The existence of auxin as a mobile growth regulator was famously inferred by Charles and Frances Darwin, as described in their 1880 book, The Power of Movement in Plants (Darwin and Darwin, 1880). However, auxin was not isolated until much later by Went (1926). Its first ever mention in Plant Physiology was in Went’s obituary, written by George Peirce, which credits Went with having “introduced into plant physiology the general conception of regulators or coordinators” (Peirce, 1936, p. 222), exemplified by the enduring value of the Cholodny-Went hypothesis, which posits that dynamic redistribution of auxin directs tropic growth (Whippo and Hangarter, 2006). Peirce continues, “ The conception of hormones is now common, and some day it may become clear!” (p. 222). Three-quarters of a century later, the picture is still rather hazy, an enticing destination shimmering indistinctly on the horizon, but it is at least in view. Perhaps it is the vanity of every generation to imagine enlightenment is just around the corner. But I think there is no better time to be an auxin biologist.
The past few decades of auxin biology have been primarily occupied with nuts and bolts and other component parts. A formidable cast of players has been assembled: receptors, transporters, synthesizers, inactivators (for recent reviews, see Chapman and Estelle, 2009; Lokerse and Weijers, 2009; Petrasek and Friml, 2009; Zhao, 2010). There are surely still some important components missing, but nonetheless, a noticeable shift in the main focus has now begun, toward understanding how the parts act and interact to “regulate and co-ordinate,” as envisaged by Went (Peirce, 1936, p. 222). How do whole plant-level patterns and behaviors emerge from the actions and interactions of the molecular components of the auxin machinery? Striking and pervasive features of the answer to this question are the extraordinary self-organizing and self-regulating properties of auxin biology, which characterize auxin action at every level (Benjamins and Scheres, 2008; Jaillais and Chory, 2010).
SELF-ORGANIZATION AT THE CELLULAR LEVEL
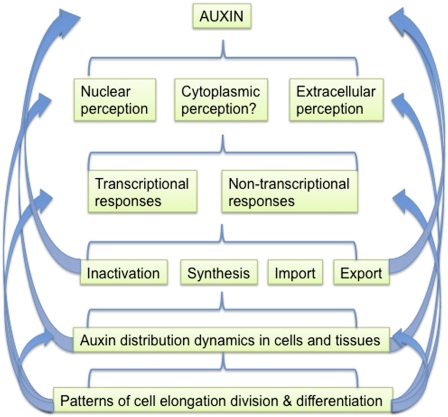
The best understood pathway for auxin signaling involves the targeted degradation of transcriptional repressors of the Auxin/INDOLE-3-ACETIC ACID (Aux/IAA) family (Chapman and Estelle, 2009). Auxin acts as a molecular glue, stabilizing the interaction between the Aux/IAAs and TRANSPORT INHIBITOR RESPONSE1 (TIR1) or closely related proteins of the AUXIN SIGNALING F-BOX PROTEIN (AFB) family (Tan et al., 2007). TIR1 and the AFBs are alternative subunits of SCF-type protein-ubiquitin ligases, targeting ubiquitinylation of the Aux/IAAs, marking them for degradation by the 26S proteosome. The degradation of the Aux/IAAs directly or indirectly changes the transcription of thousands of genes. Direct targets are genes with Auxin Response Elements in their promoters that are bound by the Auxin Response Factor (ARF) family of transcription factors. Aux/IAAs dimerize with ARFs, inhibiting transcription from these promoters, but upon their auxin-induced degradation, ARFs are freed to form ARF-ARF dimers, which in the case of activating ARFs results in transcriptional activation. Prominent among the genes activated by auxin in this way are the Aux/IAA genes, replenishing the repressor pool and reestablishing repression, and GH3 family genes, which encode auxin-inactivating enzymes. Furthermore, it is clear that the Aux/IAA-AFB signaling pathway also down-regulates auxin biosynthesis (Ljung et al., 2001) and up-regulates the expression of some auxin efflux carriers of the PIN-FORMED (PIN) family (Blilou et al., 2005; Heisler et al., 2005; Vieten et al., 2005). These transporters are not only important for auxin export from the cell but also for the directionality of this export (Wisniewska et al., 2006). They can be polarly localized in cells, which among other things is likely to create intracellular cytoplasmic gradients of auxin. The polarity of PIN proteins is regulated by the PINOID protein kinase and related members of the cAMP-dependent protein kinase/protein kinase G/protein kinase C family (Michniewicz et al., 2007). Auxin also up-regulates the transcription of PINOID, providing yet another avenue for feedback regulation in the system. Thus, this pathway is highly self-regulating through multiple feedback pathways (Fig. 1).
Figure 1.
Feedback between the auxin action machinery occurs at multiple levels.
In addition to the Aux/IAA-AFB auxin signaling pathway, which is localized to the nucleus, it is clear that there are additional routes for auxin signaling (Badescu and Napier, 2006). There is strong evidence for extracellular auxin perception, and the detection of intracellular cytoplasmic auxin gradients has also been proposed (Kramer, 2009; Payne and Grierson, 2009). At least some of these additional signaling roles are likely to involve auxin perception by AUXIN-BINDING PROTEIN1 (Napier et al., 2002; Badescu and Napier, 2006).
Likely transcription-independent responses to auxin include stimulation of the plasma membrane proton-pumping ATPase, resulting in apoplastic acidification. This has the potential to change auxin influx by increasing the concentration of protonated auxin in the apoplast, which can enter the cell passively (Petrasek and Friml, 2009). In addition, the active transport of auxin into and out of cells, by influx carriers of the AUX1/LIKE AUX1 family and efflux carriers of the PIN family, could also be affected, since both are likely energized by the plasma membrane electrochemical gradient established by the proton-pumping ATPase. In contrast, a third family of auxin transporters, the ABCBs (for ATP-binding cassette transporters of the B family), are energized directly by ATP hydrolysis, and their activity is therefore less likely to be affected.
An additional activity for auxin that is likely to be transcription independent is the inhibition of endocytosis, which results in the stabilization of PIN proteins at the plasma membrane and hence increased auxin export (Paciorek et al., 2005; Titapiwatanakun and Murphy, 2009). This role for auxin is dependent on BIG (Gil et al., 2001), a large protein, mutations in which have been implicated in the regulation of vesicle trafficking. In Drosophila, mutation in a homologous protein, PUSHOVER, disrupts vesicle trafficking at the neuromuscular junction (Richards et al., 1996), and in mammalian cells, the homologous protein p600 has been shown to interact with clathrin (Nakatani et al., 2005).
While the regulation of PIN endocytosis, and the ability of auxin to modulate it, are generally thought to be clathrin and actin mediated (Dhonukshe et al., 2007), interestingly, p600 has also been implicated in interactions with the microtubules and has been proposed to play a role in the activation of Rho GTPases (Nakatani et al., 2005). Several lines of evidence support a role for auxin in regulating the activation kinetics of Rho of Plants (ROP) GTPases, which are important regulators of vesicle trafficking and cytoskeleton coordination (Payne and Grierson, 2009; Hazak et al., 2010). This, in turn, can influence PIN protein localization. For example, the INTERACTOR OF CONSTITUTIVE ACTIVE ROP (ICR1) protein is a ROP-interacting protein that is required for targeted PIN exocytosis. Auxin affects the subcellular localization of ICR1 as well as ICR1 expression, which consequently modulates PIN localization (Hazak et al., 2010).
Thus, the outputs of all known and proposed auxin signaling pathways include local cellular feedback on auxin levels, mediated by effects on diverse targets in the auxin synthesis, inactivation, and transport machinery (Fig. 1). In addition to these reasonably direct and rapid feedback effects, auxin also influences cell division, elongation, and differentiation, all of which can, in turn, affect the auxin machinery. For example, auxin-induced elongation could change the slope of an intracellular auxin gradient and is also likely to have major impacts on the cytoskeleton, which is remodeled in response to physical stresses in the cell resulting from elongation (Hamant et al., 2008). Furthermore, a triggered differentiation event could lead to a different polarity for auxin transporters or a reprogramming of the cell’s Aux/IAA and ARF contents (Blilou et al., 2005; Lokerse and Weijers, 2009).
In this context, it is interesting to consider those elements of the system that seem less heavily enmeshed in the auxin feedback machinery. For example, members of the ARF family that repress transcription can influence auxin response without themselves being auxin regulated (Vert et al., 2008). It is also interesting that the ABCB auxin exporters are responsible for a substantial proportion of auxin export: they are much less deeply embedded in the auxin machinery than many of its other components (Benjamins and Scheres, 2008; Titapiwatanakun and Murphy, 2009). These components may act either as stable central parts of the system or as important input nodes for major system reconfiguration.
SELF-ORGANIZATION AT THE TISSUE LEVEL
The multiple auxin feedback systems operating in each cell can be coupled together at the tissue level to generate the elegant, dynamic, environmentally responsive self-organizing patterning systems that characterize plant development. These systems successfully combine plasticity, involving exquisite sensitivity to the environment, with robustness, which in some long-lived tree species supports the continued initiation of new organs over thousands of years.
Particularly well-studied examples include the root apical meristem and shoot apical meristem (SAM; Benjamins and Scheres, 2008; Vernoux et al., 2010). Both these systems are characterized by dynamic interactions between auxin and its transport network. Furthermore, in both cases, auxin induces changes in the nature of these interactions. This can be the result of a direct link between the auxin concentration and the rules for auxin-regulated auxin transport, or the link may be less direct, being mediated by auxin-induced cellular reprogramming (i.e. differentiation). For example, at the SAM, auxin distribution and redistribution patterns the formation of successive leaves with regular spacing (i.e. phyllotaxis). A combination of computational and wet lab experiments suggests that this patterning is achieved by a system in which PIN allocation in the epidermal cell layer is regulated by localizing PIN within each cell to the face abutting the neighboring cell that has the most auxin (Jönsson et al., 2006; Smith et al., 2006). This results in the transport of auxin in the meristem epidermis “up the gradient” and the consequent formation of a local auxin maximum with high PIN levels and high PIN polarization toward the auxin maximum. Leaf formation is triggered by the auxin maximum, which is accompanied by a dramatic change in PIN localization, such that PINs accumulate in the subepidermal layers of the meristem, oriented in the direction of auxin flux, toward the existing vascular strands in the stem below. The mechanism underlying the shift between these contrasting rules for PIN positioning is not known. One proposal is that the switch emerges from a combination of a direct effect of auxin concentration, with higher auxin concentrations favoring “with the flux” PIN allocation over up the gradient allocation, and different abundances of transport components in subepidermal versus epidermal tissues (Bayer et al., 2009).
Whatever its mechanistic basis, the ability of patterns such as phyllotaxis to self-organize is heavily dependent on the multiple feedback loops in the auxin machinery, coupled with transitions that change the feedback relationships as patterning proceeds, like shifting gears in a car.
SELF-ORGANIZATION AT THE WHOLE PLANT LEVEL
Zooming out further, the principles of self-organization can be seen at work at the whole plant level. One good example is in the regulation of shoot branching. Here, the ability of auxin to act in positive feedback loops to up-regulate and polarize its own transport can act as a long-range communication network, linking all the SAMs in a shoot system through their requirement to access common auxin transport pathways to the root.
This positive feedback in auxin transport is the basis for the auxin transport canalization hypothesis, which was proposed by Sachs (1968) to explain a range of phenomena in which the positioning of auxin sources and auxin sinks was observed to pattern the vascular connectivity between them. At the heart of the hypothesis is the idea that an initial low flux of auxin from a source to a sink can up-regulate and polarize auxin transport. Consistent with this idea, it is possible to observe the gradual polarization of PIN transporters in adjacent cells, forming files of cells transporting auxin from sources to sinks (Sauer et al., 2006). Furthermore, there is good evidence that such cell files define the path of vascular strand formation. Although the mechanism underlying this process is not understood, there is ample opportunity for such a system driven by combinations of the numerous regulatory interactions listed above.
Also as described above, this flux-driven polarization of PIN proteins is a critical part of the phyllotactic patterning process. For leaf initiation to proceed at the SAM, it is likely that each successive leaf must be linked into the existing vasculature in the stem in a process initiated by auxin transport canalization (Bayer et al., 2009). It is reasonable, therefore, to postulate that SAM activity depends on efficient auxin transport away from the SAM, which requires that the existing vascular-associated auxin-transporting cell files act as sinks for auxin, which in turn requires that they are efficiently transporting auxin away down the plant to the root. In this way, all active SAMs export auxin down the plant in the so-called polar auxin transport stream (PATS), ultimately through the base of the main stem and down into the root. Through the connectivity of this vascular-associated auxin transport network, every SAM in the shoot system is connected to every other one. SAMs that are exporting auxin are active, producing leaves. Those that cannot export auxin are inactive. Since the active SAMs are contributing auxin to a common PATS, they will influence its sink strength and thus the ability of further auxin sources, namely inactive SAMS, to establish auxin transport into this network, and hence to activate.
This mechanism can explain the well-documented phenomena of apical dominance and correlative inhibition, in which auxin from active apices in a shoot system can inhibit the activity of others, whether above or below them, indirectly without entering the inhibited buds (Prusinkiewicz et al., 2009). Strong evidence in support of this whole plant-level feedback-regulated network comes from the physiology of the newly identified hormone, strigolactone (Gomez-Roldan et al., 2008; Umehara et al., 2008). There is mounting evidence that strigolactone acts at least in part by modulating the competition between SAMs for access to common auxin transport routes of the PATS (Crawford et al., 2010; Liang et al., 2010).
Strigolactones are required for the auxin-mediated inhibition of shoot branching, with mutants in strigolactone biosynthesis having auxin-resistant axillary buds (for review, see Leyser, 2009). It has been proposed that strigolactones act as second messengers for auxin (Brewer et al., 2009). Consistent with this idea, auxin up-regulates the transcription of strigolactone biosynthetic genes, which could result in increased strigolactone synthesis. It is known that strigolactones are transported up the plant and that their direct application to buds can inhibit them. Together, this suggests that auxin moving in the main stem promotes strigolactone synthesis, and this strigolactone moves into the axillary buds, where it acts locally to inhibit their growth. However, strigolactones are ineffective at inhibiting the growth of isolated buds (Crawford et al., 2010; Liang et al., 2010) and have only been shown to do so in the presence of other active buds or exogenously supplied auxin. Particularly striking in this regard is the fact that when a stem segment explant carrying two buds is treated with strigolactone, it is not the case that both buds are inhibited; rather, growth is focused into a single bud. This, coupled with the demonstrated systemic auxin transport phenotypes in strigolactone mutants, suggests that strigolactones are not really inhibitors of shoot branching at all but rather modulators of auxin transport canalization and thereby regulate the competition between shoot branches for activation (Prusinkiewicz et al., 2009; Crawford et al., 2010; Liang et al., 2010). This gives them the interesting property of regulating the total number of active branches in a shoot, without dictating which branches will be active. This can be determined by other local factors, such as the local light environment (Sachs et al., 1993). This mechanism of action is entirely consistent with their likely role as a root-derived signal for low nutrient availability (Umehara et al., 2010).
Here again, plasticity and robustness in shoot system architecture are conferred by a combination of the autoregulatory self-organizing properties of the auxin machinery and the ability to change the gearing between them. In this example, the gearing is changed by an external input, namely nutrient availability, mediated by a second hormone, strigolactone. This system is part of a wider hormonal network that also involves cytokinin and illustrates the central role of the auxin machinery in coordinating the activities of diverse hormonal and environmental inputs (Leyser, 2009; Jaillais and Chory, 2010).
CONCLUSION
The power of auxin in plants is clearly wide and deep. To understand it, we need to find the missing component parts of the auxin machinery. However, recent successes have illustrated the importance of developing new tools to allow us to watch and measure the auxin machinery in action, including noninvasive probes for its various parts, coupled with live imaging (Jaillais and Chory, 2010). Furthermore we need to understand the ways in which dynamic regulatory circuitry with multiple interlocking feedback loops can function to deliver important plant properties. Many of the most interesting phenomena of plant biology, such as plasticity and robustness, seem likely to be encoded not in single genes or pathways but in the quantitative relationships between different parts of the system: their gearing. To understand the nature of these properties and how they are regulated, we need computational modeling (Jaillais and Chory, 2010). It is these approaches that are likely to be the most illuminating for those following in the footsteps of Went, “reflecting on the phenomena of growth and wondering about its cause and control” (Peirce, 1936, p. 222).
Acknowledgments
O.L.'s research is supported by the Gatsby Foundation and the Biotechnology and Biological Sciences Research Council of the United Kingdom.
References
- Badescu GO, Napier RM. (2006) Auxin: will it all end in TIRs. Trends Plant Sci 11: 217–223 [DOI] [PubMed] [Google Scholar]
- Bayer E, Smith R, Mandel T, Nakayama N, Sauer M, Prusinkiewicz P, Kuhlemeier C. (2009) Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev 23: 373–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Scheres B. (2008) Auxin: the looping star of plant development. Annu Rev Plant Biol 59: 443–465 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Brewer P, Dun E, Ferguson B, Rameau C, Beveridge C. (2009) Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol 150: 482–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman EJ, Estelle ME. (2009) Mechanism of auxin-regulated gene expression in plants. Annu Rev Genet 34: 265–285 [DOI] [PubMed] [Google Scholar]
- Crawford S, Shinohara N, Sieberer T, Williamson L, George G, Hepworth J, Müller D, Domagalska MA, Leyser O. (2010) Strigolactones enhance competition between shoot branches by dampening auxin transport. Development 137: 2905–2913 [DOI] [PubMed] [Google Scholar]
- Darwin C, Darwin F. (1880) The Power of Movement in Plants. John Murray, London [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. (2007) Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 17: 520–527 [DOI] [PubMed] [Google Scholar]
- Gil P, Dewey E, Friml J, Zhao Y, Snowden KC, Putterill J, Palme K, Estelle M, Chory J. (2001) BIG: a calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, et al. (2008) Strigolactone inhibition of shoot branching. Nature 455: 189–194 [DOI] [PubMed] [Google Scholar]
- Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P, Coroson F, Sahlin P, Boudaoud A, Meyerowitz EM, et al. (2008) Developmental patterning by mechanical signals in Arabidopsis. Nature 322: 1650–1655 [DOI] [PubMed] [Google Scholar]
- Hazak O, Bloch D, Poraty L, Sternberg H, Zhang J, Friml J, Yalovsky S. (2010) A Rho scaffold integrates the secretory system with feedback mechanisms in regulation of auxin distribution. PLoS Biol 8: e1000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM. (2005) Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 15: 1899–1911 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Chory J. (2010) Unraveling the paradoxes of plant hormone signal integration. Nat Struct Mol Biol 17: 642–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. (2006) An auxin-driven polarized transport model for phyllotaxis. Proc Natl Acad Sci USA 103: 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer EM. (2009) Auxin-regulated cell polarity: an inside job? Trends Plant Sci 14: 242–247 [DOI] [PubMed] [Google Scholar]
- Leyser O. (2009) The control of shoot branching: an example of plant information processing. Plant Cell Environ 32: 694–703 [DOI] [PubMed] [Google Scholar]
- Liang J, Zhao L, Challis R, Leyser O. (2010) Strigolactone regulation of shoot branching in chrysanthemum (Dendranthema grandiflorum). J Exp Bot 61: 3069–3078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokerse A, Weijers D. (2009) Auxin enters the matrix: assembly of response machineries for specific outputs. Curr Opin Plant Biol 12: 520–526 [DOI] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. (2001) Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. Plant J 28: 465–474 [DOI] [PubMed] [Google Scholar]
- Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F, et al. (2007) Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130: 1044–1056 [DOI] [PubMed] [Google Scholar]
- Nakatani Y, Konishi H, Vassilev A, Kurooka H, Ishiguro K, Sawada JI, Ikura T, Kormeyer SJ, Qin J, Herlitz AM. (2005) p600, a unique protein required for membrane morphogenesis and cell survival. Proc Natl Acad Sci USA 102: 15093–15098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier RM, David KD, Perrot-Rechenmann C. (2002) A short history of auxin-binding proteins. Plant Mol Biol 49: 339–348 [PubMed] [Google Scholar]
- Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof YD, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, et al. (2005) Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435: 1251–1256 [DOI] [PubMed] [Google Scholar]
- Payne RJH, Grierson CS. (2009) A theoretical model for ROP localization by auxin in Arabidopsis root hair cells. PLoS ONE 4: e8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce GJ. (1936) F. A. F. C. Went. Plant Physiol 11: 219–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrasek J, Friml J. (2009) Auxin transport routes in plant development. Development 136: 2675–2688 [DOI] [PubMed] [Google Scholar]
- Prusinkiewicz P, Crawford C, Smith R, Ljung K, Bennett T, Ongaro V, Leyser O. (2009) Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA 106: 17431–17436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Hillman T, Stern M. (1996) Mutations in the Drosophila pushover gene confer increased neuronal excitability and spontaneous synaptic vesicle fusion. Genetics 142: 1215–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs T. (1968) On the determination of the pattern of vascular tissue in peas. Ann Bot (Lond) 32: 781–790 [Google Scholar]
- Sachs T, Novoplansky A, Cohen D. (1993) Plants as competing populations of redundant organs. Plant Cell Environ 16: 765–770 [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinohl V, Friml J, Benkova E. (2006) Canalization of auxin flow by Aux/IAA-ARF-dependent feedback regulation of PIN polarity. Genes Dev 20: 2902–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS, Guyomarc’h S, Mandel T, Reinhardt D, Kuhlemeier C, Prusinkiewicz P. (2006) A plausible model of phyllotaxis. Proc Natl Acad Sci USA 103: 1301–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, Zheng CX, Robinson CV, Estelle M, Zheng N. (2007) Mechanism of perception by the TIR1 ubiquitin ligase. Nature 446: 640–645 [DOI] [PubMed] [Google Scholar]
- Titapiwatanakun B, Murphy AS. (2009) Post-transcriptional regulation of auxin transport proteins: cellular trafficking, protein phosphorylation, protein maturation, ubiquitination and membrane composition. J Exp Bot 60: 1093–1107 [DOI] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Magome H, Takeda-Kamiya N, Yamaguchi S. (2010) Contribution of strigolactones to the inhibition of tiller bud outgrowth under phosphate deficiency in rice. Plant Cell Physiol 51: 1118–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umehara M, Hanada A, Yoshida S, Akiyama K, Arite T, Takeda-Kamiya N, Magome H, Kamiya Y, Shirasu K, Yoneyama K, et al. (2008) Inhibition of shoot branching by new terpenoid plant hormones. Nature 455: 195–200 [DOI] [PubMed] [Google Scholar]
- Vernoux T, Besnard F, Traas J. (2010) Auxin at the shoot apical meristem. Cold Spring Harb Perspect Biol 2: a001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vert G, Walcher CL, Chory J, Nemhauser JL. (2008) Integration of auxin and brassinosteroid pathways by Auxin Response Factor 2. Proc Natl Acad Sci USA 105: 9829–9834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J. (2005) Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132: 4521–4531 [DOI] [PubMed] [Google Scholar]
- Went FW. (1926) On growth-accelerating substances in the coleoptile of Avena sativa. Proc Kon Ned Akad Wet 30: 10–19 [Google Scholar]
- Whippo CW, Hangarter RP. (2006) Phototropism: bending towards enlightenment. Plant Cell 18: 1110–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewska J, Xu J, Seifertová D, Brewer PB, Ruzicka K, Blilou I, Rouquié D, Benková E, Scheres B, Friml J. (2006) Polar PIN localization directs auxin flow in plants. Science 312: 883. [DOI] [PubMed] [Google Scholar]
- Zhao Y. (2010) Auxin biosynthesis and its role in plant development. Annu Rev Plant Biol 61: 49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]