [DOI] [PubMed] [Google Scholar]
- Jang JC, Leon P, Zhou L, Sheen J. (1997) Hexokinase as a sugar sensor in higher plants. Plant Cell 9: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Sheen J. (1994) Sugar sensing in higher plants. Plant Cell 6: 1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyna M, Barta A. (2004) A plethora of plant serine/arginine-rich proteins: redundancy or evolution of novel gene functions? Biochem Soc Trans 32: 561–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyna M, Lopato S, Barta A. (2003) Ectopic expression of atRSZ33 reveals its function in splicing and causes pleiotropic changes in development. Mol Biol Cell 14: 3565–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KE. (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 509–540 [DOI] [PubMed] [Google Scholar]
- Kohtz JD, Jamison SF, Will CL, Zuo P, Luhrmann R, Garcia-Blanco MA, Manley JL. (1994) Protein-protein interactions and 5′-splice-site recognition in mammalian mRNA precursors. Nature 368: 119–124 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI. (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23: 587–596 [DOI] [PubMed] [Google Scholar]
- Le Hir H, Gatfield D, Izaurralde E, Moore MJ. (2001) The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J 20: 4987–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JA, Koornneef M. (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Lin PC, Hwang SG, Endo A, Okamoto M, Koshiba T, Cheng WH. (2007) Ectopic expression of ABSCISIC ACID 2/GLUCOSE INSENSITIVE 1 in Arabidopsis promotes seed dormancy and stress tolerance. Plant Physiol 143: 745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopato S, Kalyna M, Dorner S, Kobayashi R, Krainer AR, Barta A. (1999) atSRp30, one of two SF2/ASF-like proteins from Arabidopsis thaliana, regulates splicing of specific plant genes. Genes Dev 13: 987–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, Chua NH. (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98: 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand S, McLachlin DT, Chait BT, Chua NH. (2002) ABI5 acts downstream of ABI3 to execute an ABA-dependent growth arrest during germination. Plant J 32: 317–328 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. (2007) ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. Plant J 50: 935–949 [DOI] [PubMed] [Google Scholar]
- Palusa SG, Ali GS, Reddy AS. (2007) Alternative splicing of pre-mRNAs of Arabidopsis serine/arginine-rich proteins: regulation by hormones and stresses. Plant J 49: 1091–1107 [DOI] [PubMed] [Google Scholar]
- Reddy AS. (2004) Plant serine/arginine-rich proteins and their role in pre-mRNA splicing. Trends Plant Sci 9: 541–547 [DOI] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J. (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57: 675–709 [DOI] [PubMed] [Google Scholar]
- Rook F, Corke F, Card R, Munz G, Smith C, Bevan MW. (2001) Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J 26: 421–433 [DOI] [PubMed] [Google Scholar]
- Sanford JR, Gray NK, Beckmann K, Caceres JF. (2004) A novel role for shuttling SR proteins in mRNA translation. Genes Dev 18: 755–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Green MR. (2004) A pathway of sequential arginine-serine-rich domain-splicing signal interactions during mammalian spliceosome assembly. Mol Cell 16: 363–373 [DOI] [PubMed] [Google Scholar]
- Tange TO, Nott A, Moore MJ. (2004) The ever-increasing complexities of the exon junction complex. Curr Opin Cell Biol 16: 279–284 [DOI] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM. (2002) Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiol 129: 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Maniatis T. (1993) Specific interactions between proteins implicated in splice site selection and regulated alternative splicing. Cell 75: 1061–1070 [DOI] [PubMed] [Google Scholar]
- Xiao W, Sheen J, Jang JC. (2000) The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol Biol 44: 451–461 [DOI] [PubMed] [Google Scholar]
- Zhang XN, Mount SM. (2009) Two alternatively spliced isoforms of the Arabidopsis SR45 protein have distinct roles during normal plant development. Plant Physiol 150: 1450–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Krainer AR. (2004) Involvement of SR proteins in mRNA surveillance. Mol Cell 16: 597–607 [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J. (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95: 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, Ye N, Zhang J. (2009) Glucose-induced delay of seed germination in rice is mediated by the suppression of ABA catabolism rather than an enhancement of ABA biosynthesis. Plant Cell Physiol 50: 644–651 [DOI] [PubMed] [Google Scholar]