Abstract
Recombinase-mediated DNA cassette exchange (RMCE) has been successfully used to insert transgenes at previously characterized genomic sites in plants. Following the same strategy, groups of transgenes can be stacked to the same site through multiple rounds of RMCE. A gene-silencing cassette, designed to simultaneously silence soybean (Glycine max) genes fatty acid ω-6 desaturase 2 (FAD2) and acyl-acyl carrier protein thioesterase 2 (FATB) to improve oleic acid content, was first inserted by RMCE at a precharacterized genomic site in soybean. Selected transgenic events were subsequently retransformed with the second DNA construct containing a Yarrowia lipolytica diacylglycerol acyltransferase gene (DGAT1) to increase oil content by the enhancement of triacylglycerol biosynthesis and three other genes, a Corynebacterium glutamicum dihydrodipicolinate synthetase gene (DHPS), a barley (Hordeum vulgare) high-lysine protein gene (BHL8), and a truncated soybean cysteine synthase gene (CGS), to improve the contents of the essential amino acids lysine and methionine. Molecular characterization confirmed that the second RMCE successfully stacked the four overexpression cassettes to the previously integrated FAD2-FATB gene-silencing cassette. Phenotypic analyses indicated that all the transgenes expressed expected phenotypes.
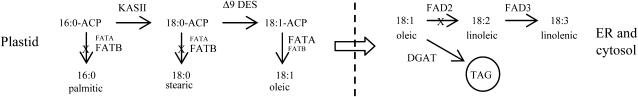
Many vegetable oils for human consumption are rich in 18-carbon ω-6 fatty acids, which, if excessively consumed, can lead to the depletion of ω-3 fatty acids in human body tissues, with numerous negative health consequences. Edible vegetable oils are also often hydrogenated to improve oxidative stability, maintain flavor, and provide necessary solid fat functionality. But hydrogenation leads to the formation of trans-unsaturated fatty acids, which have been linked to cardiovascular diseases. Healthy alternatives are oils rich in oleic acid, 18:1, that can be obtained from mutant oilseed plants with defective ω-6 desaturase (FAD2) genes. FAD2 is directly responsible for the desaturation of 18:1 to linoleic acid, 18:2 (Fig. 1). Many plants have several FAD2 genes contributing to seed 18:2 content that need to be simultaneously mutated in order to get high enough levels of 18:1 (Heppard et al., 1996; Lightner et al., 2006). However, the fatty acid contents of nonseed organs can also be affected by the FAD2 mutations, causing agronomic problems (for review, see Damude and Kinney, 2007, 2008a, 2008b).
Figure 1.
Alteration of fatty acid biosynthesis for high oleic acid and high oil production. Two genes, FATB and FAD2, are silenced, leading to the increase of 18:1 and decrease of saturated fatty acids 16:0 and 18:0. The DGAT gene encoding a key enzyme for fatty acid accumulation in oil bodies is overexpressed, leading to increased oil. ACP, Acyl carrier protein; KASII, β-ketoacyl-ACP synthase II; Δ9 DES, Δ-9 desaturase; FATA, acyl-acyl carrier protein thioesterase 1; FATB, acyl-acyl carrier protein thioesterase 2; FAD2, ω-6 desaturase; FAD3, ω-3 desaturase; DGAT, diacylglycerol acyltransferase, TAG, triacylglycerol; ER, endoplasmic reticulum. FATA or FATB in smaller font indicates a minor role for the step.
A transgenic approach that is able to simultaneously knock out several FAD2 genes in seeds only can overcome the problems associated with the FAD2 mutants mentioned above. Furthermore, additional genes involved in fatty acid biosynthesis can be simultaneously targeted (Fig. 1). The acyl-acyl carrier protein thioesterase 2 (FATB) gene, primarily responsible for the accumulation of the saturated fatty acids palmitic acid, 16:0, and stearic acid, 18:0, can be knocked out to reduce saturated fatty acids and increase 18:1 (Hitz, 2001). The diacylglycerol acyltransferase (DGAT) gene responsible for transferring a fatty acyl group from acyl-CoA to a diacylglycerol substrate to form triacylglycerol can be overexpressed to increase the overall oil content (Cahoon et al., 2007; Meyer et al., 2008). The advantage of a transgenic approach is that several genes in the fatty acid biosynthesis pathway can be simultaneously manipulated through gene silencing or overexpression using one or a few DNA constructs (Wu et al., 2005; Kinney, 2006).
Another nutritional trait of crops is the content of essential amino acids such as Lys and Trp, which are often low in cereals, and Met, Cys, and Thr, which are often low in legumes (Hesse et al., 2001; Sun and Liu, 2004; Galili et al., 2005). Both Lys and Met, including Cys and Thr intermediates, are synthesized through the Asp family biosynthesis pathway by two branches, the Lys branch and the Thr-Met branch, which compete for some common substrates. Complex feedback controls on key enzymes in the pathway, such as dihydrodipicolinate synthase (DHPS) and cystathionine γ-synthase (CGS), maintain a dynamic balance of the amino acids levels (Chiba et al., 1999; Falco et al., 1999; Falco, 2006). The overexpression of a feedback-insensitive DHPS gene alone, or combined with the knockout of Lys catabolism key enzymes Lys ketoglutarate reductase/saccharopine dehydrogenase and the overexpression of a feedback-insensitive CGS gene, can dramatically increase the levels of free Lys or both Lys and Met (Zhu and Galili, 2003, 2004; Hacham et al., 2007; Thu et al., 2007). But the increased levels of free amino acids may not necessarily be stored unless enough sink, such as in the barley (Hordeum vulgare) high Lys (BHL8), a small storage protein engineered to contain high levels of Lys and Met, is provided to capture the increased free amino acids in seeds (Roesler and Rao, 2000).
It is a great challenge through traditional plant transformation such as Agrobacterium tumefaciens infection and biolistic bombardment to place the three oil genes FAD2, FATB, and a Yarrowia DGAT1, the three amino acid genes DHPS, CGS, and BHL8, and a selection marker gene, ALS, at a single locus in a way that all of them can be properly expressed. To integrate the seven genes at a precharacterized soybean (Glycine max) genomic site, we employed the recombinase-mediated cassette exchange (RMCE) technology using the yeast FLP/FRT recombination system (Li et al., 2009). Other recombination systems, such as the bacteriophage Cre/Lox and yeast R/RS, which have been successfully used in RMCE, can also be explored for gene stacking (Ow, 2002; Nanto et al., 2005; Louwerse et al., 2007).
Two rounds of site-specific integration (SSI) transformation were done to stack the seven genes. First, a donor DNA designed to silence both the FAD2 and FATB genes was integrated in a precharacterized target site by RMCE involving FRT1 and FRT87 sites. A third FRT site, FRT12, was simultaneously introduced between the FRT1 and FRT87 sites. Selected RMCE events with desired fatty acid profiles were retransformed with the second donor DNA containing DGAT1, DHPS, BHL8, and CGS genes flanked by FRT1 and FRT12 sites. A RMCE event with expected phenotypes for all the transgenes was obtained and confirmed to have the seven genes precisely stacked at the genomic site.
RESULTS
DNA Construction and SSI Transformation
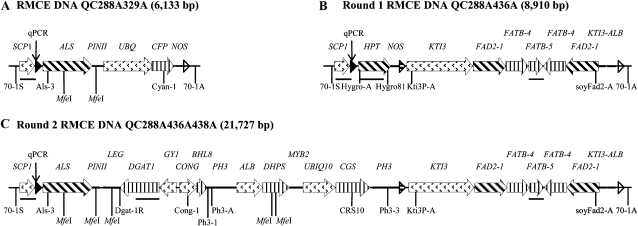
Two rounds of SSI transformation were done to stack seven transgenes, including the selection gene ALS, the three genes BHL8, DHPS, and CGS for improving essential amino acids, and the three genes DGAT1, FAD2, and FATB for high oil, high 18:1, and low 16:0 and 18:0 (Fig. 2C). While FAD2 and FATB were constructed in one cassette for gene silencing, the others were constructed as separate cassettes for overexpression with different promoters. Any transgene cassette that contains two incompatible FRT sites can be used as a target for FLP-mediated RMCE to have the intervening DNA replaced by the DNA between two corresponding FRT sites of a donor DNA construct. The QC288A329A and QC288A436A transgenes were used as targets for the two rounds of SSI transformation in which the selectable marker genes ALS and HPT were used alternately for transgenic event selection (Fig. 2, A and B).
Figure 2.
Schematics of DNA transgenes. A, The first target DNA, QC288A329A, contains the constitutive promoter SCP1 driving a mutant ALS gene. A FRT1 site (black triangle) is placed between the SCP1 promoter and the ALS gene, and a FRT87 site (white triangle) is placed at the 3′ end. B, Predicted first RMCE DNA, QC288A436A, from the transformation of QC288A329A target with the first donor, QC436. All the components between the FRT1 and FRT87 sites of QC288A329A are replaced by the components between the FRT1 and FRT87 sites of QC436. The third recombination site, FRT12 (black and white triangle), is introduced. C, Predicted second RMCE DNA, QC288A436A438A, from the retransformation of the first RMCE DNA, QC288A436A, now used as the second target, with the second donor QC438. The components between the FRT1 and FRT12 sites of QC288A436A are replaced by the components between the FRT1 and FRT12 sites of QC438. Relative positions of qPCR assays (vertical arrows), PCR primers, and MfeI-cleavage sites are marked. Black bars represent Southern hybridization probes specific to SCP1, HPT, FATB-2, and DGAT1. FLP QC292 and predicted excision DNA QC288ME, which lost all the components between the FRT1 and FRT87 sites, were described previously (Li et al., 2009).
The first round of SSI transformation was done on embryogenic callus derived from the homozygous progeny of reported RMCE plants B5-1 and B5-2 containing the QC288A329A transgenes (Li et al., 2009). Since QC288A329A contains the ALS gene, the first donor DNA construct, QC436, consisting of all the components between the FRT1 and FRT87 sites of QC288A436A, has to contain a different selectable marker gene, HPT (Fig. 2, A and B). The HPT gene and the FAD2-FATB gene-silencing cassette replaced the ALS and CFP genes of QC288A329A during the first round of RMCE to form QC288A436A. The FRT12 site was simultaneously introduced between the FRT1 and FRT87 sites. The bombardment of 11 plates of B5 embryogenic tissues with the donor QC436 and FLP QC292 DNA produced 37 hygromycin-resistant events, and 23 of them were identified to be SSI positive. So the first-round SSI transformation frequency is approximately two SSI events per bombarded plate. Four selected RMCE events, B51, B52, B53, and B54, were proliferated and used directly as the targets for the second round of SSI transformation. B52 retransformation events were subsequently abandoned, since B52 somatic embryos did not display the expected fatty acid phenotype.
The second donor DNA construct QC438 contains all the components between the FRT1 and FRT12 sites of QC288A436A438A (Fig. 2C). The ALS selectable marker gene and the FRT1 and FRT12 sites are required for RMCE to stack all the genes onto the QC288A436A target. The second round of SSI transformation was expected to be challenging for several reasons: (1) it required the embryogenic callus of newly identified transgenic events to be directly retransformed; (2) the performance of the four first-round RMCE events as targets for another round of transformation was unknown; (3) the QC438 donor DNA is larger than any previously transformed DNA; and (4) the complication of three FRT sites on both the QC288A436A target and the QC438 donor DNA had never been evaluated. There were 32 chlorsulfuron-resistant events produced, but most of them were determined to be SSI negative. Only one event, B531, was confirmed to be a gene-stacking RMCE event containing the complete QC288A436A438A transgenes (Fig. 2C).
Characterization of the First-Round RMCE Events
Somatic embryo samples of all 37 hygromycin-resistant events produced by the first SSI transformation were analyzed by four quantitative (q)PCR assays. The target qPCR is specific to the FRT1 site of QC288A329A; it checks the SCP1 and ALS junction for the copy number change of the target (Fig. 2A). The SSI qPCR is specific to the FRT1 site of QC288A436A; it checks the new SCP1 and HPT junction that resulted from a recombination event at the FRT1 site (Fig. 2B). Since the integration of transgenes is site specific for the FRT1 site, any events positive for the SSI qPCR are considered SSI positive. Only those SSI-positive events that are shown, by subsequent border-specific PCR, to also have predicted FRT87-end DNA recombination are considered as RMCE events. The donor and FLP qPCR assays are each specific to a unique region of the donor QC436 or the FLP QC292 circular plasmid DNA and were used to check for their random integration.
Since the B5 target cultures were initiated from homozygous transgenic plants, the target qPCR should identify two copies of the target QC288A329A if SSI recombination did not occur. If DNA recombination occurred on only one target chromosome, the SSI qPCR would detect one copy of SSI QC288A436A, while the target qPCR would detect a copy of the target contributed by the other target chromosome. Since the FRT1 and FRT87 sites are not completely incompatible, all components between them can possibly be excised, resulting in gene excision product QC288ME that cannot be detected by either SSI or target qPCR (Li et al., 2009). It was observed that events with one copy of SSI often no longer contained any target, probably due to gene excision. All of the 23 SSI-positive events contained a single-copy SSI; 10 of them retained one copy of the target while the remaining 13 had the other copy of the target excised. Many of the 23 SSI-positive events also contained randomly integrated donor or FLP DNA, or both. Four selected events, B51, B52, B53, and B54, are all free of the target DNA, although B51 and B52 contain the FLP DNA (Table I). B51 is likely a chimera containing only 0.2 copy of the FLP DNA.
Table I. Transgene integration and fatty acid contents of the first-round SSI events.
| Eventa | Gene Copy No. by qPCRb |
Fatty Acid Content by GCc |
|||||||
| SSI | Donor | Target | FLP | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | |
| B51 | 1.0 | 0.0 | 0.0 | 0.2 | 4.0 | 2.1 | 50.8 | 25.6 | 17.6 |
| B52 | 0.8 | 0.0 | 0.0 | 0.9 | 12.6 | 3.2 | 18.0 | 47.1 | 19.1 |
| B53 | 0.8 | 0.0 | 0.0 | 0.0 | 3.8 | 1.4 | 51.7 | 27.3 | 15.8 |
| B54 | 1.1 | 0.0 | 0.0 | 0.0 | 3.7 | 1.2 | 57.3 | 20.1 | 17.8 |
| B53-1 | 1.9 | 0.0 | 0.0 | 0.0 | 2.4 | 2.5 | 90.4 | 1.0 | 3.5 |
| B53-2 | 1.9 | 0.0 | 0.0 | 0.0 | 2.2 | 3.7 | 89.3 | 0.8 | 3.8 |
| B53-3 | 0.9 | 0.0 | 0.0 | 0.0 | 2.5 | 2.3 | 90.6 | 0.9 | 3.4 |
| B53-4 | 0.9 | 0.0 | 0.0 | 0.0 | 2.8 | 2.4 | 89.5 | 1.7 | 3.4 |
| B53-5 | 0.0 | 0.0 | 0.0 | 0.0 | 13.0 | 3.4 | 14.4 | 55.5 | 13.6 |
| B53-6 | 0.0 | 0.0 | 0.0 | 0.0 | 12.1 | 3.4 | 11.3 | 54.0 | 19.1 |
Events B51 to B54 are representative hygromycin-resistant events selected from the retransformation of homozygous target QC288A329A with the first donor QC436 and FLP QC292 DNA. B53-1 to B53-6 are segregating T1 plants derived from event B53.
Embryogenic callus or T1 plant leaf samples were analyzed by qPCR specific to the SCP1 and HPT junction of QC288A436A (SSI), a QC436-specific region (Donor), the SCP1 and ALS junction of QC288A329A (Target), and a QC292-specific region (FLP). A heat shock protein gene, HSP, was used as an endogenous control in duplex qPCR. A genomic DNA sample containing one copy of the respective transgene was used as the calibrator to calculate relative transgene copies in other samples using the Applied Biosystems 7500 system software. A value of less than 0.3 or between 0.4 and 1.3 was considered as zero or one copy. A value between 1.4 and 2.3 was considered as two copies.
Fatty acids were determined on the bulk of 10 mature somatic embryos for B51 to B54 or on T1 seed chips for B53 by GC and expressed as the percentage of total fatty acids. Fatty acid measurements by GC as described here are reproducible to approximately 3% of total fatty acids. Untransformed control somatic embryos typically contain 12.6% to 20.8% 16:0, 4.2% to 6.6% 18:0, 12.3% to 22.9% 18:1, 39.3% to 46.9% 18:2, and 12.4% to 23.5% 18:3 (Meyer et al., 2008). Increases in 18:1 to greater than 30% and decreases in 16:0 to less than 10% are indicative of successful FAD2 and FATB gene down-regulation, respectively. The chipped B53 seeds later germinated to T1 plants that provided leaf DNA for the qPCR assay.
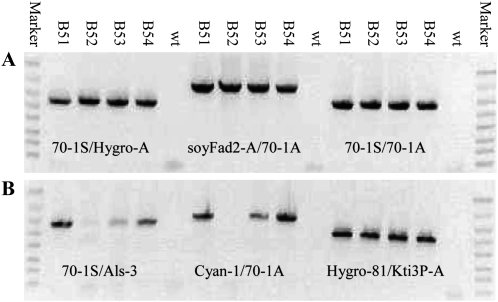
As exemplified by the four selected events B51, B52, B53, and B54 (Fig. 3), PCR assays using various primer combinations were done on SSI-positive events to confirm RMCE (Fig. 2). The four events were confirmed to be RMCE/excision events by PCR using primer sets 70-1S/Hygro-A and soyFad2-A/70-1A specific to the 5′ and 3′ borders of RMCE DNA QC288A436A and 70-1S/70-1A specific to excision DNA QC288ME (Fig. 3A). Three of the four events were also positive for PCR with primer sets 70-1S/Als-3 and Cyan-1/70-1A specific to the 5′ and 3′ borders of the target DNA QC288A329A; B52 was weakly positive for the 5′ target border PCR and negative for the 3′ target border (Fig. 3B). The results indicated that the four events, when sampled at the callus stage, were chimeras consisting of cells containing the RMCE, target, and excision. Cells lacking the RMCE DNA QC288A436A would be eliminated by extended hygromycin selection, since neither the target QC288A329A nor excision QC288ME contains a HPT gene. The third recognition site, FRT12, of QC288A436A was shown to be present in the four events using primers Hygro-81 and Kti3P-A (Fig. 3B).
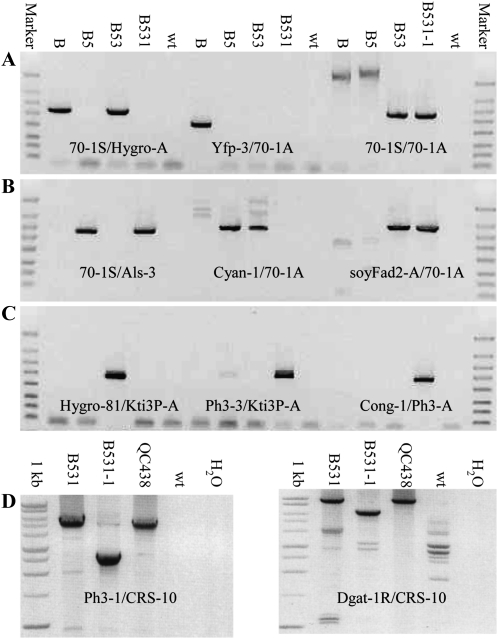
Figure 3.
Analysis of the first-round SSI events. PCR assays specific to the genomic borders of the B target site hosting different transgenes was done using combinations of the 5′ border, 3′ border, and transgene-specific primers (Fig. 2). A, Expected PCR fragments of the 5′ border (left), 3′ border (center), and excision QC288ME (right) of the first RMCE, QC288A436A, are 886, 1,116, and 986 bp. The expected 9,108-bp-long full-length QC288A436A is too large to be amplified (right). B, Expected PCR fragments of the 5′ border (left) and 3′ border (center) of target QC288A329A and the FRT12 region of RMCE QC288A436A (right) are 967, 1,180, and 693 bp. Wild-type DNA (wt) was included as a negative control. The four events were all chimeras containing the RMCE, target, and excision transgenes at the embryogenic callus stage. The FlashGel DNA markers are 4, 2, 1.25, 0.8, 0.5, 0.3, 0.2, and 0.1 kb (Lonza Rockland).
Depending on the availability of enough somatic embryos, 13 SSI-positive events were analyzed by gas chromatography (GC) to check for fatty acids. All of them, except for B52, showed reduced levels of saturated fatty acids (16:0 and 18:0) and elevated levels of 18:1, as exemplified by the four selected events (Table I). The results indicated that endogenous FAD2 and FATB genes were silenced by the FAD2-FATB cassette of the QC288AQC436A transgenes. The fatty acid levels in event B52 are similar to those in wild-type somatic embryos. It is not known why event B52 failed to exhibit the silencing phenotype even though molecular analyses indicate that B52 is an RMCE event.
T0 plants regenerated from 10 RMCE events including B53 and B54 produced T1 seeds. Fatty acid profiles were determined on T1 seed chips by GC. T1 plants germinated from the same chipped seeds were analyzed by the SSI, target, donor, and FLP-specific qPCR assays to check for transgene segregation. Mendelian segregation was observed in nine of the 10 events, and the high 18:1 and low 16:0 and 18:0 phenotypes are linked to the transgenes. Although the SSI qPCR-negative plants (null) contain the wild-type levels of fatty acids in their seeds, homozygous and hemizygous T1 plants contain similar high levels of 18:1 (approximately 90%) and low levels of 16:0 and 18:0 (approximately 5%) in their seeds, indicating that one copy of the QC288AQC436A transgenes is sufficient to suppress endogenous FAD2 and FATB genes. The qPCR results and fatty acid profiles for B53 T1 progeny, including two homozygous B53-1 and B53-2, two hemizygous B53-3 and B53-4, and two null B53-5 and B53-6, are shown as examples (Table I).
Characterization of the Second-Round RMCE Events
Putative events selected from the direct retransformation of the four QC288A436 RMCE events B51, B52, B53, and B54 with the second donor QC438 DNA were analyzed by the same four qPCR assays; however, the SSI and target qPCR were switched because the HPT gene in QC288A436A was exchanged to the ALS gene in QC288A436A438A (Fig. 2). There were only four events identified as SSI qPCR positive, including B531 and B541 shown in Table II. The other two SSI-positive events failed to survive. Border-specific PCR assays indicated that only one event, B531, was an RMCE event positive for both the 5′ and 3′ end borders specific to QC288A436A438A (data not shown).
Table II. Transgene integration and fatty acid contents of the second-round SSI events.
| Eventa | Gene Copy No. by qPCRb |
Fatty Acid Content by GCc |
Oild | |||||||
| SSI | Donor | Target | FLP | 16:0 | 18:0 | 18:1 | 18:2 | 18:3 | ||
| % | ||||||||||
| B531 | 1.2 | 0.0 | 0.0 | 0.0 | 2.4 | 2.7 | 71.1 | 15.5 | 8.2 | 7.5 |
| B532 | 0.0 | 0.7 | 1.3 | 0.0 | 3.7 | 3.3 | 68.7 | 16.7 | 7.6 | ND |
| B541 | 1.3 | 0.0 | 0.0 | 0.0 | 13.3 | 4.0 | 23.0 | 45.1 | 14.6 | 6.3 |
| B511 | 0.0 | 0.7 | 0.0 | 0.0 | 16.0 | 5.3 | 15.8 | 42.8 | 20.1 | 3.5 |
| B512 | 0.0 | 0.0 | 0.0 | 0.0 | 13.8 | 8.1 | 30.8 | 31.6 | 15.7 | 6.1 |
Events B531 to B512 are representative chlorsulfuron-resistant events selected from the retransformation of the embryogenic callus of RMCE events B51, B53, and B54 containing QC288A436A with the second donor QC438 and FLP QC292 DNA.
Embryogenic callus samples were analyzed by qPCR specific to the SCP1 and ALS junction of QC288A436A438A (SSI), a QC438-specific region (Donor), the SCP1 and HPT junction of QC288A436A (Target), and a QC292-specific region (FLP). The qPCR assays were done as described in the Table I legend.
Fatty acids in mature somatic embryos were determined by GC as described in the Table I legend and are expressed as the percentages of total fatty acids.
Oil contents in mature somatic embryos determined by NMR are presented as the percentages of total sample dry weight. Oil measurements made by NMR as described here are reproducible to approximately 1% oil of sample dry weight. Untransformed control somatic embryos typically contain 2.2% to 6.2% oil (Meyer et al., 2008). Increases in oil contents to above 7% oil of sample dry weight are indicative of a functional DGAT1 gene. Oil content for event B532 was not determined (ND) due to low tissue amounts.
Event B531 and a few others were analyzed by GC and NMR to check for fatty acids and total oil in somatic embryos (Table II). Event B531 retained the high level of 18:1 and low levels of saturated fatty acids (16:0 and 18:0) conferred by the FAD2-FATB cassette of the first RMCE DNA QC288AQC436 and showed an elevated level of total oil (7.5%), suggesting that the DGAT1 cassette of the second RMCE DNA QC288A436AQC438 was expressed. The total oil content in wild-type somatic embryos at a similar stage varies from 3% to 6%. Oil measurements on somatic embryos transformed with Yarrowia DGAT1 have been shown to be excellent predictors for increased oil in seeds, and oil contents greater than 6% typically result if a functional Yarrowia DGAT1 is expressed (Meyer et al., 2008). The SSI-negative event B532 had a high level of 18:1 and low levels of 16:0 and 18:0, suggesting that it retained the FAD2-FATB cassette of QC288A436A. Oil measurements were not completed with this event due to tissue amounts being too low; therefore, it is not known whether the copy of the donor DNA indicated by the donor qPCR contains a functional DGAT1 gene. In contrast, the SSI-positive event B541 lost the high-18:1 phenotype, suggesting that the FAD2-FATB cassette of QC28A436A was probably excised by FLP during retransformation, and oil content suggested a nonexpressed or nonfunctional DGAT1 gene. SSI-negative events B511 and B512 not only failed to show elevated oil levels but also lost the high-18:1 phenotype, suggesting that their FAD2-FATB cassette was also excised.
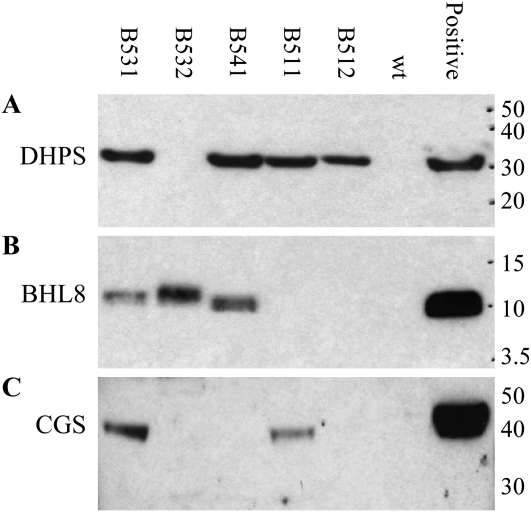
The same five events were analyzed by western blotting to check for the expression of amino acid modification transgenes DHPS, BHL8, and CGS in somatic embryos. Only event B531 expressed all three genes at levels detectable by the DHPS, BHL8, and CGS antibodies (Fig. 4). The other events expressed only one or two of the three genes. DHPS expression was detected in event B512, suggesting that only the DHPS cassette of QC438 was probably randomly integrated, which would not be detectable by either the SSI or donor qPCR assays. It is known that DNA fragmentation is common during biolistic bombardment especially for large DNA constructs.
Figure 4.
Transgene expression in the second-round SSI events. The expression of genes DHPS, BHL8, and CGS in mature somatic embryos of selected second-round SSI events was checked by western blotting. Only the RMCE event B531 expressed all three proteins DHPS (A), BHL8 (B), and CGS (C). Event B532 expressed only BHL8. Event B541 expressed both DHPS and BHL8 but not CGS. Event B511 expressed both DHPS and CGS but not BHL8. Event B512 expressed only DHPS. Positive controls containing respective genes are transgenic events from unrelated projects. The protein markers are in kD. wt, Wild type.
The above molecular and phenotypic analyses indicated that seven genes, ALS, DGAT1, BHL8, DHPS, CGS, FAD2, and FATB, were successfully stacked at a previously characterized genomic site by two rounds of SSI transformation and that all the transgenes were expressed. In the case of ALS, DGAT1, BHL8, DHPS, and CGS, the expression of the transgenes led to the production of functional proteins, and in the case of FAD2 and FATB, it led to the silencing of endogenous genes.
Molecular Characterization of the Gene-Stacking RMCE Plant
A T0 plant, B531-1, regenerated from event B531 was analyzed by the same qPCR assays previously done on embryogenic callus. Consistent with previous results (Table II), the qPCR assays confirmed that B531-1 contains one copy of the RMCE DNA QC288A436A438A and is free of any donor, target, or FLP DNA (data not shown).
The T0 plant B531-1 containing the stacked genes QC288A436A438A and its ancestors, including the original target B containing QC288A, the first-round SSI transformation target B5 containing QC288A329A, and the second-round SSI transformation target B53 containing QC288A436A, were analyzed by PCR using nine sets of primers (Fig. 2). The 70-1S/Hygro-A primer set specific to the SCP1 and HPT junction of QC288A and QC288A436A detected the expected band in B and B53 (Fig. 5A, left). The Yfp-3/70-1A primer set specific to the QC288A 3′ end detected the band expected only in B (Fig. 5A, center). The 70-1S/70-1A primer set specific to both the 5′ and 3′ end genomic borders of the B target site detected the full-length QC288A band in homozygous B, the full-length QC288A329A band in homozygous B5, and the excision band in hemizygous B53 and B531-1 (Fig. 5A, right). The full-length QC288A436A of B53 and QC288A436A438A of B531 are too large to be amplified by PCR. The 70-1S/Als-3 primer set specific to the SCP1 and ALS junction of QC288A329A and QC288A436A438A amplified the expected band in B5 and B531-1 (Fig. 5B, left). The Cyan-1/70-A primer set specific to the 3′ end of QC288A329A amplified the expected band in B5 and also in B53 (Fig. 5B, center), indicating that B53 still partially contained its parent target B5, consistent with previous PCR analysis (Fig. 3B). The soyFad2-A/70-A primer set specific to the 3′ end of both QC288A436A and QC288A436A438A amplified the expected band in B53 and B531-1 (Fig. 5B, right). The Hygro-81/Kti3P-A primer set specific to the FRT12 region of QC288A436A amplified the band expected only in B53 (Fig. 5C, left). The Ph3-3/Kti3P-A primer set specific to the FRT12 region and the Cong-1/Ph3-A primer set specific to a middle region of QC288A436A438A amplified the bands expected only in B531-1 (Fig. 5C, center and right). Figure 5D will be described later with regard to Southern hybridization analysis.
Figure 5.
Analysis of the second round RMCE event. PCR assays specific to the genomic borders and internal regions of the second RMCE DNA, QC288A436A438A, were done on RMCE T0 plant B531-1 using various primer combinations (Fig. 2). The hemizygous (RMCE/excision) B531-1 ancestors hemizygous B53, homozygous B5 and B, and wild-type DNA (wt) were included as controls. A, The expected 886-bp 5′ border of both QC288A in B and QC288A436A in B53 (left) and the 561-bp 3′ border of QC288A in B (center) were amplified. The full-length 4,742-bp QC288A of B, 6,331-bp QC288A329A of B5, and 986-bp QC288ME (excision) of B53 and B531 were amplified (right). The expected full-length 9,108-bp QC288A436A of B53 and 21,925-bp QC288A436A438A of B531-1 were too large to be amplified. B, The expected 967-bp 5′ border of both QC288A329A in B5 and QC288A436A438A in B531 (left) and the 1,180-bp 3′ border of QC288A329A in B5 (center) were amplified. The same B5 band was also amplified from B53 that contained chimerically B5 DNA QC288A329A. The expected 1,116-bp 3′ border of both QC288A436A in B53 and QC288A436A438A in B531-1 were amplified (right). C, A 693-bp fragment unique to the FRT12 region of QC288A436A was amplified only in B53 (left). An 840-bp fragment unique to the FRT12 region (center) and a 711-bp fragment unique to the 5′ region of QC288A436A438A were amplified only in B531-1 (right). The FlashGel DNA markers are 4, 2, 1.25, 0.8, 0.5, 0.3, 0.2, and 0.1 kb. D, A 2,946-bp segment covering the DHPS, MYB2 terminator, and UBIQ10 promoter of QC288A436A438A was lost in T0 plant B531-1 although intact in embryogenic callus B531. The expected 5,753-bp Ph3-1/CRS-10 band (left) and 9,216-bp Dgat-1R/CRS-10 band (right) of intact QC288A436A438A were amplified from the embryogenic callus B531 and QC438 donor DNA but not from the T0 plant B531-1 leaf DNA, which produced approximately 3-kb smaller bands. Some nonspecific bands were amplified by the long-range PCR. The 1-kb DNA markers are 10, 8, 6, 5, 4, 3, 2.5, 2, 1.5, and 1 kb (Invitrogen).
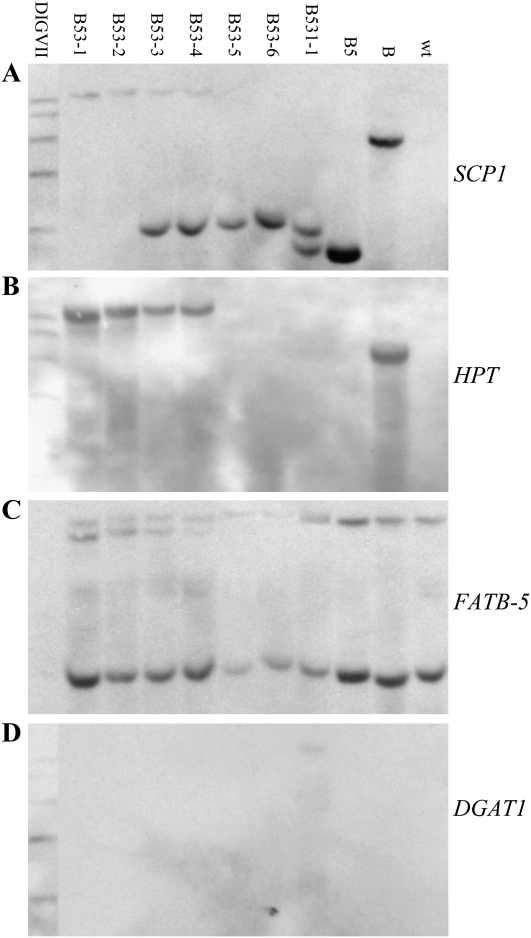
The T0 plant B531-1 together with its parent B53 segregating T1 plants (Table II) and their homozygous B5 and B ancestors were analyzed by Southern blot to confirm precise RMCE. Leaf genomic DNA was digested with MfeI and hybridized sequentially with SCP1, HPT, FATB-5, and DGAT1 probes (Fig. 2). Since the 5′ and 3′ genomic borders of the B target site had been sequenced and the QC288A transgene at this site lost 17-bp 5′ and 49-bp 3′ end sequences (data not shown), the sizes of the expected Southern bands of the transgenes are known for the samples if they are true RMCE events.
Since the SCP1 promoter is outside the FRT1 recombination site, it cannot be changed by RMCE and should always be present at the target site (Fig. 2). The SCP1 probe hybridized to the expected 12,205-bp QC288A436A-specific band in homozygous B53-1 and B53-2 and the 3,987-bp QC288ME band in excision B53-5 and B53-6. Both the 12,205- and 3,987-bp bands were detected in hemizygous B53-3 and B53-4 (Fig. 6A). The B531-1 T0 plant is indeed “hemizygous,” with one chromosomal target QC288A436A converted to excision QC288ME and the other converted to RMCE QC288A436A438A. As expected, both the QC288A436A438A-specific 3,681-bp band and the QC288ME-specific 3,987-bp band were detected in B531-1. The same 3,681-bp band, also specific to QC288A329A of the B5 ancestor, was detected in B5. The QC288A-specific 7,839-bp band was detected in B (Fig. 6A). The HPT probe hybridized to the same QC288A436A-specific 12,205-bp band in B53-1, B53-2, B53-3, and B53-4 and the QC288A-specific 7,839-bp band in B. As expected, no HPT band was detected in B53-5, B53-6, B531-1, or B5 (Fig. 6B). The FATB-5 probe hybridized to two endogenous homolog gene bands in all the samples in addition to the same QC288A436A-specific 12,205-bp band in B53-1, B53-2, B53-3, and B53-4. But the expected QC288A436A438A-specific 12,931-bp FATB-5 band was not detected. Instead, a larger band seems overlapped with the large wild-type band (Fig. 6C). The discrepancy was caused by an internal deletion of QC288A436A438A, as discussed below.
Figure 6.
Confirmation of gene stacking by Southern hybridization. Genomic DNA of B53 T1 plants, homozygous RMCE B53-1 and B53-2, hemizygous (RMCE/excision) B53-3 and B53-4, and excision B53-5 and B53-6, the T0 plant B531-1 (RMCE/excision), and homozygous B5 and B ancestor plants were digested with MfeI and hybridized sequentially with probes SCP1, HPT, FATB-5, and DGAT1. The expected sizes of Southern bands are specific to transgenes integrated at the B target site, where the transgenic QC288A lost 17-bp 5′ end and 49-bp 3′ end sequences. There is an MfeI site 2,131 bp upstream and 1,230 bp downstream, respectively, of the transgenes that contain MfeI sites (Fig. 2). A, The SCP1 probe hybridized to the expected 12,205-bp band of QC288A436A in B53-1 and B53-2, the 3,987-bp band of QC288ME in B53-5 and B53-6, and both bands in hemizygous plants B53-3 and B53-4. The same 3,987-bp QC288ME band and a 3,681-bp QC288A436A438A band were hybridized in B531-1. As expected, the same 3,681-bp band also specific to QC288A329A was detected in B5 and a 7,839-bp QC288A band was detected in B. B, The HPT probe hybridized to the same 12,205-bp QC288A436A band in B53-1, B53-2, B53-3, and B53-4 and to the same 7,839-bp QC288A band in B. C, The FATB-5 probe hybridized to two endogenous bands in all samples in addition to the same 12,205-bp QC288A436A band in B53-1, B53-2, B53-3, and B53-4. Instead of the expected 12,931-bp band of QC288A436A438A, a larger band, likely the 16,402-bp band expected from modified QC288A436A438A with its 2,946-bp DHPS-MYB2-UBIQ10 deleted, overlapped with the top wild-type band (wt) in B531-1. D, The DGAT1 probe detected in B531-1 the same 16,402-bp band of the modified QC288A436A438A instead of an expected 6,352-bp QC288A436A438A band. The DIGVII DNA markers are 8,576, 7,427, 6,106, 4,899, and 3,639 bp (Roche).
The DGAT1 probe hybridized to only B531-1 as expected, but the band is much larger than the expected 6,352-bp QC288A436A438A-specific band (Fig. 6D). To resolve this discrepancy, the entire 21,727-bp QC288A436A438A transgenes plus some 5′ and 3′ end genomic borders were amplified by PCR as five overlapping approximately 5-kb fragments (data not shown). An approximately 3-kb-long deletion involving the DHPS gene and the UBIQ10 promoter was identified only in the B531-1 T0 plant but not in its embryogenic callus B531 (Fig. 5D). Sequencing the corresponding fragments revealed that a 2,946-bp segment including the entire DHPS gene and the UBIQ10 promoter was looped out between two 165-bp repeats in B531-1. The 165-bp sequence encoding a soybean ribulose-1,5-bisphosphate carboxylase small subunit transit peptide was engineered at the 5′ end of both DHPS and CGS to target the enzymes to chloroplast. The loss of two MfeI sites contained in the DHPS region (Fig. 2C) changed the predicted 12,931-bp FATB-5 band and the 6,352-bp DGAT1 band of QC288A436A438A to one 16,402-bp band hybridized in B531-1 by both the FATB-5 and DGAT1 probes (Fig. 6, C and D).
DISCUSSION
RMCE using different recombinase systems has been achieved successfully in several plants (Nanto et al., 2005; Louwerse et al., 2007; Li et al., 2009). However, stacking multiple genes at a precharacterized genomic site using RMCE or any other technology has not been reported. Although the gene-stacking event B531 in this report has defects and cannot be used as a product, the integration of seven functional trait genes at one genomic site by two rounds of SSI transformation demonstrates the usefulness of RMCE technology in agricultural biotechnology and gene expression research.
Taking advantage of reversible DNA cassette exchange in RMCE, an RMCE product can be used as a new target for subsequent SSI transformation. If additional incompatible recognition sites can be introduced, successive rounds of RMCE can stack genes at a precharacterized genomic target site repeatedly, in theory. The work described here required three rounds of RMCE. The first round of RMCE described previously by Li et al. (2009) was an SSI transformation that switched the HPT gene of QC288A in the original target event B to the ALS gene of QC288A329A in RMCE event B5. This is necessary to ensure that the final gene-stacking event B531 contains the regulatory-acceptable ALS gene. Thus, the first round of SSI transformation in this study was done on RMCE B5 as the target to integrate the first group of trait genes, FAD2 and FATB, and meanwhile switching the selectable marker back to HPT to create RMCE B53 containing QC288A436A. The second group of trait genes, DGAT1, DHPS, BHL8, and CGS, were subsequently integrated by the second SSI transformation on B53 to create RMCE B531 containing QC288A436A438A, which consisted of the above six and ALS genes. If desired, the process can continue to stack more genes as long as additional incompatible FRT recognition sites are available. Outcrossing to wild-type plants may be necessary to rejuvenate the target lines, as they may lose vigor by repeated transformation.
The proper expression of the stacked seven genes ALS, DGAT1, DHPS, BHL8, CGS, FAD2, and FATB demonstrated that gene overexpression and silencing can be simultaneously achieved at one genomic site. One copy of hairpin-structured FAD2 and FATB is sufficient to trigger the silencing of corresponding endogenous genes. Careful evaluation of the phenotypes conferred by all the genes will help to check if the seven genes placed in the linear configuration interfere with each other. Due to the 2,946-bp internal deletion of QC288A436A438A and other defects in the T0 plant B531-1, we will not be able to study this event further. New gene-stacking events produced from other experiments will need to be evaluated to address the question.
RMCE is a complex process especially when there are two targets, one on each homologous chromosome, and the two recombinase recognition sites involved are only partially incompatible (Li et al., 2009). The process is further complicated in gene stacking by using three recognition sites and large donor DNA such as QC438 containing multiple genes with some repeated sequences. Since it is known that the three FRT sites used in B53 and B531 are not completely incompatible (Tao et al., 2007), DNA recombination can happen between any pairs of them when exposed to FLP recombinase. As a result, genes already integrated in the first-round SSI (i.e. FAD2 and FATB) can be excised during the second round of SSI transformation, as indicated by the reduced levels of 18:1 in events B541, B511, and B512 (Table II).
Furthermore, even the perfect gene-stacking RMCE event B531, confirmed at the embryogenic callus stage, lost an internal segment of the transgenes later during plant regeneration, possibly by DNA looping out between two 165-bp repeats. Although longer DNA fragment inverted repeats, such as the two FAD2-1 and FATB-4 fragments designed for gene silencing, were maintained in B531-1 T0 plants, direct repeats should be generally avoided in DNA constructs to prevent intervening DNA from being looped out via similar intrachromosomal homologous recombination that is involved in creating extrachromosomal circular DNA from tandem repeats in plant genomes (Cohen et al., 2008).
Recent developments in plant gene targeting demonstrate that endogenous genomic sites can be specifically targeted for modification through DNA double-strand break-induced homologous recombination. DNA double-strand breaks can be created with either designed zinc finger nucleases or modified homing endonucleases. Customized zinc finger nucleases have been employed to introduce successfully an herbicide resistance gene, PAT, to a tobacco (Nicotiana tabacum) endochitinase gene locus, a maize (Zea mays) inositol-1,3,4,5,6-petakisphosphate 2-kinase gene locus, or to introduce specific mutations to a tobacco acetolactate synthase gene to gain resistance to sulfonyl urea (Cai et al., 2009; Shukla et al., 2009; Townsend et al., 2009). Similarly, an engineered I-CreI endonuclease derivative designed to recognize a selected sequence adjacent to the maize LIGULELESS1 gene has been used to produce mutations with small deletions or insertions specifically at expected cleavage sites (Gao et al., 2010). Thus, the random integration problem of creating initial SSI target sites can be resolved by using double-strand break-induced homologous recombination to introduce the FRT1-FRT87 recognition sequences at specifically selected genomic sites. Then, more transgenes can subsequently be added or exchanged through FLP recombinase-mediated RMCE, which may have the advantages of being reversible, more effective and flexible, and able to deliver large transgenes. Future challenge for the application of RMCE in agricultural biotechnology is to develop highly transformable target lines that are able to accept transgenes with different preferences for optimal gene overexpression, gene silencing, tissue-specific expression, and agronomic performance.
MATERIALS AND METHODS
DNA Construction and Plant Transformation
Donor constructs QC436 containing FRT1-HPT:NOS-FRT12+KTI3:FAD2-1-FATB-4-FATB-5-FATB-4-FAD2-1:KTI3-ALB-FRT87 and QC438 containing FRT1-ALS:PINII+GY1:DGAT1:LEG+CONG:BHL8:PH3+ALB:DHPS:MYB2+UBIQ10:CGS:PH3-FRT12 were made following standard molecular cloning procedures using components from existing DNA constructs (Falco et al., 1999; Roesler and Rao, 2000; Hitz, 2001; Kinney et al., 2004; Falco, 2006; Lightner et al., 2006; Meyer et al., 2008; Li et al., 2009; Xing et al., 2010). Soybean (Glycine max) embryogenic cultures were initiated from homozygous progeny of RMCE plants B5-1 and B5-2 containing the QC288A329A transgenes SCP1-FRT1:ALS:PINII+UBQ:CFP:NOS-FRT87 as the target (Li et al., 2009). The first donor DNA QC436 and the FLP expression DNA QC292 SCP1:FLP:PINII were cotransformed at 9:3 ratios following the biolistic transformation protocol using 30 μg mL−1 hygromycin B for transgenic event selection (Li et al., 2009). The embryogenic cultures of selected QC436 RMCE events were directly retransformed with the second donor DNA QC438 and the same FLP expression DNA QC292 at 9:3 ratios using 90 ng mL−1 chlorsulfuron (DuPont) for transgenic event selection. All the donor and FLP DNAs used are circular plasmid DNA.
qPCR Analysis
Gene-specific qPCR assays were done on somatic embryo or leaf DNA samples using the same primer/probe sets specific to the original target QC288A, the first target QC288A329A (i.e. RMCE event B5), and the FLP DNA QC292 (Li et al., 2009). The first donor QC436-specific qPCR assay used a new primer, 409-1F (5′-CGACGGTATCGATAAGCTTGTTAAC-3′), and the previous primer Hygro-116R and probe Hygro-79T. The second donor QC438-specific qPCR used primers 409-1F, Als-163R, and probe Als-110T. SSI-specific qPCR assays used the same QC288A-specific primer/probe set of QC288A-1F, Hygro-116R, and probe Hygro-79T for the first-round RMCE QC288A436A and the same QC288A329A-specific primer/probe set of QC288A-1F, Als-163R, and probe Als-110T for the second-round RMCE QC288A436A438A.
PCR Analysis
PCR assays were similarly done on somatic embryo or leaf samples using some primers previously described (Li et al., 2009). The 5′ border PCR assays used primers 70-1S and Als-3 for QC288A329A and QC288A436A438A or 70-1S and Hygro-A for QC288A436A. The 3′ border PCR assays used primers Cyan-1 and 70-1A for QC288A329A or soyFad2-A (5′-GAAGGGTCAAACCCACAACATCATC-3′) and 70-1A for both QC288A436A and QC288A436A438A. The FRT12 site of QC288A436A was checked with primers Hygro81 (5′-CCGAGGGCAAAGGAATAGTGAGG-3′) and Kti3P-A (5′-GGCGGGGTTGATATATTTATACACACC-3). The FRT12 site of QC288A436A438A was checked with primers PH3-3 (5′-CAATCGTTTAGCCTTGCTGGACG-3′) and Kti3P-A. A QC288A436A438A middle region was checked with primers Cong-1 (5′-TCAACACCCGTCAAACTGCATG-3′′) and Ph3-A (5′-GCATTCCATAAGCCGTCACGATTC-3′). A QC288A436A438A internal deletion was checked by two PCR assays with primers Ph3-1 (5′-TGAATCGTGACGGCTTATGGAATG-3′) and CRS10 (5′-AGGAGTGCAGAATCAGATCAG-3′) and primers Dgat-1R (5′-CTGGTTCTGCTTGTAGTTGTAGGCC-3′) and CRS10. The expected sizes of all PCR bands are given in the figure legends.
Fatty Acid and Oil Analysis
Soybean seeds or somatic embryos were ground and their fatty acid compositions were determined by GC. Approximately 5 mg of embryo powder was incubated while shaking with 50 μL of 0.25 m trimethylsulfonium hydroxide in methanol and 0.5 mL of hexane for transesterification in a GC vial at room temperature for 30 min. Fatty acid methyl esters (5 μL injected from the hexane layer) were separated and quantified using a Hewlett-Packard 6890 gas chromatograph fitted with an Omegawax 320 fused silica capillary column (Supelco). The oven temperature was programmed to hold at 220°C for 2.6 min, increase to 240°C at 20°C min−1, and then hold for an additional 2.4 min. Carrier gas was supplied by a Whatman hydrogen generator. Retention times were compared with those for methyl esters of commercially available standards (Nu-Chek Prep), and the peaks corresponding to 16:0, 18:0, 18:1, 18:2, and 18:3 were analyzed using the Chemstation software (Agilent).
The oil content was analyzed on remaining embryo powder by NMR using a Maran Ultra NMR system. The samples were weighed and scanned in a Cobra 800 NMR robot (Adept). The oil content was determined by comparing the NMR readings with standard curves and expressed as the percentage of the total sample weight.
Western Blotting
Total proteins were extracted from 5 mg of lyophilized somatic embryo powder in a buffer containing 50 mm Tris, pH 7.5, 10 mm β-mercaptoethanol, and 0.1% SDS. The proteins were resolved on 12% NuPAGE Tris-Glycine protein gels and blotted to nitrocellulose membranes using the XCell SureLock Novex Mini-Cell system (Invitrogen). Protein loadings and MultiMark weight markers (Invitrogen) were checked by staining the blots with Ponceau S (Sigma). The blots were then incubated at 4°C overnight with DHPS, BHL8, or CGS antibodies (rabbit) diluted 1:1,500 in 5% dry milk dissolved in TBS buffer (20 mm Tris, 150 mm NaCl, pH 7.5) followed by four 5-min washes in TTBS buffer (TBS with 500 μL L−1 Tween 20; Sigma). Specific proteins were detected using the horseradish peroxidase-labeled mouse antibody of the Lumi-Light western blotting (mouse/rabbit) kit (Roche). Signals were captured on BioMax films (Eastman Kodak).
Southern Hybridization Analysis
Soybean genomic DNA was prepared from leaf samples and analyzed by Southern hybridization with digoxigenin-labeled probes (Li et al., 2009). DNA was digested with MfeI and hybridized with a 581-bp SCP1 probe (Li et al., 2009), a 588-bp HPT probe made by PCR with primers Hpt-1 (5′-TTCAGCTTCGATGTAGGAGGGCG-3′) and Hpt-2 (5′-GATGTTGGCGACCTCGTATTGGG-3′), a 459-bp FATB-5 probe made with primers TE2-F (5′-GGTGAAATCTTGACAAGAGCTTCCAG-3′) and TE2-R (5′-CACAATCTCAGCACCATTTTCCAG-3′), and a 616-bp DGAT1 probe made with primers Dgat1-F (5′-CGTCTCTCTGTGCATGCTTATTCAG-3′) and Dgat-1R using the PCR digoxigenin probe synthesis kit (Roche).
Acknowledgments
We thank our colleagues Dave Peterson, Chris Scelonge, Alex Lyznik, Bill Gordon-Kamm, and Dennis Bidney, whose work on FLP-FRT RMCE in maize provided many of the components and concepts used to develop the system in soybean, and Lingxia Huang for western and Kevin Ripp for GC assays.
References
- Cai CQ, Doyon Y, Ainley WM, Miller JC, DeKelver RC, Moehle EA, Rock JM, Lee YL, Garrison R, Schulenberg L, et al. (2009) Targeted transgene integration in plant cells using designed zinc finger nucleases. Plant Mol Biol 69: 699–709 [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Shockey JM, Dietrich CR, Gidda SK, Mullen RT, Dyer JM. (2007) Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Curr Opin Plant Biol 10: 236–244 [DOI] [PubMed] [Google Scholar]
- Chiba Y, Ishikawa M, Kijima F, Tyson RH, Kim J, Yamamoto A, Nambara E, Leustek T, Wallsgrove RM, Naito S. (1999) Evidence for autoregulation of cystathionine γ-synthase mRNA stability in Arabidopsis. Science 286: 1371–1374 [DOI] [PubMed] [Google Scholar]
- Cohen S, Andreas Houben A, Segal D. (2008) Extrachromosomal circular DNA derived from tandemly repeated genomic sequences in plants. Plant J 53: 1027–1034 [DOI] [PubMed] [Google Scholar]
- Damude HG, Kinney AJ. (2007) Engineering oilseed plants for a sustainable, land-based source of long chain polyunsaturated fatty acids. Lipids 42: 179–185 [DOI] [PubMed] [Google Scholar]
- Damude HG, Kinney AJ. (2008a) Enhancing plant seed oils for human nutrition. Plant Physiol 147: 962–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damude HG, Kinney AJ. (2008b) Engineering oilseeds to produce nutritional fatty acids. Physiol Plant 132: 1–10 [DOI] [PubMed] [Google Scholar]
- Falco SCinventor (July 4, 2006) Chimeric genes and methods for increasing the lysine and threonine content of the seeds of plants. US Patent Application No. 7071383 [Google Scholar]
- Falco SC, Guida AD, Locke MEHinventors (June 15, 1999) Nucleic acid fragments, chimeric genes and methods for increasing the methionine content of the seeds of plants. US Patent Application No. 591241 [Google Scholar]
- Galili G, Amir R, Hoefgen R, Hesse H. (2005) Improving the levels of essential amino acids and sulfur metabolites in plants. Biol Chem 386: 817–831 [DOI] [PubMed] [Google Scholar]
- Gao H, Smith J, Yang M, Jones S, Djukanovic V, Nicholson MG, West A, Bidney D, Falco SC, Jantz D, et al. (2010) Heritable targeted mutagenesis in maize using a designed endonuclease. Plant J 61: 176–187 [DOI] [PubMed] [Google Scholar]
- Hacham Y, Song L, Schuster G, Amir R. (2007) Lysine enhances methionine content by modulating the expression of S-adenosylmethionine synthase. Plant J 51: 850–861 [DOI] [PubMed] [Google Scholar]
- Heppard EP, Kinney AJ, Stecca KL, Miao GH. (1996) Developmental and growth temperature regulation of two different microsomal ω-6 desaturase genes in soybeans. Plant Physiol 110: 311–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse H, Kreft O, Maimann S, Zeh M, Willmitzer L, Höfgen R. (2001) Approaches towards understanding methionine biosynthesis in higher plants. Amino Acids 20: 281–289 [DOI] [PubMed] [Google Scholar]
- Hitz WDinventor (August 7, 2001) Nucleotide sequences of canola and soybean palmitoyl-ACP thioesterase genes and their use in the regulation of fatty acid content of the oils of soybean and canola plants. US Patent Application No. RE37317 [Google Scholar]
- Kinney AJ. (2006) Metabolic engineering in plants for human health and nutrition. Curr Opin Biotechnol 17: 130–138 [DOI] [PubMed] [Google Scholar]
- Kinney AJ, Cahoon EB, Damude HG, Hitz WD, Kolar CW, Liu ZBinventors (August 26, 2004) Production of very long chain polyunsaturated fatty acids in oilseed plants. US Patent Application No. WO2004071467 [Google Scholar]
- Li Z, Xing A, Moon BP, McCardell RP, Mills K, Falco SC. (2009) Site-specific integration of transgenes in soybean via recombinase-mediated DNA cassette exchange. Plant Physiol 151: 1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner JE, Okuley JJ, Hitz WD, Kinney AJ, Yadav N, Perez Grau Linventors (September 12, 2006) Genes for microsomal delta-12 fatty acid desaturases and hydroxylases from plants. US Patent Application No. 7105721 [Google Scholar]
- Louwerse JD, Van Lier MCM, Van der Steen DM, De Vlaam CMT, Hooykaas PJJ, Vergunst AC. (2007) Stable recombinase-mediated cassette exchange in Arabidopsis using Agrobacterium tumefaciens. Plant Physiol 145: 1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Hitz WD, Yadav NS, Damude HGinventors (December 12, 2008) DGAT genes from Yarrowia lipolytica for increased seed storage lipid production and altered fatty acid profiles in soybean. US Patent Application No. WO2008147935 [Google Scholar]
- Nanto K, Yamada-Watanabet K, Ebinuma H. (2005) Agrobacterium-mediated RMCE approach for gene replacement. Plant Biotechnol J 3: 203–214 [DOI] [PubMed] [Google Scholar]
- Ow DW. (2002) Recombinase-directed plant transformation for the post-genome era. Plant Mol Biol 48: 183–200 [PubMed] [Google Scholar]
- Roesler K, Rao G. (2000) A single disulfide bond restores thermodynamic and proteolytic stability to an extensively mutated protein. Protein Sci 9: 1642–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla VK, Doyon Y, Miller JC, DeKelver RC, Moehle EA, Worden SE, Mitchell JC, Arnold NL, Gopalan S, Meng X, et al. (2009) Precise genome modification in the crop species Zea mays using zinc-finger nucleases. Nature 459: 437–441 [DOI] [PubMed] [Google Scholar]
- Sun SM, Liu Q. (2004) Transgenic approaches to improve the nutritional quality of plant proteins. In Vitro Cell Dev Biol Plant 40: 155–162 [Google Scholar]
- Tao Y, Bidney D, Gordon-Kamm WJ, Lyznik LAinventors (January 25, 2007) Modified FRT recombination sites and methods of use. US Patent Application No. WO2007011733 [Google Scholar]
- Thu TT, Dewaele E, Trung LQ, Claeys M, Jacobs M, Angenon G. (2007) Increasing lysine levels in pigeonpea (Cajanus cajan (L.) Millsp) seeds through genetic engineering. Plant Cell Tissue Organ Cult 91: 135–143 [Google Scholar]
- Townsend JA, Wright DA, Winfrey RJ, Fu F, Maeder ML, Joung JK, Voytas DF. (2009) High-frequency modification of plant genes using engineered zinc-finger nucleases. Nature 459: 442–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Truksa M, Datla N, Vrinten P, Bauer J, Zank T, Cirpus P, Heinz E, Qiu X. (2005) Stepwise engineering to produce high yields of very long-chain polyunsaturated fatty acids in plants. Nat Biotechnol 23: 1013–1017 [DOI] [PubMed] [Google Scholar]
- Xing A, Moon BP, Mills KM, Falco SC, Li Z. (2010) Revealing frequent alternative polyadenylation and widespread low level transcription read-through of novel plant transcription terminators. Plant Biotechnol J 8: 772–782 [DOI] [PubMed] [Google Scholar]
- Zhu X, Galili G. (2003) Increased lysine synthesis coupled with a knockout of its catabolism synergistically boosts lysine content and also transregulates the metabolism of other amino acids in Arabidopsis seeds. Plant Cell 15: 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Galili G. (2004) Lysine metabolism is concurrently regulated by synthesis and catabolism in both reproductive and vegetative tissues. Plant Physiol 135: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]