Abstract
Background
Studies have found a modest association between depression and obesity, especially in women. Given the substantial genetic contribution to both depression and obesity, we sought to determine whether shared genetic influences are responsible for the association between these 2 conditions.
Methods
Data were obtained from 712 monozygotic and 281 dizygotic female twin pairs who are members of the community-based University of Washington Twin Registry. The presence of depression was determined by self-report of doctor-diagnosed depression. Obesity was defined as body mass index of ≥ 30, based on self-reported height and weight. Generalized estimating regression models were used to assess the age-adjusted association between depression and obesity. Univariate and bivariate structural equation models estimated the components of variance attributable to genetic and environmental influences.
Results
We found a modest phenotypic association between depression and obesity (Odds Ratio = 1.6, 95% Confidence Interval = 1.2-2.1). Additive genetic effects contributed substantially to depression (57%) and obesity (81%). The best fitting bivariate model indicated that 12% of the genetic component of depression is shared with obesity.
Conclusions
The association between depression and obesity in women may be in part due to shared genetic risk for both conditions. Future studies should examine the genetic, environmental, social, and cultural mechanisms underlying the relationship between this association.
Keywords: Depression, Genetics, Heritability, Obesity, Twins
INTRODUCTION
Depression and obesity are prevalent, complex and costly public health concerns [1-3]. Approximately 10% of the U.S. population suffers from depression annually [4], and the prevalence of obesity is rising with up to 30% of the population obese and 5% extremely obese [5]. Both conditions are associated with negative health outcomes such as cardiovascular disease and diabetes [6-14].
Many studies have examined the association between depression and obesity, yet findings are inconsistent. While some studies have reported obese individuals have 5 times the risk of major depression than the non-obese [3, 15-18], others have not reproduced these findings [19-22]. The relationship between depression and obesity also may be, to some extent, sex dependent [23]. For example, four studies have shown that middle-aged men appear to be less depressed than their normal weight counterparts [24-27]. However, in a recent large epidemiological study obese men were at increased risk for mood and anxiety disorders [28]. Conversely, obese and overweight women consistently are at great risk of experiencing depression across the lifespan [28-33]. These sex differences suggest that environmental factors influence the co-morbidity of depression and obesity. However, both depression [34] and obesity [35] have a strong genetic basis and shared genetic risk factors are possible. In that regard, the involvement of some of the same genes in the mechanisms underlying both conditions [36-43], highlights the potential for a shared genetic vulnerability to both depression and obesity.
Twin studies offer a unique opportunity to investigate shared disease etiology by evaluating the relative contributions of genetic and environmental factors to more than one condition. Despite the known substantial genetic contribution to both depression and obesity, no twin study has examined the potential for shared genetic influences for both conditions. Our goals were to: 1) determine if depression and obesity are associated in female twins from a community-based twin registry; 2) assess the genetic influence on each trait; and 3) estimate the magnitude of shared genetic influence that could explain the association between depression and obesity.
MATERIALS AND METHODS
Sample
The University of Washington Twin Registry is a community-based sample of twins derived from the drivers’ license applications of the Washington State Department of Licensing. Detailed description of recruitment strategy, response rate, representativeness of the Registry twins is presented elsewhere [44]. In short, drivers’ license numbers in Washington State are derived from a person’s name and date of birth, thus, the Department of Licensing asks every new applicant if s/he is a twin to avoid issuing duplicate license and identification numbers to twins. The University of Washington Twin Registry receives lists of license applicants who are twins, and each member of the pair is invited to join the Registry and complete a health survey. The brief survey contains items on demographics, habits, doctor-diagnosed health conditions, symptoms, healthcare use, and various abridged, standardized measures of physical and mental health. While the Registry is an ongoing collection of participants, data presented here were obtained from 2002-2006. All Registry procedures and data collection involved in this study were approved by the University of Washington Institutional Review Board. Informed consent was obtained from all twins.
Self-report measures
Questions about childhood similarity that correctly classify zygosity with an accuracy of 95 – 98% compared with biological indicators were used to determine zygosity [45-48]. Sociodemographic factors included age, sex, race, education, and marital status. To determine lifetime depression, twins were given a list of conditions including depression and asked: “Has your doctor ever told you that you have any of the following conditions?” Body mass index (BMI) was calculated based on height and weight (weight/height2), and obesity was defined as a BMI of ≥ 30 kg/m2. All questions were asked concurrently.
Statistical analyses
Descriptive statistics for demographic and health characteristics were calculated using means and standard deviations for continuous variables and percents for categorical variables. To investigate the overall age-adjusted association of depression and obesity, we fit a generalized estimating regression model to account for the lack of independence of members of twin pairs. Further, the association between depression and obesity in monozygotic (MZ) and dizygotic (DZ) pairs was assessed by 3 types of polychoric correlations: phenotypic, twin, and cross-twin, cross-trait. Phenotypic correlations measure the association of depression and obesity within individuals, whereas twin correlations examine the within pair similarity for a trait. Cross-twin, cross-trait correlations assess the degree of association for 2 traits, for example, the relationship of depression in twin 1 and obesity in twin 2, as well as depression in twin 2 and obesity in twin 1.
Classical twin analyses compare phenotypic similarity in MZ twins and DZ twins; greater phenotypic similarity in MZ than DZ twins indicates a genetic component in the parameter of interest. We used univariate structural equation modeling to estimate the additive genetic (A), common environmental (C), and unique environmental (E) influences on depression and obesity individually [49]. Models were fitted assuming an additive genetic correlation of 1.0 for MZ and 0.5 for DZ twins, a shared environmental correlation of 1.0 for all twins, and a unique environmental correlation of 0.0 for all twins. Modeling began by estimating parameters for the full model (ACE), and then reduced models were constructed by removing specific parameters. The goodness-of-fit of each reduced model was compared with the full model using a likelihood ratio test. We present parameter estimates, 95% confidence intervals, and goodness-of-fit statistics for the full model (ACE), and models in which all variance was attributable to genetic and specific environmental factors (AE), and common and specific environmental factors (CE). Parameters were removed from the model if doing so did not result in a significant degradation of model fit (p ≤ 0.05). Models were also evaluated using Akaike’s Information Criterion [50], where a lower value indicates a superior fit. Finally, the proportions of variance due to additive genetics, common environment, and unique environment were estimated from the final best-fitting model.
Structural equation modeling can also be used to estimate the variability in 2 or more phenotypes due to shared vulnerabilities. We used bivariate structural equation modeling to estimate shared genetic and environmental vulnerabilities with a full Cholesky decomposition that specified a general multivariate covariance structure and allowed for both specific and shared influences on depression and obesity. We identified the final best-fitting, most parsimonious model by removing parameters that did not significantly degrade the fit of the model based on likelihood ratio tests and the Akaike Information Criteria [50] .We present goodness-of-fit statistics for the full and reduced bivariate models of depression and obesity, and trait-specific and shared variance components for the best-fitting model.
Descriptive analyses and polychoric correlations were computed using Stata 9.2 for Windows (Stata Corp LP, 2006). Structural equation models were fit using MxGui version 1.4.06 (Department of Psychiatry, Virginia Commonwealth University, 2003). A p-value of 0.05 was considered criteria for a significant degradation of model fit.
RESULTS
Descriptive characteristics
Table 1 presents the demographic and health characteristics of 712 MZ and 281 DZ female twin pairs that had complete data and were included in the analyses by depression status. The sample included 481 twins with self-reported doctor-diagnosed depression and 1,505 non-depressed twins. The depressed and non-depressed groups were similar in age, education, race, marital status, zygosity, and mean BMI. Overall, 19% of depressed twins and 12% of non-depressed twins were classified as obese. There was a positive age-adjusted association between depression and obesity (Odds Ratio = 1.6, 95% Confidence Interval = 1.2-2.1).
Table 1.
Demographic and health characteristics of depressed and non-depressed female twins
| Characteristic | Depressed (n = 481) |
Not depressed (n = 1,505) |
|---|---|---|
| Demographic | ||
| Age, mean years (SD) | 33.9 (14.1) | 31.0 (14.7) |
| Education, mean years (SD) | 13.7 (2.5) | 13.7 (2.2) |
| White, % | 89 | 85 |
| Married or cohabitating, % | 54 | 58 |
| Zygosity, % | ||
| Monozygotic | 69 | 73 |
| Dizygotic | 31 | 27 |
| Health | ||
| Body mass index, mean kg/m2(SD) | 25.1 (5.7) | 24.1 (4.8) |
| Body mass index, % | ||
| < 18.5 (underweight) | 6 | 4 |
| 18.5 – 24.99 (normal) | 56 | 65 |
| 25.0 – 29.99 (overweight) | 20 | 19 |
| ≥ 30.0 (obese) | 19 | 12 |
Polychoric correlations
Table 2 presents phenotypic, twin, and cross-twin, cross-trait polychoric correlations for depression and obesity by zygosity. Phenotypic correlations ranged from 0.14-0.20 in MZ twins and from 0.09-0.23 in DZ twins. Larger MZ than DZ twin correlations for depression (0.55 versus 0.36) and obesity (0.81 versus 0.51) indicated a genetic basis for each trait. The higher cross-twin, cross-trait correlations in MZ compared with DZ pairs suggested modest shared genetic influences on both traits.
Table 2.
Polychoric correlations for depression and obesity in female twin pairs by zygosity
| Twin 1 |
Twin 2 |
|||
|---|---|---|---|---|
| Depression | Obesity | Depression | Obesity | |
| Monozygotic | ||||
| Twin 1 | ||||
| Depression | 1.00 | |||
| Obesity | 0.20 (0.04, 0.36)* | 1.00 | ||
| Twin 2 | ||||
| Depression | 0.55 (0.44, 0.65)† | 0.08 (−0.09, 0.25)‡ | 1.00 | |
| Obesity | 0.14 (−0.01, 0.30)‡ | 0.81 (0.73, 0.89)† | 0.14 (−0.02, 0.30)* | 1.00 |
| Dizygotic | ||||
| Twin 1 | ||||
| Depression | 1.00 | |||
| Obesity | 0.09 (−0.14, 0.31)* | 1.00 | ||
| Twin 2 | ||||
| Depression | 0.36 (0.17, 0.54)† | 0.02 (−0.20, 0.25)‡ | 1.00 | |
| Obesity | 0.06 (−0.17, 0.29)‡ | 0.51 (0.32, 0.69)† | 0.23 (0.01, 0.45)* | 1.00 |
Phenotypic correlation between depression and obesity;
Twin correlation;
Cross-twin, cross-trait correlation; Depression measure was self-report of a doctor diagnosis; Obesity measure was BMI ≥ 30.0 kg/m2.
Univariate structural equation modeling
Table 3 shows the results of the univariate structural equation models for depression and obesity. The best fitting model for depression included both additive genetic effects (57%) and unique environmental effects (43%). Similarly, the best fitting model for obesity included additive genetic effects (81%) and unique environmental exposures (19%).
Table 3.
Univariate structural equation models of depression and obesity in female twin pairs
| Estimates of variance components† (95% Confidence Intervals) |
Test of model fit | ||||||
|---|---|---|---|---|---|---|---|
| Model* | Additive genetic (A) |
Common environment (C) |
Unique environment (E) |
χ 2 | df | P value | AIC‡ |
| Depression | |||||||
| ACE | 0.42 (0.00, 0.65) | 0.14 (0.00, 0.51) | 0.44 (0.35, 0.55) | – | – | – | – |
| AE | 0.57 (0.46, 0.66) | – | 0.43 (0.34, 0.54) | 0.49 | 1 | 0.48 | −1.51 |
| CE | – | 0.50 (0.40, 0.58) | 0.50 (0.42, 0.60) | 3.92 | 1 | 0.05 | 1.92 |
| Obesity | |||||||
| ACE | 0.59 (0.20 – 0.87) | 0.21 (0.00, 0.56) | 0.20 (0.13, 0.30) | – | – | – | – |
| AE | 0.81 (0.72, 0.88) | – | 0.19 (0.12, 0.28) | 1.08 | 1 | 0.30 | −0.92 |
| CE | – | 0.71 (0.62, 0.78) | 0.29 (0.22, 0.38) | 9.57 | 1 | 0.002 | 7.57 |
ACE refers to a model that includes additive genetics (A), common environment (C), and unique environment (E), AE only includes additive genetics and unique environment, and CE only includes common and unique environment;
Proportion of variance caused by additive genetics, shared environment, and unique environment according to each model;
Akaike‘s information criterion (AIC) is a global measure of goodness of fit; the best-fitting and most parsimonious models are shown in bold.
Bivariate structural equation modeling
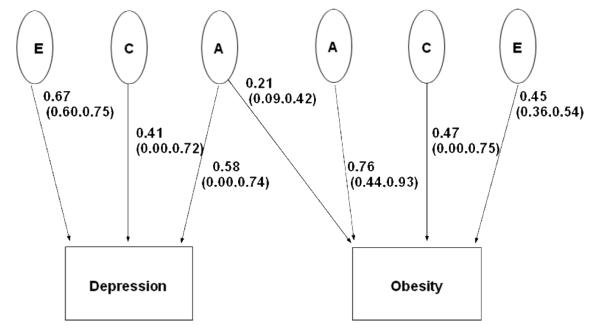
The best-fitting, most parsimonious bivariate model included only shared additive genetic influences for depression and obesity as presented in Table 4. Based on the best fitting model, we estimated that 12% of the genetic component of depression is shared with obesity. Figure 1 illustrates the full model with standardized pathway coefficients and the relative magnitude of the genetic, common and unique environmental effects on both traits.
Table 4.
Bivariate structural equation models of depression and obesity in female twin pairs
| Estimates of variance components (95% Confidence Intervals) |
Test of model fit | ||||||
|---|---|---|---|---|---|---|---|
| Shared component | Additive genetic (A) |
Common environment (C) |
Unique environment (E) |
χ 2 | df | P value | AIC* |
| ACE | – | – | – | – | |||
| Depression | 0.38 (0.03, 0.64) | 0.16 (0.05, 0.48) | 0.45 (0.35, 0.56) | ||||
| Obesity | 0.59 (0.20, 0.84) | 0.21 (0.00, 0.56) | 0.20 (0.13, 0.29) | ||||
| CE | 0.75 | 1 | 0.39 | −1.25 | |||
| Depression | 0.36 (0.00, 0.61) | 0.18 (0.08, 0.54) | 0.46 (0.36, 0.57) | ||||
| Obesity | 0.57 (0.19, 0.84) | 0.23 (0.00, 0.58) | 0.20 (0.14, 0.30) | ||||
| AE | 0.01 | 1 | 0.92 | −1.99 | |||
| Depression | 0.38 (0.01, 0.64) | 0.17 (0.00, 0.52) | 0.45 (0.35, 0.56) | ||||
| Obesity | 0.58 (0.20, 0.69) | 0.22 (0.00, 0.56) | 0.20 (0.13, 0.29) | ||||
| AC | 1.16 | 1 | 0.28 | −0.84 | |||
| Depression | 0.38 (0.01, 0.64) | 0.16 (0.00, 0.51) | 0.45 (0.35 (0.56) | ||||
| Obesity | 0.57 (0.20, 0.86) | 0.21 (0.00, 0.56) | 0.20 (0.13, 0.29) | ||||
| A | 1.32 | 2 | 0.52 | −2.68 | |||
| Depression | 0.38 (0.02, 0.64) | 0.17 (0.00, 0.51) | 0.45 (0.36, 0.56) | ||||
| Obesity | 0.58 (0.20, 0.87) | 0.22 (0.00, 0.57) | 0.20 (0.13, 0.29) | ||||
| C | 3.24 | 2 | 0.20 | −0.76 | |||
| Depression | 0.37 (0.00, 0.60) | 0.17 (0.07, 0.53) | 0.45 (0.36, 0.57) | ||||
| Obesity | 0.58 (0.20, 0.82) | 0.22 (0.02, 0.57) | 0.20 (0.16, 0.30) | ||||
| E | 5.73 | 2 | 0.06 | 1.27 | |||
| Depression | 0.36 (0.00, 0.63) | 0.17 (0.00, 0.53) | 0.46 (0.36, 0.58) | ||||
| Obesity | 0.56 (0.18, 0.85) | 0.22 (0.00, 0.42) | 0.21 (0.15, 0.31) | ||||
| None | 12.89 | 3 | 0.01 | 6.89 | |||
| Depression | 0.38 (0.00, 0.64) | 0.16 (0.00, 0.53) | 0.45 (0.36, 0.57) | ||||
| Obesity | 0.59 (0.20, 0.86) | 0.21 (0.00, 0.56) | 0.20 (0.13, 0.30) | ||||
Akaike‘s information criterion (AIC) is a global measure of goodness of fit; the best-fitting and most parsimonious model is shown in bold.
Figure 1.
Path diagram depicting additive genetic effects shared by depression and obesity plus additive genetic (A), common environmental (C), and unique environmental (E) effects unique to each trait. The parameter estimates and 95% confidence intervals are path coefficients, indicating the relative importance of the latent variables A, C, and E to depression and obesity.
DISCUSSION
To our knowledge, this is the first study to examine the shared genetic contribution to depression and obesity in a twin population. We found that a self-reported doctor diagnosis of depression was modestly associated with obesity, as well as significant genetic components to depression and obesity in female twins. Our analyses also suggested that the association between depression and obesity in women may be partially due to shared genetic risk factors for both conditions.
Our findings support previous research on the genetic basis of depression and obesity, separately. A meta analysis of twin studies found that the heritability of major depression is approximately 37% with minimal to no common environmental influences on the phenotype [51]. Similarly, a recent study with the largest twin sample to date estimated the heritability of major depression to be 38%, with heritability higher in women (42%) than in men (29%) [34]. Obesity also manifests a strong familial influence with the prevalence 2-8 times higher in families of obese individuals than in the population at large [52]. Heritability estimates for obesity range from 50% to 90% depending on the population [53], with higher estimates in twin studies (50%-80%) than in adoption studies (10%-30%) [52].
Both depression and obesity appear to be polygenic with multiple genes contributing to the development of each condition. Although results from linkage and association studies require replication, a recent meta-analysis of genetic studies on major depression identified 6 likely depression susceptibility genes [54]. Other studies have focused on genes involved in the serotonergic pathway in the brain [55], and polymorphisms in the promoter region of the 5-hydroxylase-tryptamine transporter (5-HTT) protein have been associated with personality traits that may predispose individuals to depression [56] or interact with environmental factors such as life stress to result in depression [57]. Likewise, studies on the genetics of obesity have revealed hundreds of associated genes [58-61], with variants of the fat mass and obesity associated gene (FTO) strongly associated with adiposity in multiple populations [62-65].
The association between depression and obesity could be due to several factors [1], including the influence of each trait on the other [66, 67], shared environmental determinants, or shared genetic factors. We found a modest overlap in the genetic risk factors that increase liability to both depression and obesity. In contrast, a recent family-based study did not detect an association between symptoms of depression and measures of body composition (e.g., BMI) in a genetically isolated community in the southwest of The Netherlands [68]. Although more research is needed to reconcile these disparate results, our findings are consistent with association studies demonstrating that the glucocorticoid receptor gene, the corticotrophin releasing hormone receptor gene, the serotonin 2A and 2C receptor genes, and the dopamine receptor D4 gene are linked to both depression and obesity [36-43]. In addition, polymorphisms in the norepinephrine transporter gene have been associated with both depression and feeding behavior [69]. Finally, disturbances in the hypothalamic-pituitary-adrenal axis, immune functioning, and the serotonin/dopamine pathways in both conditions [70-72], raise the possibility that both depression and obesity are influenced by gene-by-environment interactions. Such interactions are encompassed within the significant additive genetic component noted in this study. Environmental and behavioral factors such as emotional eating and physical inactivity also may play a role in the influence of each trait on the other [23, 73-75]. Clearly, identifying modifiable environmental factors and examining the genetic, environmental, social, and cultural mechanisms underlying the relationship between depression and obesity can lead to more effective prevention and treatment strategies for both conditions.
This study has several limitations. First, because our variables were intended as screening items on a large survey, we were limited in our assessments of depression and obesity. The potentially higher level of error in these brief assessments would be expected to reduce genetic effects estimates. Therefore, our findings are likely a conservative estimate of the shared genetic contribution to depression and obesity. Second, the use of self-reported doctor diagnosed depression could have resulted in response bias or misclassification of depression. One study noted that self-report of a physician diagnosis of depression underestimated current depressive symptoms, though both self-reported physician diagnosis and depressive symptoms were independently associated with obesity [76], and therefore appear to be valid measures to assess the relation of depression and obesity. While the self-reported rates of doctor diagnoses depression in our sample were consistent with previously reported population-based rates of lifetime depression in women [77], nonetheless, our findings should be replicated with symptom- or diagnosis-based measures of depression. In addition, no measure of current depression was available, thus our findings are restricted to the association between lifetime depression and obesity. Second, BMI ascertained through self-reported height and weight may underestimate true BMI or be differentially reported by twins with and without depression. Although women tend to underreport their weight, subjective assessments of weight do not appear to be affected by depression or obesity [78]. Thus, potential misclassification due to self-report is unlikely to significantly affect the estimates of association and would not be expected to differ between MZ and DZ twin pairs. Finally, while the rates of obesity in this sample are in line with those of White, well-educated women in Washington State [79], our results may not be generalizable to twin populations with different racial or educational backgrounds.
CONCLUSIONS
Our findings from a community-based sample of twins provide evidence for a modest shared genetic vulnerability to depression and obesity in women. Although future research should confirm these results using standardized clinical criteria to establish the diagnosis of depression and objective data to determine BMI, these results highlight the need for more systematic approaches to genetic association studies pertaining to depression and obesity. Such research may eventually yield new insights into the common pathophysiology and risk factors for these conditions. Additionally, examining the environmental, social, and cultural mechanisms in these 2 conditions jointly can identify targets for effective prevention and treatment strategies for individuals with comorbid depression and obesity.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health awards R01AR051524 (Dr. Afari) and U19AI38429 (Dr. Buchwald). Dr. Afari also is supported by the VA Center of Excellence for Stress and Mental Health. Dr. Schur is supported by K23 DK070826. We also thank the twins who are taking part in the University of Washington Twin Registry for their time and enthusiasm. The authors have no conflicts of interest to declare.
REFERENCES
- 1.Stunkard AJ, Faith MS, Allison KC. Depression and obesity. Biol Psychiatry. 2003 Aug 1;54(3):330–337. doi: 10.1016/s0006-3223(03)00608-5. [DOI] [PubMed] [Google Scholar]
- 2.Kress AM, Peterson MR, Hartzell MC. Association between obesity and depressive symptoms among U.S. Military active duty service personnel, 2002. J Psychosom Res. 2006 Mar;60(3):263–271. doi: 10.1016/j.jpsychores.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Simon GE, Von Korff M, Saunders K, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry. 2006 Jul;63(7):824–830. doi: 10.1001/archpsyc.63.7.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994 Jan;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 5.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002 Oct 9;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 6.Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006 Apr;331(4):166–174. doi: 10.1097/00000441-200604000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Katon W, Lin EH, Kroenke K. The association of depression and anxiety with medical symptom burden in patients with chronic medical illness. Gen Hosp Psychiatry. 2007 Mar-Apr;29(2):147–155. doi: 10.1016/j.genhosppsych.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults - The Evidence Report. Obes Res. 1998 Sep;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 9.Schulz R, Beach SR, Ives DG, Martire LM, Ariyo AA, Kop WJ. Association between depression and mortality in older adults: the Cardiovascular Health Study. Arch Intern Med. 2000 Jun 26;160(12):1761–1768. doi: 10.1001/archinte.160.12.1761. [DOI] [PubMed] [Google Scholar]
- 10.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001 Jun;24(6):1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 11.Pohjasvaara T, Vataja R, Leppavuori A, Kaste M, Erkinjuntti T. Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur J Neurol. 2001 Jul;8(4):315–319. doi: 10.1046/j.1468-1331.2001.00182.x. [DOI] [PubMed] [Google Scholar]
- 12.Robbins J, Hirsch C, Whitmer R, Cauley J, Harris T. The association of bone mineral density and depression in an older population. J Am Geriatr Soc. 2001 Jun;49(6):732–736. doi: 10.1046/j.1532-5415.2001.49149.x. [DOI] [PubMed] [Google Scholar]
- 13.Koenig HG, George LK, Larson DB, McCullough ME, Branch PS, Kuchibhatla M. Depressive symptoms and nine-year survival of 1,001 male veterans hospitalized with medical illness. Am J Geriatr Psychiatry. 1999 Spring;7(2):124–131. [PubMed] [Google Scholar]
- 14.Abramson J, Berger A, Krumholz HM, Vaccarino V. Depression and risk of heart failure among older persons with isolated systolic hypertension. Arch Intern Med. 2001 Jul 23;161(14):1725–1730. doi: 10.1001/archinte.161.14.1725. [DOI] [PubMed] [Google Scholar]
- 15.Kasen S, Cohen P, Chen H, Must A. Obesity and psychopathology in women: a three decade prospective study. Int J Obes (Lond) 2007 Sep 25; doi: 10.1038/sj.ijo.0803736. [DOI] [PubMed] [Google Scholar]
- 16.Pine DS, Goldstein RB, Wolk S, Weissman MM. The association between childhood depression and adulthood body mass index. Pediatrics. 2001 May;107(5):1049–1056. doi: 10.1542/peds.107.5.1049. [DOI] [PubMed] [Google Scholar]
- 17.Roberts RE, Kaplan GA, Shema SJ, Strawbridge WJ. Are the obese at greater risk for depression? Am J Epidemiol. 2000 Jul 15;152(2):163–170. doi: 10.1093/aje/152.2.163. [DOI] [PubMed] [Google Scholar]
- 18.Roberts RE, Deleger S, Strawbridge WJ, Kaplan GA. Prospective association between obesity and depression: evidence from the Alameda County Study. Int J Obes Relat Metab Disord. 2003 Apr;27(4):514–521. doi: 10.1038/sj.ijo.0802204. [DOI] [PubMed] [Google Scholar]
- 19.Friedman MA, Brownell KD. Psychological correlates of obesity: moving to the next research generation. Psychol Bull. 1995 Jan;117(1):3–20. doi: 10.1037/0033-2909.117.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Faubel M. Body image and depression in women with early and late onset obesity. J Psychol. 1989 Jul;123(4):385–395. doi: 10.1080/00223980.1989.10542993. [DOI] [PubMed] [Google Scholar]
- 21.Hallstrom T, Noppa H. Obesity in women in relation to mental illness, social factors and personality traits. J Psychosom Res. 1981;25(2):75–82. doi: 10.1016/0022-3999(81)90093-3. [DOI] [PubMed] [Google Scholar]
- 22.Onyike CU, Crum RM, Lee HB, Lyketsos CG, Eaton WW. Is obesity associated with major depression? Results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2003 Dec 15;158(12):1139–1147. doi: 10.1093/aje/kwg275. [DOI] [PubMed] [Google Scholar]
- 23.Dragan A, Akhtar-Danesh N. Relation between body mass index and depression: a structural equation modeling approach. BMC Med Res Methodol. 2007;7:17. doi: 10.1186/1471-2288-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palinkas LA, Wingard DL, Barrett-Connor E. Depressive symptoms in overweight and obese older adults: a test of the “jolly fat” hypothesis. J Psychosom Res. 1996 Jan;40(1):59–66. doi: 10.1016/0022-3999(95)00542-0. [DOI] [PubMed] [Google Scholar]
- 25.Stewart AL, Brook RH. Effects of being overweight. Am J Public Health. 1983 Feb;73(2):171–178. doi: 10.2105/ajph.73.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crisp AH, Queenan M, Sittampaln Y, Harris G. ‘Jolly fat’ revisited. J Psychosom Res. 1980;24(5):233–241. doi: 10.1016/0022-3999(80)90013-6. [DOI] [PubMed] [Google Scholar]
- 27.Crisp AH, McGuiness B. Jolly fat: relation between obesity and psychoneurosis in general population. Br Med J. 1976 Jan 3;1(6000):7–9. doi: 10.1136/bmj.1.6000.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barry D, Pietrzak RH, Petry NM. Gender differences in associations between body mass index and DSM-IV mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Ann Epidemiol. 2008 Jun;18(6):458–466. doi: 10.1016/j.annepidem.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon GE, Ludman EJ, Linde JA, et al. Association between obesity and depression in middle-aged women. Gen Hosp Psychiatry. 2008 Jan-Feb;30(1):32–39. doi: 10.1016/j.genhosppsych.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sachs-Ericsson N, Burns AB, Gordon KH, et al. Body mass index and depressive symptoms in older adults: the moderating roles of race, sex, and socioeconomic status. Am J Geriatr Psychiatry. 2007 Sep;15(9):815–825. doi: 10.1097/JGP.0b013e3180a725d6. [DOI] [PubMed] [Google Scholar]
- 31.Erickson SJ, Robinson TN, Haydel KF, Killen JD. Are overweight children unhappy?: Body mass index, depressive symptoms, and overweight concerns in elementary school children. Arch Pediatr Adolesc Med. 2000 Sep;154(9):931–935. doi: 10.1001/archpedi.154.9.931. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter KM, Hasin DS, Allison DB, Faith MS. Relationships between obesity and DSM-IV major depressive disorder, suicide ideation, and suicide attempts: results from a general population study. Am J Public Health. 2000 Feb;90(2):251–257. doi: 10.2105/ajph.90.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Istvan J, Zavela K, Weidner G. Body weight and psychological distress in NHANES I. Int J Obes Relat Metab Disord. 1992 Dec;16(12):999–1003. [PubMed] [Google Scholar]
- 34.Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006 Jan;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- 35.Cummings DE, Schwartz MW. Genetics and pathophysiology of human obesity. Annu Rev Med. 2003;54:453–471. doi: 10.1146/annurev.med.54.101601.152403. [DOI] [PubMed] [Google Scholar]
- 36.Challis BG, Luan J, Keogh J, Wareham NJ, Farooqi IS, O’Rahilly S. Genetic variation in the corticotrophin-releasing factor receptors: identification of single-nucleotide polymorphisms and association studies with obesity in UK Caucasians. Int J Obes Relat Metab Disord. 2004 Mar;28(3):442–446. doi: 10.1038/sj.ijo.0802564. [DOI] [PubMed] [Google Scholar]
- 37.Lerer B, Macciardi F, Segman RH, et al. Variability of 5-HT2C receptor cys23ser polymorphism among European populations and vulnerability to affective disorder. Mol Psychiatry. 2001 Sep;6(5):579–585. doi: 10.1038/sj.mp.4000883. [DOI] [PubMed] [Google Scholar]
- 38.Lin RC, Wang XL, Dalziel B, Caterson ID, Morris BJ. Association of obesity, but not diabetes or hypertension, with glucocorticoid receptor N363S variant. Obes Res. 2003 Jun;11(6):802–808. doi: 10.1038/oby.2003.111. [DOI] [PubMed] [Google Scholar]
- 39.Liu Z, Zhu F, Wang G, et al. Association study of corticotropin-releasing hormone receptor1 gene polymorphisms and antidepressant response in major depressive disorders. Neurosci Lett. 2007 Mar 6;414(2):155–158. doi: 10.1016/j.neulet.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 40.Leon S Lopez, Croes EA, Sayed-Tabatabaei FA, Claes S, Van Broeckhoven C, van Duijn CM. The dopamine D4 receptor gene 48-base-pair-repeat polymorphism and mood disorders: a meta-analysis. Biol Psychiatry. 2005 May 1;57(9):999–1003. doi: 10.1016/j.biopsych.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 41.Poston WS, 2nd, Ericsson M, Linder J, et al. D4 dopamine receptor gene exon III polymorphism and obesity risk. Eat Weight Disord. 1998 Jun;3(2):71–77. doi: 10.1007/BF03339991. [DOI] [PubMed] [Google Scholar]
- 42.Rosmond R, Bouchard C, Bjorntorp P. 5-HT2A receptor gene promoter polymorphism in relation to abdominal obesity and cortisol. Obes Res. 2002 Jul;10(7):585–589. doi: 10.1038/oby.2002.79. [DOI] [PubMed] [Google Scholar]
- 43.van West D, Van Den Eede F, Del-Favero J, et al. Glucocorticoid receptor gene-based SNP analysis in patients with recurrent major depression. Neuropsychopharmacology. 2006 Mar;31(3):620–627. doi: 10.1038/sj.npp.1300898. [DOI] [PubMed] [Google Scholar]
- 44.Afari N, Noonan C, Goldberg J, et al. University of Washington Twin Registry: construction and characteristics of a community-based twin registry. Twin Res Hum Genet. 2006 Dec;9(6):1023–1029. doi: 10.1375/183242706779462543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin Genet. 1989 Jun;35(6):423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- 46.Magnus P, Berg K, Nance WE. Predicting zygosity in Norwegian twin pairs born 1915-1960. Clin Genet. 1983 Aug;24(2):103–112. doi: 10.1111/j.1399-0004.1983.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 47.Reed T, Plassman BL, Tanner CM, Dick DM, Rinehart SA, Nichols WC. Verification of self-report of zygosity determined via DNA testing in a subset of the NAS-NRC twin registry 40 years later. Twin Res Hum Genet. 2005 Aug;8(4):362–367. doi: 10.1375/1832427054936763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torgersen S. The determination of twin zygosity by means of a mailed questionnaire. Acta Genet Med Gemellol (Roma) 1979;28(3):225–236. doi: 10.1017/s0001566000009077. [DOI] [PubMed] [Google Scholar]
- 49.Neale M, Cardon L. Methodology for the study of twins and families. Kluwer Academic Press; Dordrecht, the Netherlands: 1992. [Google Scholar]
- 50.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:327–332. [Google Scholar]
- 51.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000 Oct;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 52.Perusse L. [Genetics of human obesity: results from genetic epidemiology studies] Ann Endocrinol (Paris) 2000 Dec;61(Suppl 6):24–30. [PubMed] [Google Scholar]
- 53.Barsh GS, Farooqi IS, O’Rahilly S. Genetics of body-weight regulation. Nature. 2000 Apr 6;404(6778):644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- 54.Lopez-Leon S, Janssens AC, Gonzalez-Zuloeta Ladd AM, et al. Meta-analyses of genetic studies on major depressive disorder. Mol Psychiatry. 2008 Aug;13(8):772–785. doi: 10.1038/sj.mp.4002088. [DOI] [PubMed] [Google Scholar]
- 55.Christiansen L, Tan QH, Iachina M, et al. Candidate gene polymorphisms in the serotonergic pathway: Influence on depression symptomatology in an elderly population. Biological Psychiatry. 2007 Jan;61(2):223–230. doi: 10.1016/j.biopsych.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 56.Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996 Nov;274(5292):1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 57.Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003 Jul;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 58.Bouchard C. Genetics of obesity in humans: current issues. Ciba Found Symp. 1996;201:108–115. doi: 10.1002/9780470514962.ch7. discussion 115-107, 188-193. [DOI] [PubMed] [Google Scholar]
- 59.Yang W, Kelly T, He J. Genetic epidemiology of obesity. Epidemiol Rev. 2007;29:49–61. doi: 10.1093/epirev/mxm004. [DOI] [PubMed] [Google Scholar]
- 60.Rankinen T, Zuberi A, Chagnon YC, et al. The human obesity gene map: the 2005 update. Obesity (Silver Spring) 2006 Apr;14(4):529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- 61.Li S, Loos RJ. Progress in the genetics of common obesity: size matters. Curr Opin Lipidol. 2008 Apr;19(2):113–121. doi: 10.1097/MOL.0b013e3282f6a7f3. [DOI] [PubMed] [Google Scholar]
- 62.Thorleifsson G, Walters GB, Gudbjartsson DF, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009 Jan;41(1):18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- 63.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009 Jan;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007 Jul;3(7):e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007 May 11;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Blaine B. Does depression cause obesity?: A meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008 Nov;13(8):1190–1197. doi: 10.1177/1359105308095977. [DOI] [PubMed] [Google Scholar]
- 67.Atlantis E, Baker M. Obesity effects on depression: systematic review of epidemiological studies. Int J Obes (Lond) 2008 Jun;32(6):881–891. doi: 10.1038/ijo.2008.54. [DOI] [PubMed] [Google Scholar]
- 68.Choy WC, Lopez-Leon S, Aulchenko YS, et al. Role of shared genetic and environmental factors in symptoms of depression and body composition. Psychiatr Genet. 2009 Feb;19(1):32–38. doi: 10.1097/YPG.0b013e328320804e. [DOI] [PubMed] [Google Scholar]
- 69.Bonisch H, Bruss M. The norepinephrine transporter in physiology and disease. Handb Exp Pharmacol. 2006;(175):485–524. doi: 10.1007/3-540-29784-7_20. [DOI] [PubMed] [Google Scholar]
- 70.Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998 Aug 1;44(3):151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 71.Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2003 Jan 1;116B(1):103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 72.Bornstein SR, Schuppenies A, Wong ML, Licinio J. Approaching the shared biology of obesity and depression: the stress axis as the locus of gene-environment interactions. Mol Psychiatry. 2006 Oct;11(10):892–902. doi: 10.1038/sj.mp.4001873. [DOI] [PubMed] [Google Scholar]
- 73.Dallman M, Pecoraro N, la Fleur S. Chronic stress and comfort foods: Self-medication and abdominal obesity. Brain, Behavior, and Immunity. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Wise L, Adams-Campbell L, Palmer J, Rosenberg L. Leisure time physical activity in relation to depressive symptoms in the Black Women’s Health Study. Annals of Behavioral Medicine. 2006;32:68–76. doi: 10.1207/s15324796abm3201_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strine TW, Mokdad AH, Dube SR, et al. The association of depression and anxiety with obesity and unhealthy behaviors among community-dwelling US adults. Gen Hosp Psychiatry. 2008 Mar-Apr;30(2):127–137. doi: 10.1016/j.genhosppsych.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Fan AZ, Strine TW, Huang Y, et al. Self-rated depression and physician-diagnosed depression and anxiety in Florida adults: Behavioral Risk Factor Surveillance System, 2006. Prev Chronic Dis. 2009 Jan;6(1):A10. [PMC free article] [PubMed] [Google Scholar]
- 77.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993 Oct-Nov;29(2-3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 78.Jeffery RW, Finch EA, Linde JA, et al. Does clinical depression affect the accuracy of self-reported height and weight in obese women? Obesity (Silver Spring) 2008 Feb;16(2):473–475. doi: 10.1038/oby.2007.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Drewnowski A, Rehm CD, Solet D. Disparities in obesity rates: analysis by ZIP code area. Soc Sci Med. 2007 Dec;65(12):2458–2463. doi: 10.1016/j.socscimed.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]