Abstract
OBJECTIVES
There is limited and conflicting data regarding the role of esophageal hypersensitivity in the pathogenesis of functional chest pain (FCP). We examined esophageal sensori-motor properties, mechanics and symptoms in subjects with FCP.
METHODS
Esophageal balloon distension test (EBDT) was performed using impedance planimetry in 189 (m/f = 57/132) consecutive subjects with noncardiac, non-reflux chest pain, and 36 (m/f = 16/20) healthy controls. The biomechanical and sensory properties of subjects with and without esophageal hypersensitivity were compared to controls. The frequency, intensity and duration of chest pain were assessed. RESULTS: 143 (75 %) subjects had esophageal hypersensitivity and 46 (25%) had normal sensitivity. Typical chest pain was reproduced in 105/143 (74%) subjects. Subjects with hypersensitivity demonstrated larger cross-sectional area (CSA) (p<0.001), decreased esophageal wall strain (p<0.001) and distensibility (p<0.001), and lower thresholds for perception (p<0.01), discomfort (p<0.01) and pain (p<0.01) compared to those without hypersensitivity or healthy controls. Chest pain scores (mean ± SD) for frequency, intensity and duration were 2.5 ± 0.3, 2.2 ± 0.2 and 2.2 ± 0.2 respectively, and were similar between the two patient groups.
CONCLUSIONS
75% of subjects with FCP demonstrate esophageal hypersensitivity. Visceral hyperalgesia and sensori-motor dysfunction of the esophagus play a key role in the pathogenesis of chest pain.
Keywords: Esophagus, Functional chest pain, sensory and biomechanical properties, Balloon distension test
INTRODUCTION
Noncardiac chest pain (NCCP) or functional chest pain (FCP) is characterized by recurrent, often debilitating chest pain with no clear etiology. Often individuals with this condition receive a negative cardiac evaluation with annual cost estimates exceeding $8 billion, just for ruling out coronary artery disease.(1) This problem is further compounded by significant effects on the quality of life and consumption of significant amounts of health care resources.(2)
The exact mechanism for chest pain is unclear, but several factors have been proposed. Currently, most experts believe that functional gastrointestinal disorders with abdominal or chest pain may be a consequence of one or more mechanisms that include abnormal motility, visceral hypersensitivity,(3-8) microscopic inflammation (9), brain-gut interactions(6, 10), psychosocial factors(11, 12), genetic susceptibility(13-15) and postinfectious neuromuscular and neurotransmitter dysfunction (16, 17). Several neurotransmitters such as serotonin(18), N-methyl-d-aspartate (5) and adenosine (19, 20) have been proposed as mediators for chest pain.
Esophageal hypersensitivity, either due to peripheral or central sensitization has been postulated as a biomarker and a mechanism for functional chest pain (4, 14). This stems from observations of balloon distension test and electrical stimulation that have revealed esophageal hypersensitivity in up to 83% of individuals(21-25). Although some studies have suggested that 50-70% of such subjects have altered sensory perception(21, 26, 26, 27), others have reported a low yield (10-20%) or no difference between controls and patients.(22, 23, 28). However, most of these studies were performed in either small numbers of subjects, typically less than 25, subjects were poorly characterized or the studies were compromised by methodological problems.
We hypothesized that esophageal hypersensitivity causes chest pain in subjects with otherwise unexplained and non-cardiac chest pain. Thus, the aims of this study were to prospectively assess sensori-motor and biomechanical properties of the esophagus in a large consecutive group of subjects with unexplained chest pain, to characterize their symptoms of chest pain and to compare the sensori-motor properties with healthy controls.
MATERIALS AND METHODS
SUBJECTS
Over a 10 year period (1997-2007), we examined consecutive subjects with unexplained chest pain who were referred to the motility laboratory after angiography, stress thallium or stress technetium 99mMIBI tests revealed either normal or insignificant coronary artery disease. Subjects were only included if they reported at least one episode of chest pain per week, and for at least three months. In addition, each subject had gastrointestinal evaluations that included normal upper gastrointestinal endoscopy with esophageal biopsy, normal esophageal manometry, and either had a normal 24 hr pH study or no symptomatic response to a 6-week course of BID proton pump inhibitor therapy. They were recruited from an outpatient setting and had no other significant comorbid illnesses, including pulmonary and musculoskeletal sources for chest pain. Additionally, studies were also performed on healthy volunteers, who had no significant past medical history including no previous thoracic or gastrointestinal surgery, were not taking medication, and had a normal physical examination. All subjects gave written informed consent for the study, which was approved by the Human Investigation Review Board.
All subjects were required to fill out a chest pain symptom questionnaire using a likert-like scale, in which they scored the frequency, intensity and severity of chest pain episodes. The frequency of chest pain was scored as “0” for none, “1” for less than one episode per week, “2” for one episode per week, and “3” for more than one episode per week. The intensity of chest pain was scored as “0” for none, “1” for mild, “2” for moderate, and “3” for severe. The duration of chest pain episodes was scored as “0” for none, “1” for less than 10 minutes, “2” for 10-30 minutes, and “3” for greater than 30 minutes.
Biomechanical and Sensory Properties
We used impedance planimetry to perform balloon distension and to examine the esophageal sensori-motor and biomechanical properties. Impedance planimetry has been described in detail previously (3, 29-32). This method consists of a sensing and a signal processing system. In brief, the sensing system comprised of a flexible plastic probe, 6 mm in diameter, with four ring electrodes and a 4.5 cm long latex balloon that was attached to a leveling container. By raising or lowering the height of the leveling container, a dilute electrolyte solution (0.018% NaCl) was infused into the balloon to achieve its inflation or deflation. The probe also contained three water perfusion side holes for measuring intraluminal pressures. The signal processing system consisted of a generator that gave a constant alternating current of 100 micro amps at 5 KHz, amplifier, impedance detector, analogue-digital converter and a computer (29, 30, 32).
Study Protocol
All subjects came for the study after an overnight fast. Oropharyngeal anesthesia was achieved with a local spray of tetracaine (pontocaine®, Hospira, Lake Forest, IL). The lubricated probe was passed through the mouth until the tip was located 55 cm from the teeth. The subject was asked to lie supine on a bed that was tilted, so that the head side was raised by 30°. The catheter was gradually withdrawn until the balloon lay across the lower esophageal sphincter. The lower esophageal sphincter was identified as a zone of high resting pressure that relaxed with wet swallows. The catheter was pulled out further and taped in position such that the center of the balloon lay 10 cm above the lower esophageal sphincter. All measurements were performed at this level. After a rest period of ten minutes, the balloon pressure was zeroed to the resting intraesophageal pressure by adjusting the height of the leveling container that was situated behind a screen adjacent to the subject. The subjects were therefore blinded to the level of inflation. Next, by raising the leveling container in steps of 5 cm H2O, the balloon pressure was increased up to 65 cm H2O or until the maximum tolerable pressure. Following each distension, the balloon was deflated and re-inflated after a 3-minute rest period. Each inflation was maintained for 3-5 minutes and/or until the CSA measurements on the screen reached a new stable baseline between reactive contractions. At this steady state, the CSA was measured and subjects were asked to score their sensory responses on a Likert scale as follows: Grade “0” for no sensation, Grade “1” for a first sensation of fullness or distension, Grade “2” for discomfort (tolerable), and Grade “3” for pain. During inflations, subjects were asked to refrain from swallowing and to signal the onset of any swallows.
MEASUREMENTS & DATA ANALYSIS
Sensory responses
Balloon CSA was measured according to Ohms Law as described previously (20-22). The computer software provided an output that was directly proportional to the CSA. The data for CSA and intraluminal pressure were stored on disks. The records, visualized off-line on a computer, were analyzed with a software program by one of the authors. The balloon pressure that induced first sensations of fullness, discomfort and pain were noted for each subject and the mean values for the threshold pressures that induced each sensation were calculated. Subjects were considered to have esophageal hypersensitivity if they reported first perception at ≤ 30 cm H2O distension pressure and pain at ≤ 50 cm H2O distension pressure (3). Based on the sensory threshold data, subjects were categorized into those with a hypersensitive esophagus and those with a normosensitive esophagus. The mean thresholds for first perception, discomfort and pain were compared between controls and subjects with hypersensitive or normosensitive esophagus.
Biomechanical Parameters
Cross sectional area (CSA)
At each level of balloon inflation, the radius was calculated from the new steady state baseline CSA. Since previous studies(29, 30) showed that the measurements of CSA conform to the linear portion of the calibration curve up to 40 cm H2O, the biomechanical parameters were calculated up to inflations of 40 cm H2O.
Circumferential wall tension (T)
The total force applied to stretch a segment of the wall was calculated (29) as T = r × dP, where r is the balloon radius (r = square root of the CSA × P–1) and dP is the transmural pressure difference (Law of Laplace). Transmural pressure was defined as the difference between the balloon pressure and the resting pressure in the esophagus, with the assumption that esophageal intraluminal pressure at rest is the same as intrathoracic pressure.
Circumferential Wall Strain
This is a ratio that refers to the relative deformity produced by the application of stress (20). Strain was calculated (29, 30) for each level of inflation as (e) = (rp - r5) r5-1, where rp is the balloon radius at a given pressure and r5 is the radius at 5 cm H2O inflation pressure. The relationship between the changes in wall tension and the changes in strain were plotted during stepwise inflations.
Esophageal Wall Reactivity
Balloon distension induced reactive contractions of the esophageal wall that produced a transient decrease in the cross-sectional area. Reactivity was measured as the difference in height between the steady-state cross-sectional area and the minimum cross-sectional area that was observed during each balloon inflation.
STATISTICS
Means were compared using two tailed Student's t test with Welch's correction for unequal variances, one way analysis of variance (ANOVA), or the Kruskall- Wallis test, where appropriate, using a commercially available software package (Prism 3.0; GraphPad Software, Inc., San Diego, California, USA). Correlations were assessed by calculating Spearman's correlation coefficients for non- parametric data. A p-value < 0.05 was considered significant.
RESULTS
Demographics
Over a 10 year period, 606 subjects with unexplained, non-cardiac chest pain were referred to the GI clinic for evaluation. Among this group, 189 (57 men and 132 women; mean age 47 yrs; range = 30-74) fulfilled our inclusion and exclusion criteria and were enrolled in this study. The remaining subjects were excluded because either their chest pain was explained by cardiac, pulmonary or psychiatric illnesses, or they had gallbladder disease, peptic ulcer disease, gastroesophageal reflux disease, previous abdominal surgeries (Nissen, gastric, etc) or had significant comorbid illnesses (stroke, COPD, etc). In addition, 36 (16 men and 20 women; mean age 43 yrs; range = 24-63) healthy volunteers were enrolled as a control group.
Characteristics of Chest Pain
Our subjects reported frequent episodes of chest pain with a mean (± SD) frequency score of 2.5 ± 0.3. This translates in to at least one episode of chest pain per week. The intensity and duration of pain were rated as 2.2 ± 0.2 and 2.2 ± 0.2 respectively. These data indicate that the chest pain was frequent, moderately severe in intensity and typically lasted between 10 to 30 minutes. In addition, both the hypersensitive and the normosensitive groups had similar baseline chest pain characteristics. The hypersensitive group had chest pain mean frequency score of 2.6 ± 0.2, intensity of 2.2 ± 0.3 and duration of 2.2 ± 0.2. These characteristics were similar to the normosensitive group with pain frequency of 2.4 ± 0.3, intensity and duration of 2.2 ± 0.2 and 2.3 ± 0.2 respectively.
Esophageal Sensory Properties
One hundred and forty three (75%) subjects were found to have hypersensitive esophagus. In this group, 105 (74%) subjects reported that their typical chest pain was reproduced during the balloon distension test. The remaining 46 (25%) subjects had a negative test and were designated as having a normo-sensitive esophagus (Fig. 1).
Fig. 1.
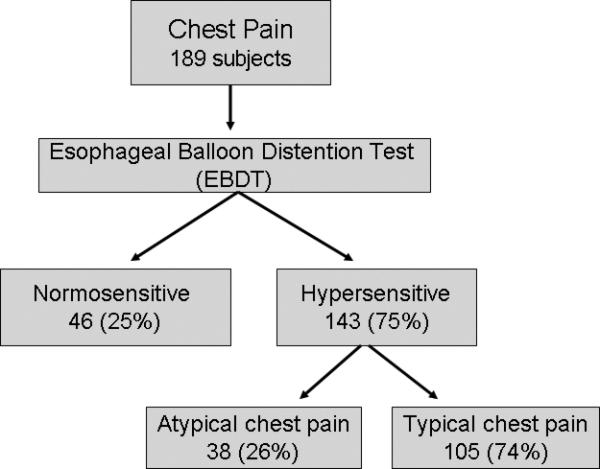
Consort diagram displaying the incidence of esophageal visceral hypersensitivity during esophageal balloon distension test.
The threshold pressures at which balloon distension was first perceived was significantly lower (p<0.001) in subjects with esophageal hypersensitivity (13.4 ± 8 cm H2O) than controls (30 ± 5 cm H2O) [Fig. 2]. Likewise, the threshold for discomfort (21 ±10 cm H2O versus 53 ± 5 cm H2O) and pain (32 ± 13 vs. 62 ± 2 cm H2O) were significantly lower (p<0.001) in subjects with hypersensitivity than controls. The sensory thresholds for first perception, discomfort and pain were significantly lower (p<0.01) for hypersensitive patients compared to normosensitive patients (Fig 2). In addition, there were no differences in the sensory thresholds between subjects with normosensitive esophagus and controls (Fig. 2). Furthermore, there were no statistically significant differences in males and females among both the controls and patient groups for sensory thresholds (p> 0.05); therefore, gender did not affect esophageal sensory thresholds.
Fig. 2.
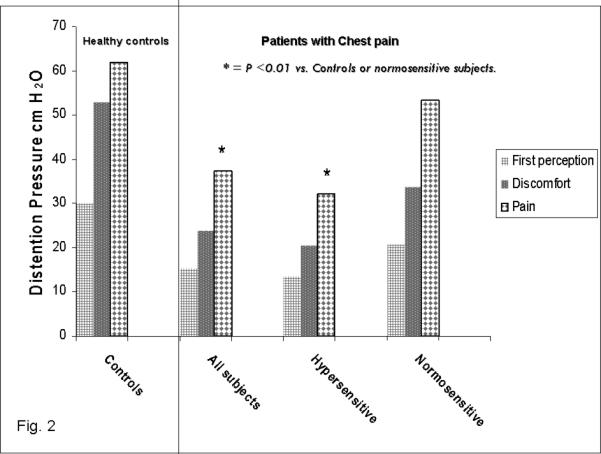
Thresholds for first sensory perception, discomfort and pain in all subject with chest pain, hypersensitive, normosensitive esophagus and healthy controls. Overall, patients had significantly lower thresholds than controls. Subjects with hypersensitive esophagus had significantly lower (p<0.01) thresholds for first perception, discomfort and pain than controls and subjects with normo-sensitive esophagus.
Esophageal Biomechanical Parameters
Cross Sectional Area (CSA)
Intermittent balloon inflations produced a linear increase in CSA of the esophagus in all three groups of subjects (Fig. 3). The mean (±SD) CSA in subjects with hypersensitivity increased from 190 ± 52 mm2 to 525 ± 167 mm2 and was significantly higher (p<0.05) than that observed in the other two groups (Fig. 3). There was no difference between subjects with normosensitive esophagus and controls.
Fig. 3.
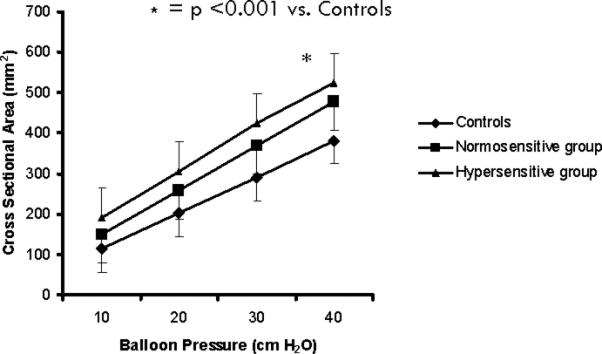
Graded balloon distensions induced a linear increase in CSA in all three groups of subjects. CSA was significantly higher in hypersensitive group compared to those without and healthy controls.
Circumferential Wall Strain
Balloon distension produced an increase in esophageal wall strain from 0.3 ± 0.2 to 1.1 ± 0.1 in subjects with esophageal hypersensitivity. This was significantly lower than seen in subjects with chest pain and normo-sensitive esophagus (0.3 ± 0.1 to 1.3 ± 0.3) and from controls (0.2 ± 0.1 to 1.3 ± 0.3) (p<0.001). This suggests that the esophagus was less deformable in subjects with esophageal hypersensitivity when compared to the other two groups.
Circumferential Wall Tension-Strain relation
In subjects with esophageal hypersensitivity, balloon distension caused an exponential increase in wall tension from 78 ± 12 to 529 ± 90 mm/cm H2O, and in wall strain from 0.3 ± 0.2 to 1.1 ± 0.4. Stepwise increments in balloon pressure were also associated with a linear rise in the circumferential tension-strain relationship, in subjects with hypersensitivity and in controls. However, the tension-strain association for subjects with esophageal hypersensitivity was significantly shifted (p <0.001) to the left (Fig. 4). This suggests that the esophagus is less distensible in subjects with esophageal hypersensitivity.
Fig. 4.
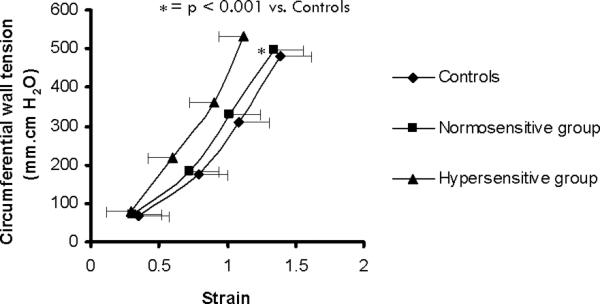
Tension-Strain relationship: stepwise increments in balloon pressure were associated with linear rise in the circumferential tension-strain relationship in the three groups. However, it was significantly shifted to the left in subjects with hypersensitive esophagus. This suggests lower esophageal distensibility in hypersensitive group.
Esophageal Reactivity
Balloon distension produced an increase in esophageal reactivity from 84 ± 50 to 194 ± 100 mm2 in subjects with esophageal hypersensitivity, from 58 ± 25 to 167 ± 63 mm2 in subjects with chest pain and normo-sensitive esophagus and from 54 ± 38 to 150 ± 71 mm2 in controls. This increase was significantly higher in subjects with esophageal hypersensitivity than the other two groups (p<0.001) (Fig. 5).
Fig. 5.
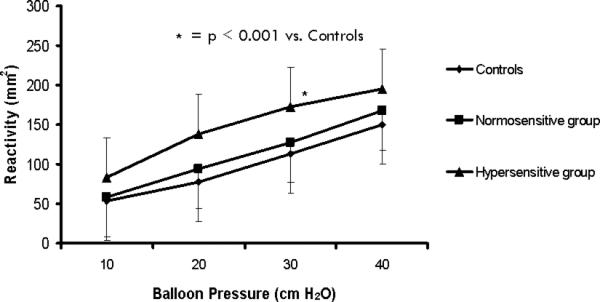
Graded balloon distensions caused an increase in esophageal wall reactivity in all three groups, but it was significantly higher in subjects with hypersensitive esophagus compared to those without and healthy controls.
DISCUSSION
In this prospective study, our objectives were to examine the symptom patterns and define the esophageal sensori-motor function in subjects with functional chest pain. We found that our subjects reported significant and bothersome chest pain that was characterized by more than one episode of moderately intense chest pain per week that typically lasted for 10-30 minutes. Previously, it has been reported that this degree of intense and prolonged duration of chest pain is related to both increased morbidity and a diminished quality of life, and often requires urgent medical evaluation and treatment in emergency rooms and doctors offices (2, 33). Furthermore, a high proportion of these subjects have coexisting other functional GI disorders such as dyspepsia and IBS (34) which further adds to the complexity of their care and towards the overall healthcare burden. Thus, in these individuals, in order to optimize health care and reduce unnecessary health care costs, it is important to define the mechanism(s) for chest pain.
Visceral hypersensitivity is a phenomenon in which the conscious perception of a visceral stimulus is enhanced, independent of the stimulus intensity. In our study, we found that 75% of subjects with unexplained chest pain had esophageal hypersensitivity. The thresholds for sensory perceptions were significantly different between healthy controls and subjects with esophageal hypersensitivity. Additionally, 105 subjects (74%) reported that the balloon distension test reproduced the typical chest pain that they had experienced at home. These findings confirm and extend previous observations that visceral hypersensitivity plays an important role in the pathogenesis of esophageal chest pain. (3)
In addition to the sensory dysfunction, we found that subjects with hypersensitivity also demonstrated that the esophageal CSA was significantly larger than that of healthy controls or those without hypersensitivity. The precise reason for the larger luminal CSA is unclear, but this phenomenon has been consistently observed in several studies (3, 35). One possible explanation could be an adaptive relaxation of the resting tone of the esophageal wall in response to the underlying hypersensitivity. Another possibility could be the effect of aging(35). However, there was no difference in age between subjects with or without hypersensitivity. Hence, this finding must represent a true pathophysiological change in the biomechanical property of the esophageal wall.
Furthermore, the esophageal wall strain, a property that describes the deformability of the esophageal wall was significantly decreased in subjects with esophageal hypersensitivity suggesting that the esophageal wall was stiff. Also, the curve which describes the tension-strain relationship was significantly shifted to the left, suggesting that the esophagus was less distensible in subjects with esophageal hypersensitivity. These findings confirm and extend previous observations that subjects with esophageal hypersensitivity exhibit a stiffer and less compliant esophageal wall (3). In contrast, subjects with chest pain and normosensitive esophagus had CSA and tension-strain characteristics that were similar to healthy controls. This is a new finding. Hence, the changes in biomechanical properties of the esophageal wall are specific and innate to the group of subjects with visceral hyperalgesia. It also seems likely from a mechanical point of view that there is an association between the increased CSA and increased wall stiffness, i.e. a larger CSA results in increased wall tension, which from a stress balance will increase the wall thickness or change the wall constituents to stiffer materials.(51)
The neurophysiological basis for the hypersensitivity is unclear; proposed mechanisms include abnormally heightened peripheral responses to normal sensory inputs and abnormal cognitive processing of such inputs. One possible mechanism is that balloon distension activates the “in series” tension receptors that are possibly located in the muscle layers of the esophagus(36). It is believed that these receptors may be sensitized in subjects with visceral hyperalgesia (37). Consequently, pain is induced at lower levels of distension and at thresholds that are not normally perceived as noxious. Several candidate substances have been proposed that may modulate esophageal sensori-motor function (38-41). For example, adenosine, an endogenous nucleotide, exerts its effects on the peripheral and central nervous system through specific, cell-surface–associated receptors (42-45). In humans, exogenous administration of adenosine has been shown to modulate or induce somatic or visceral pain (46, 47) probably due to peripheral sensitization or activation of nociceptive afferents. In a recent controlled study, we found that the esophageal sensory thresholds decreased after adenosine infusion suggesting that adenosine may induce visceral hyperalgesia (19). Likewise, we have shown that theophylline, an adenosine receptor antagonist, significantly increases esophageal pain thresholds, and reduces the number of chest pain episodes in NCCP subjects (48, 49). In addition to these peripheral mechanisms, Sarkar et al reported that the development of hyperexcitability of dorsal horn neurons, can occur either because of irritation of peripheral tissue or descending neuronal influences originating in the brainstem. These findings suggest a central role for the pathogenesis of chest pain.(5)
Is the hypersensitivity or the hyperreactivity of the esophageal smooth muscle predominantly responsible for chest pain? In one study, following atropine, the esophageal wall relaxed and its reactivity decreased but the sensory thresholds were unchanged(4). This suggested that the hypersensitivity or visceral hyperalgesia of the esophageal wall is the predominant mechanism for functional chest pain(4). Furthermore, the esophagus is not uniformly hypersensitive (32, 50). In one study, 2/3rd of subjects with functional chest pain were hypersensitive at both levels whereas 1/3rd were hypersensitive either at the smooth muscle or the striated muscle portion (50). Hence, the balloon distension test should be performed at both levels, particularly in subjects with a negative test at the smooth muscle segment.
Although not widely available, the esophageal balloon distension test can be developed at a small cost and may prove to be more cost-effective than empiric therapy, repeated endoscopies or cardiac reassessment. In USA, there is an approved CPT code for performing the Esophageal balloon distension test (91040). The technique is simple, safe, inexpensive, comprehensive, and reproducible (30). Balloon distension test can provide an objective method of evaluation for new drugs that modify neuromuscular function.
In conclusion, by using the balloon distension test we found that 75% of a large series of subjects with significant and persistent unexplained/functional chest pain demonstrated a hypersensitive esophagus. These findings clearly establish that visceral hyperalgesia and sensori-motor dysfunction of the esophagus play a major role in the pathogenesis of unexplained, functional, noncardiac chest pain.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Xing Zhao and Ranjit Mudipalli, MD for technical assistance, and Bridget Zimmerman, PhD for her advice with statistical analysis.
Funding:
Dr. Rao is supported by Grant R01 DK057100, National Institutes of Health. This work was also supported by a Clinical Research Grant from the American College of Gastroenterology.
Footnotes
Portions of this work were presented at Digestive Disease Week 2007 and published as abstracts; Gastroenterology 2007, 132:4,S1:p8; Gastroenterology, 2007, 132:4,S1:p47.
References
- 1.Hutter AM, Jr, Amsterdam EA, Jaffe AS. 31st bethesda conference. emergency cardiac care. task force 2: Acute coronary syndromes: Section 2B--chest discomfort evaluation in the hospital. J Am Coll Cardiol. 2000;35:853–862. [PubMed] [Google Scholar]
- 2.Achem SR, DeVault KR. Recent developments in chest pain of undetermined origin. Curr Gastroenterol Rep. 2000;2:201–209. doi: 10.1007/s11894-000-0062-4. [DOI] [PubMed] [Google Scholar]
- 3.Rao SS, Gregersen H, Hayek B, Summers RW, Christensen J. Unexplained chest pain: The hypersensitive, hyperreactive, and poorly compliant esophagus. Ann Intern Med. 1996;124:950–958. doi: 10.7326/0003-4819-124-11-199606010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rao SS, Hayek B, Summers RW. Functional chest pain of esophageal origin: Hyperalgesia or motor dysfunction. Am J Gastroenterol. 2001;96:2584–2589. doi: 10.1111/j.1572-0241.2001.04101.x. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar S, Aziz Q, Woolf CJ, Hobson AR, Thompson DG. Contribution of central sensitisation to the development of non-cardiac chest pain. Lancet. 2000;356:1154–1159. doi: 10.1016/S0140-6736(00)02758-6. [DOI] [PubMed] [Google Scholar]
- 6.Mertz H, Morgan V, Tanner G, Pickens D, Price R, Shyr Y, Kessler R. Regional cerebral activation in irritable bowel syndrome and control subjects with painful and nonpainful rectal distention. Gastroenterology. 2000;118:842–848. doi: 10.1016/s0016-5085(00)70170-3. [DOI] [PubMed] [Google Scholar]
- 7.Schmulson M, Chang L, Naliboff B, Lee OY, Mayer EA. Correlation of symptom criteria with perception thresholds during rectosigmoid distension in irritable bowel syndrome patients. Am J Gastroenterol. 2000;95:152–156. doi: 10.1111/j.1572-0241.2000.01677.x. [DOI] [PubMed] [Google Scholar]
- 8.Camilleri M, Talley NJ. Pathophysiology as a basis for understanding symptom complexes and therapeutic targets. Neurogastroenterol Motil. 2004;16:135–142. doi: 10.1111/j.1365-2982.2004.00516.x. [DOI] [PubMed] [Google Scholar]
- 9.Spiller RC. Inflammation as a basis for functional GI disorders. Best Pract Res Clin Gastroenterol. 2004;18:641–661. doi: 10.1016/j.bpg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Kim DY, Camilleri M. Serotonin: A mediator of the brain-gut connection. Am J Gastroenterol. 2000;95:2698–2709. doi: 10.1111/j.1572-0241.2000.03177.x. [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999;45(Suppl 2):II25–30. doi: 10.1136/gut.45.2008.ii25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palsson OS, Drossman DA. Psychiatric and psychological dysfunction in irritable bowel syndrome and the role of psychological treatments. Gastroenterol Clin North Am. 2005;34:281–303. doi: 10.1016/j.gtc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Park MI, Camilleri M. Genetics and genotypes in irritable bowel syndrome: Implications for diagnosis and treatment. Gastroenterol Clin North Am. 2005;34:305–317. doi: 10.1016/j.gtc.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Locke GR, 3rd, Zinsmeister AR, Talley NJ, Fett SL, Melton LJ., 3rd Familial association in adults with functional gastrointestinal disorders. Mayo Clin Proc. 2000;;75:907–912. doi: 10.4065/75.9.907. [DOI] [PubMed] [Google Scholar]
- 15.Levy RL, Jones KR, Whitehead WE, Feld SI, Talley NJ, Corey LA. Irritable bowel syndrome in twins: Heredity and social learning both contribute to etiology. Gastroenterology. 2001;121:799–804. doi: 10.1053/gast.2001.27995. [DOI] [PubMed] [Google Scholar]
- 16.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: Postal survey of patients. BMJ. 1997;314:779–782. doi: 10.1136/bmj.314.7083.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okhuysen PC, Jiang ZD, Carlin L, Forbes C, DuPont HL. Post-diarrhea chronic intestinal symptoms and irritable bowel syndrome in north american travelers to mexico. Am J Gastroenterol. 2004;99:1774–1778. doi: 10.1111/j.1572-0241.2004.30435.x. [DOI] [PubMed] [Google Scholar]
- 18.Varia I, Logue E, O'connor C, Newby K, Wagner HR, Davenport C, Rathey K, Krishnan KR. Randomized trial of sertraline in patients with unexplained chest pain of noncardiac origin. Am Heart J. 2000;140:367–372. doi: 10.1067/mhj.2000.108514. [DOI] [PubMed] [Google Scholar]
- 19.Remes-Troche JM, Chahal P, Mudipalli R, Rao SS. Adenosine modulates oesophageal sensorimotor function in humans. Gut. 2008 doi: 10.1136/gut.2006.116699. [DOI] [PubMed] [Google Scholar]
- 20.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 21.Barish CF, Castell DO, Richter JE. Graded esophageal balloon distention. A new provocative test for noncardiac chest pain. Dig Dis Sci. 1986;31:1292–1298. doi: 10.1007/BF01299805. [DOI] [PubMed] [Google Scholar]
- 22.Cannon RO, 3rd, Cattau EL, Jr, Yakshe PN, Maher K, Schenke WH, Benjamin SB, Epstein SE. Coronary flow reserve, esophageal motility, and chest pain in patients with angiographically normal coronary arteries. Am J Med. 1990;88:217–222. doi: 10.1016/0002-9343(90)90145-4. [DOI] [PubMed] [Google Scholar]
- 23.Nevens F, Janssens J, Piessens J, Ghillebert G, De Geest H, Vantrappen G. Prospective study on prevalence of esophageal chest pain in patients referred on an elective basis to a cardiac unit for suspected myocardial ischemia. Dig Dis Sci. 1991;36:229–235. doi: 10.1007/BF01300762. [DOI] [PubMed] [Google Scholar]
- 24.Lasch H, DeVault KR, Castell DO. Intraesophageal balloon distention in the evaluation of sensory thresholds: Studies on reproducibility and comparison of balloon composition. Am J Gastroenterol. 1994;89:1185–1190. [PubMed] [Google Scholar]
- 25.Gregersen H, Kassab G. Biomechanics of the gastrointestinal tract. Neurogastroenterol Motil. 1996;8:277–297. doi: 10.1111/j.1365-2982.1996.tb00267.x. [DOI] [PubMed] [Google Scholar]
- 26.Richter JE, Barish CF, Castell DO. Abnormal sensory perception in patients with esophageal chest pain. Gastroenterology. 1986;91:845–852. doi: 10.1016/0016-5085(86)90685-2. [DOI] [PubMed] [Google Scholar]
- 27.Deschner WK, Maher KA, Cattau EL, Jr, Benjamin SB. Intraesophageal balloon distention versus drug provocation in the evaluation of noncardiac chest pain. Am J Gastroenterol. 1990;85:938–943. [PubMed] [Google Scholar]
- 28.Ghillebert G, Janssens J, Vantrappen G, Nevens F, Piessens J. Ambulatory 24 hour intraoesophageal pH and pressure recordings v provocation tests in the diagnosis of chest pain of oesophageal origin. Gut. 1990;31:738–744. doi: 10.1136/gut.31.7.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregersen H, Djurhuus JC. Impedance planimetry: A new approach to biomechanical intestinal wall properties. Dig Dis. 1991;9:332–340. doi: 10.1159/000171320. [DOI] [PubMed] [Google Scholar]
- 30.Rao SS, Hayek B, Summers RW. Impedance planimetry: An integrated approach for assessing sensory, active, and passive biomechanical properties of the human esophagus. Am J Gastroenterol. 1995;90:431–438. [PubMed] [Google Scholar]
- 31.Orvar KB, Gregersen H, Christensen J. Biomechanical characteristics of the human esophagus. Dig Dis Sci. 1993;38:197–205. doi: 10.1007/BF01307535. [DOI] [PubMed] [Google Scholar]
- 32.Patel RS, Rao SS. Biomechanical and sensory parameters of the human esophagus at four levels. Am J Physiol. 1998;275:G187–91. doi: 10.1152/ajpgi.1998.275.2.G187. [DOI] [PubMed] [Google Scholar]
- 33.Katz PO, Codario R, Castell DO. Approach to the patient with unexplained chest pain. Compr Ther. 1997;23:249–253. [PubMed] [Google Scholar]
- 34.Mudipalli RS, Remes-Troche JM, Andersen L, Rao SS. Functional chest pain: Esophageal or overlapping functional disorder. J Clin Gastroenterol. 2007;41:264–269. doi: 10.1097/01.mcg.0000225521.36160.1b. [DOI] [PubMed] [Google Scholar]
- 35.Rao SS, Mudipalli RS, Mujica VR, Patel RS, Zimmerman B. Effects of gender and age on esophageal biomechanical properties and sensation. Am J Gastroenterol. 2003;98:1688–1695. doi: 10.1111/j.1572-0241.2003.07589.x. [DOI] [PubMed] [Google Scholar]
- 36.Cervero F, Janig W. Visceral nociceptors: A new world order? Trends Neurosci. 1992;15:374–378. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- 37.Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- 38.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–692. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 39.Broekaert D, Fischler B, Sifrim D, Janssens J, Tack J. Influence of citalopram, a selective serotonin reuptake inhibitor, on oesophageal hypersensitivity: A double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2006;23:365–370. doi: 10.1111/j.1365-2036.2006.02772.x. [DOI] [PubMed] [Google Scholar]
- 40.Takeda T, Kassab G, Liu J, Nabae T, Mittal RK. Effect of atropine on the biomechanical properties of the oesophageal wall in humans. J Physiol. 2003;547:621–28. doi: 10.1113/jphysiol.2002.028795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barlow JD, Gregersen H, Thompson DG. Identification of the biomechanical factors associated with the perception of distension in the human esophagus. Am J Physiol Gastrointest Liver Physiol. 2002;282:G683–9. doi: 10.1152/ajpgi.00134.2001. [DOI] [PubMed] [Google Scholar]
- 42.Sawynok J. Adenosine receptor activation and nociception. Eur J Pharmacol. 1998;347:1–11. doi: 10.1016/s0014-2999(97)01605-1. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burnstock G, Wood JN. Purinergic receptors: Their role in nociception and primary afferent neurotransmission. Curr Opin Neurobiol. 1996;6:526–532. doi: 10.1016/s0959-4388(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 45.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 46.Sylven C, Beermann B, Jonzon B, Brandt R. Angina pectoris-like pain provoked by intravenous adenosine in healthy volunteers. Br Med J (Clin Res Ed) 1986;293:227–230. doi: 10.1136/bmj.293.6541.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- 48.Rao SS, Mudipalli RS, Remes-Troche JM, Utech CL, Zimmerman B. Theophylline improves esophageal chest pain--a randomized, placebo-controlled study. Am J Gastroenterol. 2007;102:930–938. doi: 10.1111/j.1572-0241.2007.01112.x. [DOI] [PubMed] [Google Scholar]
- 49.Minton NA, Henry JA. Pharmacodynamic interactions between infused adenosine and oral theophylline. Hum Exp Toxicol. 1991;10:411–418. doi: 10.1177/096032719101000608. [DOI] [PubMed] [Google Scholar]
- 50.Rao SS, Hayek B, Mudipalli R, Gregersen H. Does esophageal function vary at the striated and smooth muscle segments in functional chest pain? Am J Gastroenterol. 2002;97:2201–2207. doi: 10.1111/j.1572-0241.2002.05973.x. [DOI] [PubMed] [Google Scholar]
- 51.Gregersen H. Bionechanics of the gastrointestinal tract. Spinger verlag; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


