Abstract
Strategies of saccadic planning must take into account both the required level of accuracy of the saccades, and the time and resources needed to plan and execute the movements. To determine relationships between accuracy and time, we studied sequences of saccades made to scan a set of stationary targets located at the corners of an imaginary square. Target separation and size varied. The time taken to complete saccadic sequences increased with the required level of precision, in agreement with the classical Fitts’s Law (1954) relationship. This was mainly due to the use of error-correcting secondary saccades, whose frequency increased with target separation and decreased with target size. Increases in the time spent fixating near each target did not increase the accuracy of the next primary saccade in the sequence. Instead, secondary saccades were the principal means of correcting landing errors of primary saccades. The results are consistent with a scanning strategy that discourages careful planning of individual saccades in favor of increasing the rate of saccadic production (i.e., exploration), using secondary saccades as needed to correct saccadic landing errors.
Keywords: eye movements, saccades, saccadic planning, saccadic latency, saccadic sequences, speed-accuracy tradeoff, latency-accuracy tradeoff, Fitts’s Law
1. Introduction
Saccadic eye movements play a crucial role in the performance of natural visual tasks by bringing the line of sight to selected, task-relevant objects or locations. Strategies of saccadic planning must take into account both the required level of accuracy of the saccades, as well as the time and resources needed to plan and execute the movements. An ideal strategy will bring the line of sight to selected targets without placing undue burden on the processing resources that are needed for other visual or cognitive aspects of the task.
An important component of any saccadic strategy is the decision about how to trade-off the rates at which saccades are made for their spatial accuracy. Prior studies of a variety of saccadic tasks has revealed a preference for speed over accuracy, that is, a preference to scan at a brisk rate even when this resulted in frequent or large saccadic landing errors, which required correction by secondary saccades. This strategy was preferred to one of improving the accuracy of primary saccades by scanning at a slower pace (Araujo, Kowler & Pavel, 2001; Cohen, Schnizter, Gersch, Singh & Kowler, 2007; Coëffé O’Regan, 1987; Viviani & Swenson, 1982; Hooge & Erklens, 1999). Such preferences have been observed when saccadic targets are presented among surrounding non-targets competing for attention. The present study investigates whether, and how, trade-offs between scanning rate and saccadic accuracy apply to sequences of saccades in the absence of a difficult selection requirement.
Options to trade-off scanning rate and accuracy might be expected to apply to saccades even in the absence of a difficult selection requirement because trade-offs between speed and accuracy are characteristic of rapid aimed movements. Trade-offs have been shown to conform to the classical relationship known as Fitts’s Law (Fitts, 1954). According to Fitt’s Law the time to complete a movement aimed to a target will increase with both the movement distance and the required level of precision. Specifically:
| (1) |
where MT is movement time, S is the traveled distance, and D is the diameter of the target in which the movement must land (A and B are constants). The quantity log 2 (2S / D) is Fitts’s Index of Difficulty (ID). Fitts’s Law has been found to hold for a variety of voluntary aimed movements (for reviews, see Plamondon & Alimi, 1997; Wright & Meyer, 1983). In an influential analysis of Fitts’s Law, Meyer, Abrams, Kornblum, Wright & Smith (1988) showed that behavior compatible with Fitts’s Law can be explained by trade-offs between the time devoted to a primary movement and the frequency of secondary submovements.
Fitts’s Law has been found to apply to saccadic waveforms, with increases in peak saccadic velocity (due to increases in amplitude) associated with a greater scatter of landing positions (Abrams, Meyer & Kornblum, 1989; Harris & Wolpert, 1998; Al-Aidroos, Fischer, Adam & Pratt, 2008). However, the relevance of Fitts’s Law, and speed-accuracy tradeoffs more generally, to the performance of saccadic sequences has not been determined. Grosjean, Shiffrar & Knoblich (2007), for example, showed that Fitts’s Law can apply to the perception of a sequence of arm movements, but assumed on the basis of a preliminary report (Chi & Lin, 1997) that that Fitts’s Law would not apply to the sequences of saccades made when viewing the movements.
The prior studies of Fitts’s Law did not consider the contribution of latency, a decision that is appropriate for limb movements (Fitts & Peterson, 1964; Klapp, 1975), but not necessarily for saccades. Saccadic latency includes the time needed to carry out sensory processing and decision-making (Carpenter & Williams, 1995; Palmer, Huk & Shadlen, 2005). Just as perceptual judgments of location become more precise with increasing processing time (Pizlo, Rosenfield & Epelboim, 1995), longer saccadic latencies may result in better saccadic precision (i.e., reduced scatter of endpoints) and better saccadic accuracy (smaller average error) (Kowler & Blaser, 1995; Lemij & Collewijn, 1989). Increases in latency have been shown to improve saccadic accuracy and precision when targets are surrounded by distractors (Cohen et al., 2007; Coëffé & O’Regan, 1987; Ottes, Van Gisbergen & Eggermont, 1985; Viviani & Swensson, 1982), but the effects of increased latency on accuracy and precision when targets are in isolation has not been determined. Jin and Reeves (2009), for example, showed that the saccadic gap effect could not be explained by latency-accuracy trade-offs.
A recent study of saccades with implications for latency/accuracy relationships is Harwood, Madelain, Krauzlis & Wallman (2008), who discussed the issue in terms of spatial scale. In Harwood et al.’s experiments, subjects were instructed to make a saccade to the center of stimulus (a rotating segmented ring) when it stepped to an unpredictable location. Prior studies had shown that latencies of saccades remain about the same across a large range of target diameters (Kowler & Blaser, 1995) and target step sizes (Frost & Poppel, 1976; Heywood & Churcher, 1980; Pratt, Dodd & Welsh, 2006; but see Dick, Ostendorf, Kraft & Ploner, 2004), with latencies increasing only when step size falls below about 1 deg (Kowler & Anton, 1987; Wyman & Steinman, 1973; Kalesnykas & Hallett, 1994). By contrast, Harwood et al. (2008) showed that saccadic latencies were modulated by the ratio of the target step size to target diameter, with latencies decreasing as the ratio of step size to diameter increased. Interestingly, Harwood et al.’s results, in which smaller targets at larger eccentricities led to a decrease in saccadic latencies, is opposite to Fitts’s Law, if we assume that Fitts’s Law applies to latencies as well as to movement times.
The diverse pattern of results summarized above shows that key relationships between saccadic latency and spatial accuracy, and the implications of such relationships for planning of saccades during the performance of visual tasks, are unresolved.
The goal of the present study is to create a more unified approach to understanding the relationship between saccadic latency, accuracy and precision during the performance of saccadic sequences, incorporating the roles of target size and eccentricity. This work differs from many previous studies of saccadic planning in a number of ways:
First, saccades were made to targets in fixed, known locations. This method minimizes uncertainty about target timing and location that may affect the planning of saccades made to follow random target step displacements. For example, greater uncertainty about target location encourages a strategy of planning saccades on the basis of the past history of target displacements, and not just the displacement in the present trial (Kapoula, 1985; Kowler & Blaser, 1995).
Second, the present study focuses on sequences of saccades (e.g., Zingale & Kowler, 1987; Inhoff, 1986; Gersch, Kowler & Dosher, 2004; Gersch, Schnitzer, Dosher & Kowler, 2009; Viviani & Swenson, 1982; Hooge & Erklens, 1996, 1998, 1999), rather than a single saccade to one isolated target. Sequences are more representative of natural visual tasks and may shed light on options and strategies for controlling scanning rate and spatial precision in relatively realistic situations.
Third, by examining performance of saccadic sequences, this study considers multiple factors: the in-flight time of the saccades, the saccadic latency (i.e., the duration of the pauses between successive saccades), and any secondary saccades that might occur during intervals between successive saccades. The distinction between in-flight time and pause duration is a major difference between saccadic eye movements and many other sequential motor behaviors. In other motor behaviors, such as tapping or wrist rotations, sensory feedback can be processed during the movement. Thus, it could be assumed that there is no intermediate pause between the end of a primary submovement and the start of secondary submovement (Meyer et al., 1988; Saunders & Knill, 2005). For saccadic eye movements, on the other hand, the movement is so rapid that saccades cannot be reprogrammed on the basis of new visual information once initiated (Chen-Harris, Joiner, Ethier, Zee & Shadmehr, 2008), thus the saccadic pause interval (latency) is the main source of visual feedback, and should not be ignored in a study of speed/accuracy relationships.
Fitts’s paradigm allows for the study of the effects of target size and target eccentricity within the same sequential scanning task. It is important to emphasize that our goal in investigating the applicability of Fitts’s Law to sequence of saccades is not to attempt a strict comparison with manual responses (which is problematic, given the many differences between saccades and other aiming movements, e.g., Stritzke, Trommershäuser, & Gegenfurtner, 2009), but rather to use Fitts’s paradigm as a launching pad for understanding the role of latency/accuracy trade-offs in saccadic planning.
2. Experiment 1
2.1 Methods
2.1.1. Stimulus display
Stimuli were displayed on a Dell P793 CRT monitor (13 deg × 12 deg; viewing distance 115 cm, 1.46 pixels/min arcs; refresh rate 75 Hz, non-interlaced).
There were 9 different types of stimuli which were defined by different target separations and target diameters. Each stimulus display contained 4 identical target circles, which were located at the corners of an imaginary square. Circles were black, drawn on a gray background set to 54 cd/m2. Target separation was defined as the distance between the centers of two adjacent circles. The diameter of the target circles were set to one of four values (15, 45, 90 or 180 min arc), and separation was set to one of three values (64, 127 or 255 min arc). In order to avoid the superimposing of targets (large targets and small separations), only 9 combinations of size and separation were tested (see Table 1). The experimental condition on each trial was selected randomly from the 9 possible conditions.
Table 1.
Target separations (S) and target diameters (D )in Experiment 1
| S (min arc) | D (min arc) | ID= log 2 (2S /D) |
|---|---|---|
| 64 | 15 | 3.09 |
| 45 | 1.51 | |
| 127 | 15 | 4.08 |
| 45 | 2.50 | |
| 90 | 1.50 | |
| 255 | 15 | 5.09 |
| 45 | 3.50 | |
| 90 | 2.50 | |
| 180 | 1.50 |
2.1.2 Procedure
Before each trial, one of the target circles, randomly selected, was displayed on the screen (Fig. 1a, left). Subjects were instructed to fixate the circle and press a button to start the trial when ready. After the button press, the other three target circles appeared (Fig. 1a, right).
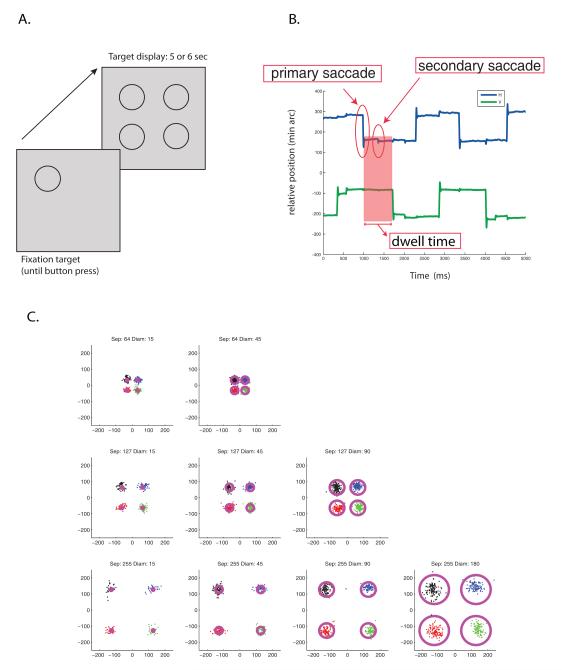
Figure 1.
A: Sequence of frames in a trial. The first frame contained the fixation circle and the second frame the experiment display of 4 targets. In the actual experiment, any of four targets can be the fixation target. B: Sample eye trace. Blue line represents horizontal eye position and green line represents vertical eye position. This eye trace shows an example of a secondary saccade following the primary saccade. The shaded area shows the dwell time (see text, section 2.2.1) between successive primary saccades. C: Representative endpoints of primary saccades from subject JW superimposed on displays of 4 targets for each target separation and diameter. Data were collapsed across the 4 different possible start locations.
Subjects were instructed to choose their own direction of scan (either clockwise or counterclockwise). Directions were maintained throughout the experiment. They were instructed to begin from the initial fixation circle and to look at each target circle in sequence at a brisk, yet comfortable pace, this is, to scan as fast as possible without skipping targets or feeling that the rate was high enough to be uncomfortable. They were told to aim successive saccades at each target and not to miss or skip any. They were not told to aim at any particular location within a target. The stimulus remained on the display for either 5 or 6 seconds depending on the subject. These durations were determined in a preliminary session to be sufficient to allow subjects to complete at least 2 loops around the four targets. Fig. 1b shows a sample eye trace, and Fig. 1c shows all of the target sizes and separations with a representative set of saccadic endpoints superimposed. The data in Fig. 1c were collapsed across the four possible starting locations.
2.1.3. Subjects
Four subjects (LM, AW, JW and SLC) were tested, all with normal, uncorrected vision, and all naïve to the experimental design and hypothesis.
2.1.4. Eye movement recording
Horizontal and vertical movements of the right eye were recorded using a Generation IV Double Purkinje Image Tracker (Crane & Steele, 1978). The left eye was covered and the head was stabilized with a dental biteboard. The tracker’s voltage output was fed on-line through a low pass 100 Hz filter to a 12-bit analog to digital converter (ADC). The ADC, controlled by a PC, sampled the eye’s position every 2 ms. The digitized voltages were stored for analysis. Tracker noise level was measured with an artificial eye after the tracker had been adjusted so as to have the same first and fourth image reflections as the average subject’s eye. Filtering and sampling rate were the same as those used in the experiment. Noise level, expressed as a standard deviation of position samples, was 0.4′ for horizontal and 0.7′ for vertical positions. Recordings were made with the tracker’s automatically movable optical stage (auto-stage) and focus servo disabled.
The beginning and ending positions of saccades were detected off-line by means of a computer algorithm employing an acceleration criterion (Gersch et al., 2004). Saccadic duration was the time between detected onset and offset, including any ‘overshoots’ at the end of saccades (Steinman, Haddad, Skavenski, & Wyman, 1973; Cornsweet & Crane, 1973). Values of the criterion was determined empirically for individual observers by examining a large sample of analog recordings of eye positions
2.1.5. Data Analysis
‘Loop duration’ was defined as the time spent completing a loop around the four targets. The first saccade in the loop was defined as the first initiated saccade after all targets appeared. The last saccade in the loop was defined as the final saccade returning back to the initial target circles, including any secondary saccades following the large primary saccade between successive targets. Loop duration extended from the beginning of the latency interval of the first saccade to the offset of the final saccade in a loop. In the majority of trials subjects completed 2 loops around the set of 4 targets. The time of onset of the second loop was set to the time of completion of the first loop.
Most of the analyses to be described were based on individual segments of a loop, that is, properties of the primary and secondary saccades made to take the line of sight from one target to the next. The primary saccade was the first saccade from target (N) to the next target (N+1). Primary saccades were often followed by a secondary saccade (see Fig. 1b). The time between consecutive primary saccades was termed the ‘dwell time’, which was the time spent looking near each target. Thus, dwell time includes any secondary saccades that may occur.
2.1.6. Numbers of trials tested and excluded
All subjects were tested 23-36 experimental sessions, where sessions contained 50 trials each, leading to a total of 1150 trials for LM, 1350 trials for AW, 1150 trials for JW and 1800 trials for SLC. About 4 to 5 sessions were tested each day.
Loops could be discarded for a number of reasons: Loss of tracker lock ( .3% for LM, JW and SLC; 2.2% for AW), latency of initial saccade < 100 ms (.3% for LM; 3% for AW; 7% for JW and 14% for SLC), or failure to complete the loop or stay on the path (i.e., skipped a target or changed direction) (3% for LM; 1.6% for AW; 9% for JW; 13% for SLC). The data reported were based on a total of 2226 loops for LM, 2516 for AW, 1928 for JW and 2602 for SLC. Some of the analyses to be reported were based on individual segments of a loop, where a segment is defined as the primary saccade made between successive targets. Each loop contained 4 segments.
2.2. Results
We first examine performance of the saccadic sequences to determine whether it is consistent with the classical Fitts’s Law relationship (see Introduction). Then, we will examine the effect of saccadic duration, latency, and secondary saccades on performance, focusing on the relationships between both latency and secondary saccades on spatial accuracy and precision.
2.2.1. Time to complete the saccadic sequence increased with Fitts’s Index of Difficulty
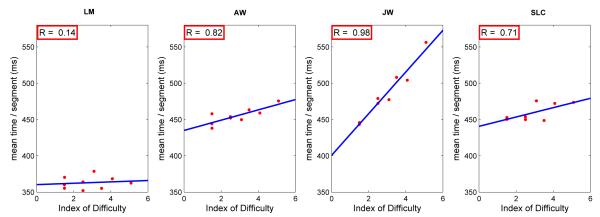
Fitts’s Law states that the time to complete a movement increases with the Index of Difficulty (ID) (eq. 1). Figure 2 plots the time per segment of a loop as a function of ID, where “time per segment” is defined as the time to complete the loop around the targets divided by 4, the number of targets in the loop. The results show that, in agreement with Fitts’s Law, time/segment increased with ID. There were differences among subjects in the magnitude of the effects, with subject LM showing a very shallow slope.
Figure 2.
Mean time per segment of a loop as a function of the Index of Difficulty(ID) where ID is defined as log 2 (2S / D), with S the separation and D the target diameter. Data from 4 subjects. Each datum point is based on approximately 640-1150 observations.
Two aspects of the saccadic sequence, the duration of the large primary saccade, and the time between successive primary saccades, could have accounted for the effects of ID. Analyses showed that each contributed in a different way.
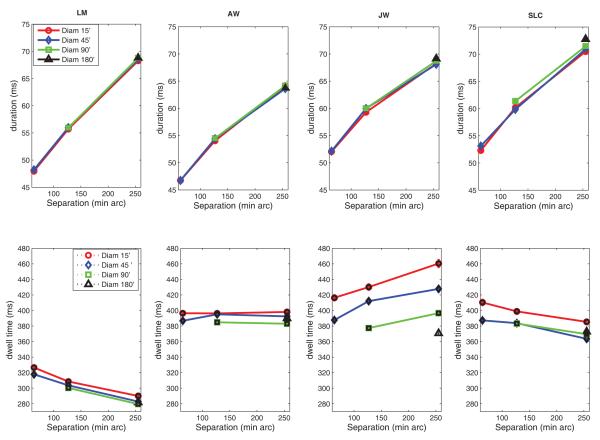
The duration of the primary saccade increased with target separation and was not affected by target size (Fig. 3, top). This was expected, given the well known dependence of saccadic duration on amplitude (Collewijn, Erkelens, & Steinman, 1988).
Figure 3.
Top: Mean saccadic duration, measured from the onset of the saccade to the offset, including any dynamic overshoots, as a function of target separation for the different target sizes. Standard errors are smaller than the plotting symbols. Each datum point is based on approximately 640-1150 observations. Bottom: Mean dwell time as a function of target separation for different target sizes. Dwell time was defined as the time between successive primary saccades (includes duration of secondary saccades). Error bars when shown are +/− 1 SE; otherwise SE’s are smaller than the plotting symbols. Each datum point is based on approximately 640-1150 observations.
Dwell time, which we define as the time spent looking at or near each target between consecutive primary saccades, including any secondary saccades that may have occurred, were longer for smaller targets (Fig. 3, bottom). Effects of separation were more complicated. Dwell time either increased, decreased, or did not change with target separation, depending on the subject. Decreases in dwell time with separation (see subjects LM and SLC, Fig. 3, bottom) cancelled to some extent the increases in scanning time due to effects of separation on the duration of the saccade (Fig. 3, top).
Dwell time, as we have defined it (see above), included any secondary saccades that occurred between successive primary saccades. Secondary saccades proved to have an important role in performance, and will be described in detail below.
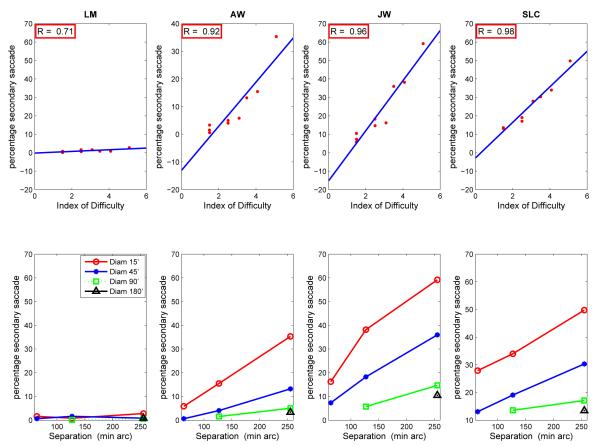
2.2.2. Secondary saccades increased in frequency with the Index of Difficulty
The frequency of secondary saccades was more consistently related to ID, target separation, and target size, than either of the two measures described above (saccadic duration or dwell time). The frequency of secondary saccades was calculated as the number of primary saccades followed by secondary saccades divided by total number of primary saccades. (Dwells with more than one secondary saccade were rare, less than 2 % for JW, and less than 0.05% for the other subjects.) Figure 4 shows that secondary saccades became more frequent with increasing ID, with frequency increasing as either target separation increased, or as target size decreased. Except for LM (who rarely made secondary saccades), secondary saccades occurred in about 5-10% of the dwells for the easiest case (smallest separation; largest size) and in about 40-50% of the dwells for the most difficult case (largest separation; smallest size). The majority (about 75%, across subjects and conditions) of the secondary saccades were corrective, meaning that they brought the line of sight closer to target center.
Figure 4.
Top: Frequency of secondary saccades as a function of the Index of Difficulty. Bottom: Frequency of secondary saccades as a function of target separation for different target sizes. Each datum point is based on approximately 640-1150 observations.
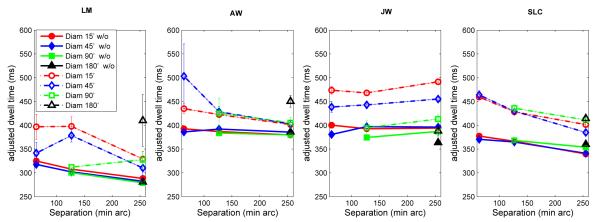
Secondary saccades prolonged dwell times. Figure 5 shows that adjusted dwell times, defined as dwell times with the in-flight time of the secondary saccades subtracted, were only about 50-75 ms longer for dwells with secondary saccades than for dwells without secondary saccades. The increase due to secondary saccades is about one-third to one-half of the typical saccadic latency.
Figure 5.
Mean adjusted dwell times for dwells that did (dashed line) and did not (solid line) contain secondary saccades as a function of target separation for different target sizes. Adjusted dwell times are defined as dwell times (Fig.3) minus the in-flight times of any secondary saccades. For dwells without secondary saccades, nothing was subtracted. Bars show +/− 1 SE; otherwise, SE’s are smaller than the plotting symbols.
Figure 5 also shows that when adjusted dwell times with and without secondary saccades were examined separately, both usually decreased with separation and neither showed consistent effects of target size. This means that the systematic increases in dwell time with decreasing target size (Fig. 3) can be attributed mainly to the effects of the increased frequency of time-consuming secondary saccades (including both their latency and in-flight time), not to a more general tendency to pause longer before initiating primary saccades to smaller targets.
Large target separations also led to more secondary saccades (Fig. 4), but this did not result in an overall increase in dwell time (Fig. 3), except for JW. For the remaining 3 subjects, the delay due to the additional secondary saccades as target separation increased was offset by the decrease in adjusted dwell time with increasing separation (Fig. 5).
Why were secondary saccades needed? They were not needed to correct for consistent saccadic undershoots because in our task primary saccades did not consistently undershoot the targets (Fig. S1). The errors of primary saccades that required correction were due to spatial imprecision, not to consistent undershoots. This was confirmed by showing that secondary saccades occurred with about equal frequency following primary saccades that either overshot or undershot the targets (Table S1). Secondary saccades were also not prompted by increases in the velocity of primary saccades (which also could lead to more variability of landing positions) because the average velocity of primary saccades was about the same regardless of whether the primary saccade was followed by a secondary saccade (Table S2).
Perhaps secondary saccades were needed because subjects rushed, and made short-latency primary saccades that missed the target, thus requiring secondary saccades in order to achieve better accuracy. Such a result would signal a trade-off between the time devoted to planning primary saccades and the occurrence of secondary saccades, analogous to the trade-offs Meyer et al. (1988) observed for wrist rotations. To examine this possibility, the latency of primary saccades was determined for four groups of primary saccades, with the groups defined according to the type (primary or secondary) of both the prior and the subsequent saccade. For this analysis the latency of the primary saccade was defined as the time from the offset of the immediately prior saccade (primary or secondary) until the onset of the current primary saccade.
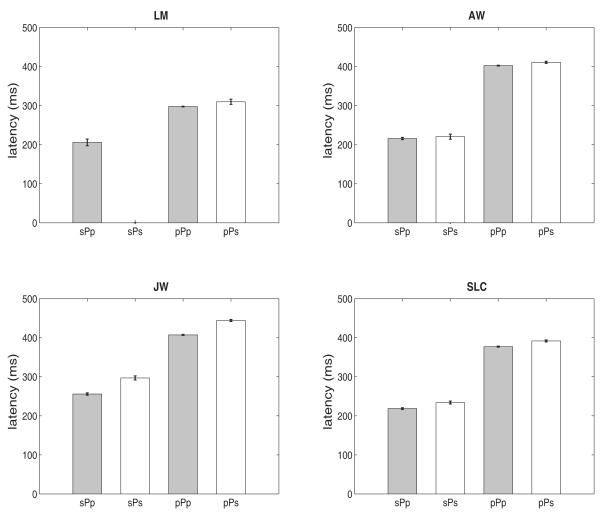
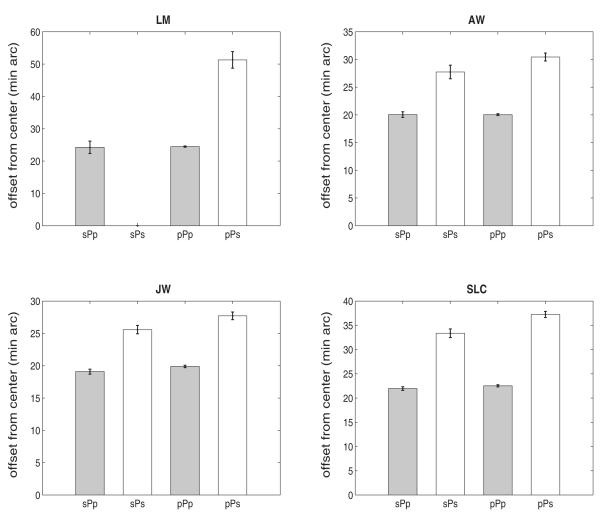
If short-latency primary saccades led to a higher proportion of secondary saccades, then primary saccades that were followed by secondary saccades should have shorter latencies than primary saccades that were followed by another primary saccade. Figure 6 shows that, contrary to this hypothesis, the latency of primary saccades followed by a secondary saccade (denoted as pPs or sPs, open bars, in Fig. 6) was the same as or slightly greater than the latency of primary saccades followed by another primary saccade (pPp or sPp, filled bars, Fig. 6). One factor that did predict the occurrence of secondary saccades was the spatial precision of the primary saccades. Figure 7 shows that the average vector error of the primary saccade (defined as the 2D offset of the saccadic landing position relative to target center) was larger for primary saccades followed by secondary saccades (pPs, sPs, open bars, Fig. 7) than for primaries followed by another primary (sPp, pPp, filled bars, Fig. 7). The type of prior saccade (primary or secondary) was not important, despite the large differences in latencies as a function of the prior saccade shown in Fig. 6.
Figure 6.
Mean latency (+/− 1 SE) of primary saccades that were preceded and followed by primary saccades (pPp), preceded by primary and followed by secondary saccades (pPs), preceded by secondary and followed by primary saccades (sPp), and preceded and followed by secondary saccades (sPs), collapsed across all target sizes and separations. Subject LM did not have any saccades in the sPs category. Bars are +/− 1 SE.
Figure 7.
Mean offset of primary saccadic endpoints from target center (+/− 1 SE) for primary saccades that were preceded and followed by primary saccades (pPp), preceded by primary and followed by secondary saccades (pPs), preceded by secondary and followed by primary saccades (sPp), and preceded and followed by secondary saccades (sPs), collapsed across all target sizes and separations. Subject LM did not have saccades in the sPs category. Bars are +/− 1 SE.
2.2.3. Within a given target size and separation, there was no reduction in landing error with increased latencies or dwell times
One of the main motivations for this study was to determine whether increases in time devoted to planning the primary saccades improved their spatial accuracy. Before examining this issue we need to consider how to best represent the time devoted to planning the primary saccades. We considered two measures:
dwell time, defined above as the time between successive primary saccades, with “adjusted dwell time” equal to dwell time minus the in-flight time of any secondary saccades.
the latency of the primary saccade, defined above as the time between the offset of the saccade preceding a primary saccade (whether the preceding saccade is primary or secondary) and the onset of the primary saccade.
Using the second measure, the latency of the primary saccade, as the index of saccadic planning time assumes the planning of a primary saccades starts only after the prior saccade (primary or secondary) is completed. We already saw, however, that this assumption is questionable. Dwells with secondary saccades were only about 50-75 ms longer than dwells without secondary saccades (Fig. 5). Since this increase is much less than typical saccadic latency, it implies there was a temporal overlap in the planning of primary saccades and secondary saccades (McPeek, Skavenski & Nakayama, 2000; Ramakrishnan, Chokhandre & Murthy, 2010). For example, initiating the planning of the primary saccade to a new target may not need to wait for the conclusion of any preceding secondary saccade (which typically corrected landing errors with respect to the current target), but rather could begin as soon as the prior primary saccade was concluded. This is supported by the result in Fig. 6, which shows that the nature of the preceding saccade had a large effect on latency. Latencies were about 100-200 ms shorter when the prior saccade was a secondary saccade than when the prior saccade was a primary saccade. This implies that planning the primary saccade did not wait until the prior secondary saccade was completed. If it had, we would expect that the latencies of primary saccades would be about the same regardless of the type of prior saccade. For these reasons, the adjusted dwell time (see definition above) may be a better index of the time taken to plan the primary saccade.
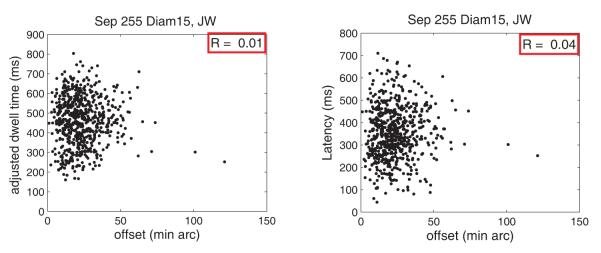
Figure 8 shows representative scatter plots that examine the relationships between each of these measures and the offset error of the primary saccade. Figure 8a shows the adjusted dwell time prior to each primary saccade vs. the offset error of the primary saccade, and Fig. 8b shows the latency of the primary saccade vs. the offset error of the primary saccade. “Offset error” refers to the 2-dimensional offset error of the primary saccade, excluding the contribution of any secondary saccades that might follow. Data are shown for subject JW for the smallest target and largest separation. The correlations (r) between either measure and offset error are near zero. Correlations for all other target sizes, separations and subjects showed similar patterns, with correlations ranging from −0.08 to 0.14. The adjusted dwell times shown in Fig. 8a exclude the flight time of any secondary saccades. The pattern of results was the same when flight time of secondary saccades was included. Given that adjusted dwell time includes some of the planning time associated with any secondary saccades, we also examined dwells without secondary saccades and found that results were the same, that is, correlations were low (range −0.07 to 0.13). These results show that increasing the pause time prior to the primary saccade did not improve its accuracy.
Figure 8.
Representative scatter plots showing A: adjusted dwell time vs. offset error; B: Latency of primary saccades vs. offset error. Data are for subject JW, target separation 255′ and diameter 15′. Each scatter plot is based on 644 observations.
2.2.4. Secondary saccades were corrective
The analyses described in the prior section show that increases in the estimated time used to plan the primary saccades did not succeed in bringing the line of sight closer to the target. Secondary saccades, on the other hand, did. To verify the corrective role of the secondary saccades, we analyzed the two dimensional scatter of landing positions for both initial saccades and final landing positions, where ‘initial’ landing position refers to the landing position of the primary saccade leaving from target (n) and heading to target (n+1), and ‘final’ landing position refers to the landing position of the last saccade at target (n+1). Thus, if there were secondary saccades, the final saccade would be the last secondary saccade. On the other hand, if there were no secondary saccades, the final saccade would be the same as the initial saccade. (Instances of more than one secondary saccade in a dwell were rare, see 2.2.2)
Scatter was quantified by the bivariate contour ellipse area (BCEA) (see Steinman, 1965; Vishwanath & Kowler, 2004, for other examples), where
| (2) |
( σH is the standard deviation of the horizontal offset error; σV is the standard deviation of the vertical offset error, and ρ is the correlation coefficient between the horizontal and vertical offset errors). The value of k was set to be 1.125, which corresponds to BCEA containing 68 % of the landing positions.
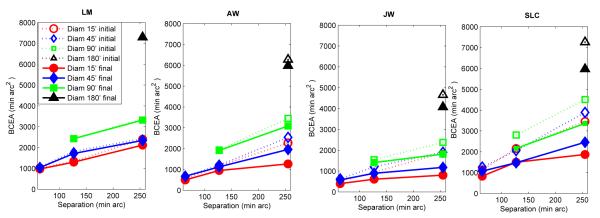
Figure 9 shows that scatter was smaller for final saccades than for initial saccades, showing that secondary saccades were corrective. (Fig. S2 shows that same pattern of results when final saccades were restricted only to those cases where the final saccade was a secondary saccade.) Figure 9 also shows that there were effects of target separation. It was expected that the scatter of initial landing positions would increase with eccentricity due to either sensory encoding or motor factors (Van Opstal & Van Gisbergen, 1989). But it was surprising that secondary saccades became less effective as target separation increased (as shown by the increase in the scatter of final landing positions with increasing separation in Fig. 9). This result suggests that there was an adjustment in criterion as to what constituted an acceptable landing location, with the region of acceptable landing scaling up with target separation. This scaling may represent a sacrifice of some level of accuracy to avoid further prolonging the scanning time with additional corrective saccades.
Figure 9.
Bivariate Contour Ellipse Area (BCEA) (measure of 2D scatter of landing position) for initial (primary) saccades (dashed line) and the final saccade following any secondary saccades (solid line) as a function of target separation for the different target sizes. Each datum point is based on approximately 640-1150.
2.2.5. Offset error preceding secondary saccades
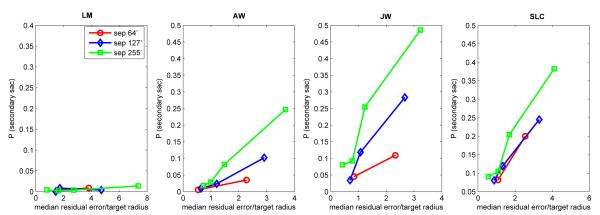
The results so far revealed a strong dependence on secondary saccades for improving the accuracy of the landing location. How far did the line of sight have to land from the target before a secondary saccade became likely? Harwood et al. (2008) considered an analogous issue for primary saccades, and reported that the probability of making a saccade to track a target step displacement depended on the ratio of target step size to target diameter, with smaller ratios leading to fewer saccades and to longer saccadic latencies. The secondary saccades in the present experiment occurred under conditions similar to many of the primary saccades studied by Harwood et al. because in both situations the line of sight was relatively near the center of the target before the saccade occurred.
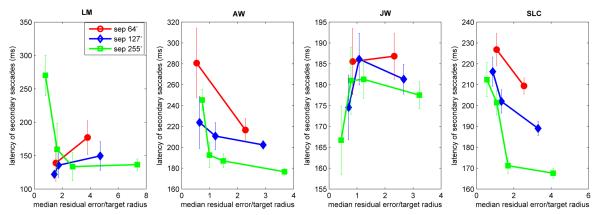
We found that our secondary saccades had similar properties as the saccades reported by Harwood et al. (2008), in that both the occurrence and the latency of the saccades could be predicted by the ratio of error to target radius. In our case, ‘error’ refers, not to the step size of a target displacement, but to the average offset error left behind by the primary saccade, where ‘offset error’ was defined as the distance of the line of sight to target center. Figure 10 shows that secondary saccades were infrequent (<10%) for ratios <1, i.e., cases where the line of sight landed within the target’s boundary. The occurrence of secondary saccades increased as the ratio of error to target radius increased. In addition, the latency of secondary saccades generally decreased as the error/size ratio increased (Fig. 11).
Figure 10.
Frequency of secondary saccades as a function of the ratio of median landing offset error of primary saccades relative to the radius of the target for the different target separations. Each datum point is based on approximately 640-1150 observations.
Figure 11.
Mean latency of secondary saccades as a function of the ratio of the median landing offset error of primary saccades relative to the radius of the target for the different target separations. Vertical error bars represent +/− 1 SE of latency of secondary saccades. Each datum point is based on approximately 40-619 observations, except for LM, who rarely makes secondary saccades (N<20 per datum point).
The pattern of results for secondary saccades are similar to those of Harwood et al. (2008) for primary saccades. One interesting new feature found for the secondary saccades was that once the ratio of landing error to target radius exceeded 1, the probability of making a secondary saccade also depended on the initial target separation, with larger initial separations leading to an increased proportion of secondary saccades (Fig. 10). These effects of separation show that “global” or contextual effects, namely, initial target separation, contributed to performance, and not just the immediate retinal conditions.
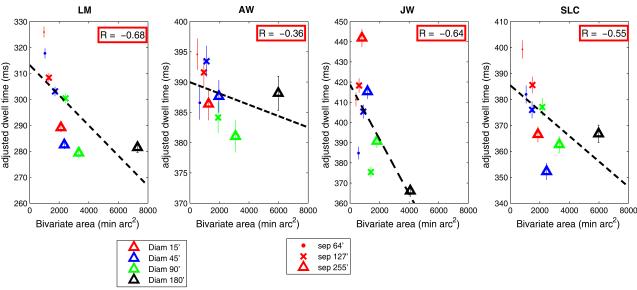
2.2.6. Across all target sizes and separations, conditions with longer dwell times were associated with less scatter of landing locations
Figure 12 summarizes the overall relationship between adjusted dwell time and the precision of saccades across all conditions. Each plotting symbol shows average adjusted dwell time (dwell time minus travel time of any secondary saccades) vs. the scatter of the final landing positions (bivariate area of final saccadic landing positions, from Fig. 9) for the different target sizes and separations.
Figure 12.
Mean adjusted dwell time as a function of average scatter of landing position of the final saccades for the different target sizes and separations. Symbol color represents target size; symbol shape represents target separation. Each datum point is based on approximately 640-1150 observations.
Figure 12 shows that conditions that encouraged longer dwell times were associated with a smaller scatter of endpoints. For variations in target size, this relationship was due to the increased frequency of error-correcting and time-consuming secondary saccades with the smaller targets (Figs. 4,5). For variations in target separation, the relationship between dwell time and scatter reflected both the decreases in dwell time with increased target separation (Fig. 5) as well as the greater tolerance for larger landing errors as separation increased (Fig. 9).
2.2.7. Summary
Fitts’s Law applied to sequences of saccades in that the time to complete the sequences increased with Fitts’s Index of Difficulty (ID) (Figs. 2 and 3). The effect was due mainly to the occurrence of secondary saccades for smaller targets. The frequency of secondary saccades was the measure that correlated most closely with ID (Fig. 4), and the occurrence of secondary saccades both corrected the landing errors of the primary saccades (Fig. 9) and prolonged the dwell times between successive primary saccades (Fig. 5). There was no evidence that increasing the dwell time preceding primary saccades, or increasing the latency of primary saccades, within a given condition, improved the spatial accuracy or precision of the primary saccades (Fig. 8). The use of secondary saccades, not increases in the time devoted to planning the primary saccades, was the principal means of bringing the line of sight closer to targets (Fig. 9).
The results also showed a clear aversion to increasing the time spent dwelling near each target. When the target separation increased, subjects preferred to tolerate landing error – landing further from the target – rather than prolonging dwell time by making additional secondary saccades (Fig. 9).
Perhaps longer dwell times failed to reduce landing errors of primary saccades because the range of observed dwell times was too small. In order to evaluate this possibility, a second experiment was run in which a larger range of dwell times were encouraged by instructing subject to adopt different paces of scanning.
3. Experiment 2
Experiment 2 used the same task as Experiment 1, but the instructions were changed to elicit a wider range of scanning rates, which might make it possible to observe relationships between dwell time and accuracy that may not have been apparent for the range of dwell times in Experiment 1. Three new subjects were tested. As a preview, although the range of observed dwell times was much larger, the pattern of results of Experiment 2 was quite similar to those of Experiment 1.
3.1. Methods
3.1.1. Subjects
Three new subjects were tested (DW, JS and EN). All had normal vision and no correction, and were naïve as to the experimental design and hypothesis.
3.1.2. Stimulus display and procedure
The stimuli were displayed on Viewsonic G90fb monitor. Movements of the right eye were recorded by an Eyelink 1000 (SR Research) tracker (tower mount) with head held by a chin and head rest. Eyelink 1000 noise level exceeds slightly that of the SRI Dual Purkinje Tracker used in Experiment 1 (see Collewijn & Kowler, 2008, Fig. 7). Viewing was monocular.
Stimuli were much the same as in Experiment 1. The diameter of the target circles were set to one of four values (15, 45, 90 or 180 min arc), and the separation was to one of four values (64, 128, 256 or 512 min arc). For the comparability of Index of Difficulty across experiments, purpose, not all combinations of size and separation were tested. A total of 10 conditions were tested, as listed in Table 2. The sequences of frames during trials were the same as Experiment 1. Trial length was 6 seconds.
Table 2.
Target separations (S) and target diameters (D) in Experiment 2
| S (min arc) | D (min arc) | ID= log 2 (2S /D) |
|---|---|---|
| 64 | 15 | 3.09 |
| 128 | 15 | 4.09 |
| 45 | 2.51 | |
| 256 | 15 | 5.09 |
| 45 | 3.51 | |
| 90 | 2.51 | |
| 512 | 15 | 6.09 |
| 45 | 4.51 | |
| 90 | 3.51 | |
| 180 | 2.51 |
3.1.3. Instructions
Two types of sessions were run, denoted “fast” and “slow”. For the initial experimental sessions, subjects were instructed to make sequences of saccades at a brisk yet comfortable pace (same instruction as in Experiment 1). If they made saccades at rapid pace (defined as 3 or more loops around the 4 targets/trial), these sessions would be defined as the “fast” conditions. Subjects DW and JS fell into this category, and in subsequent sessions they were asked to scan at a slower pace (about 2-3 loops/trial). Subject EN initially made about 2.5 loops/trial. This was defined as his “slow” pace, and in subsequent sessions he was asked to speed up (3.5-4 loops/trial). Fast and slow sessions were tested alternately.
3.1.4. Experimental sessions
Each experimental session contained 40 trials and subjects were tested in 4-5 sessions/day. The experimental condition (target size and separation) on each trial was selected randomly from the 10 possible conditions shown in Table 2.
3.2. Results
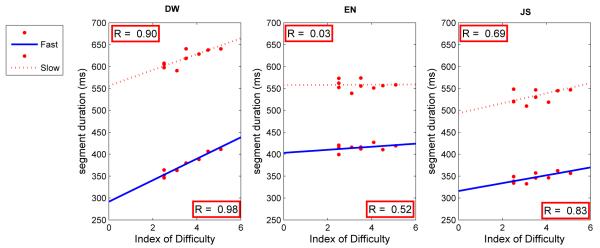
Time/segment in the slow condition was about 50% longer than in the fast condition. Time/segment in the fast condition was comparable to that found in Experiment 1. Time/segment increased with ID for both fast and slow conditions for subjects DW and JS. For subject EN, time/segment was generally flat across ID, particularly in the slow condition (Fig. 13).
Figure 13.
Mean time per segment of a loop as a function of the Index of Difficulty(ID) in fast (solid line) and slow (dashed line) conditions for three subjects in Experiment 2. Each datum point is based on approximately 340-500 observations.
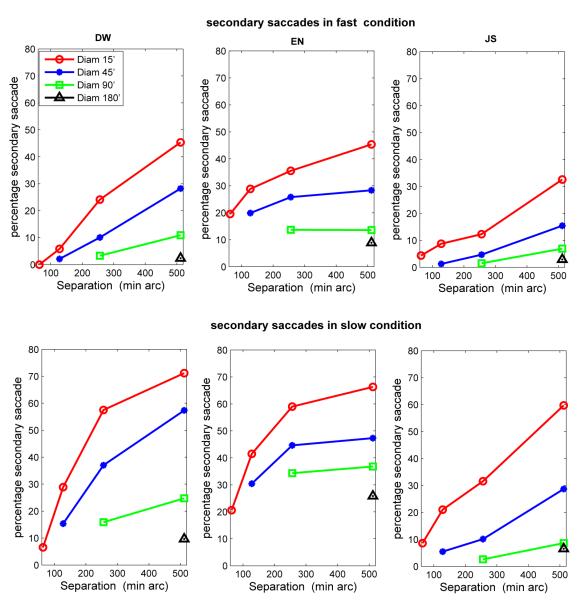
As in Experiment 1, secondary saccades became more frequent as either target separation increased, or as target size decreased (Fig. 14). There were more secondary saccades in the slow condition than in the fast condition, and many dwells in the slow condition contained more than one secondary saccade. Also, as in Experiment 1, correlations between either the latency of the primary saccade, or the adjusted dwell time prior to a primary saccade, and the landing error of the primary saccade were low ( r = −.13 to .19, except for subject JS in the slow condition, separation 64′, diameter 15′, where r = .31).
Figure 14.
Frequency of secondary saccades as a function of target separation for the different target sizes for fast (top) and slow (bottom) conditions in Experiment 2. Each datum point is based on approximately 340-500 observations.
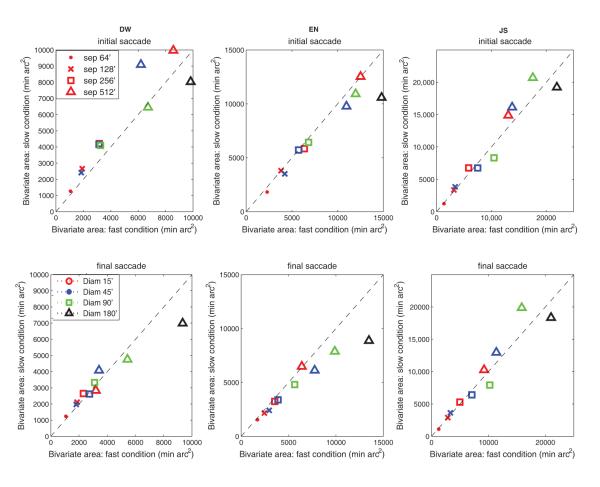
In order to investigate that whether prolonging the scanning time improved saccadic precision, the 2-dimensional scatter of landing positions (BCEA, eq. 2) in the fast and slow conditions were compared. Figure 15 shows both the initial and final scatter of landing positions, with scatter in the fast condition plotted against scatter in the slow condition. For initial saccades, most of data points fell near the diagonal, showing that despite the large difference in dwell times across the fast and slow conditions (dwells were 260-380 ms under the fast condition; 460-560 ms under the slow condition), there was no reduction in the scatter of initial saccadic landing positions. For the final saccades, scatter was smaller in the slow condition for DW and EN for the largest separation, reflecting the contribution of the additional secondary saccades. These results show that providing additional time to plan saccades had no benefit for the precision of the primary saccadic movements, and little effect, beyond allowing time for more corrections, on the final movements. Thus, even when dwell times were greatly prolonged, the extra time was not used to enable the primary saccade to reach the target more accurately. The extra time did allow for additional secondary saccades, which reduced landing errors for the largest target separations.
Figure 15.
Bivariate contour ellipse area (a measure of the scatter of landing positions) in the fast vs. slow conditions for different target sizes and separations. Symbol color represents target size; symbol shape represents target separation. Top graphs: initial saccades (before any secondary saccades). Bottom graphs: Final saccades (after any secondary saccades). Each datum point is based on approximately 340-500 observations.
4.Discussion
4.1. Speed/accuracy trade-offs and saccades
Fitts’s Law represents a trade-off: achieving greater levels of spatial precision of an aimed movement requires more time. The present study found such a relationship between the time to complete saccadic sequences and the spatial precision of the landing positions: achieving greater levels of saccadic precision required more time. This was not due to a connection between saccadic latencies and saccadic precision. Instead, two other types of relationships between time and precision were observed. First, secondary saccades reduced landing offset errors at the cost of a modest increase in the dwell time between successive primary saccades. Second, the tolerance for landing errors increased (for large target separations) in order to avoid prolonging scanning time with additional corrective saccades.
In general, improving the spatial precision of movements at the expense of movement time can be achieved by applying a variety of different strategies. For example, either: (1) reducing the variability of a movement by slowing movement speed; (2) taking more time to plan the movement (longer latency), or (3) making additional secondary submovements, could all lead to improved spatial precision at the expense of time. Studies of aimed motor behaviors (other than saccades) showed effects of the first and third strategy above, namely, slowing movement speed and making additional submovements were both effective in improving spatial precision (e.g., Meyer et al., 1988). For sequences of saccades, however, only the third strategy listed above, making secondary submovements, emerged as the principal way of trading off time in order to achieve the required level of precision. This result applies to the case studied here in which targets were presented without nearby and distracting non-targets. When non-targets are present, an increase in planning time (latency) can improve the ability to land on the selected target and avoid influence of surrounding non-targets (Cohen et al., 2007; Coëffé & O’Regan, 1987; Ottes, Van Gisbergen & Eggermont, 1985), (although, as will be discussed below, this is not necessarily the preferred option).
The conclusion that secondary saccades was the preferred way to reduce saccadic landing errors was supported by the findings that the latency or the dwell time preceding primary saccades did not predict either the magnitude of the landing error of the primary saccades, or the occurrence of secondary saccades. Taking more time to plan primary saccades did not improve accuracy. We did not, however, ask subjects to try to make primary saccades as accurately as possible, rather we asked them to look at each target, so we cannot rule out that additional time or effort would have succeeded in improving the accuracy of the primary saccades had such attempts been made. Nevertheless, we did find that in no case over the 6 subjects tested across the two experiments, including conditions in Experiment 2 where plenty of time was available, was there evidence that increased dwell time or latency improved the accuracy of primary saccades. The preferred option in all cases was to improve accuracy of landing by means of secondary saccades.
This strategy of relying on secondary saccades to clean up spatial offset errors would be the only feasible option if either: (a) the variability of the landing positions of primary saccades was limited by spatial factors and could not be reduced by increasing latency or applying more deliberate efforts, or (b) the time or effort involved in improving the accuracy of any given primary saccade would have been too great to warrant use of such a strategy. Although either option could explain the results, the first possibility seems more likely because even when ample time was provided (Experiment 2) the spatial precision of primary saccades did not improve.
4.2. Saccadic vs. perceptual localization
The finding that increasing the latency or the dwell time preceding primary saccades did not improve saccadic precision is not consistent with findings from perceptual localization tasks, where increased processing time improved the precision of perceptual judgments of target separation, even for processing times comparable to those we observed for saccades (Pizlo et al., 1995). The inconsistent results may be due to use of different streams of visual information for saccades and for perceptual localization (e.g., Hanson, 1978; Goodale & Milner, 1992). Alternatively, perceptual localization involved judgments of the relative spatial position of two targets, whereas for saccadic tasks, only the location of a single target was relevant. It is also possible that the latency/accuracy tradeoffs found for perceptual localization tasks could involve decision stages that may not be relevant to saccadic planning.
4.3. Global and contextual factors affected saccadic planning
The probability of making secondary saccades depended on local retinal conditions, specifically, the ratio between retinal error and target size, with larger ratios producing more secondary saccades and shorter secondary saccadic latencies. This result is analogous to Harwood et al.’s (2008) findings for primary saccades. In addition to these local retinal effects, however, the frequency of secondary saccades also increased with the initial target separation. That is, given the same ratio between retinal error and target size, the larger the primary saccade, the more likely a secondary saccade would occur. This increase in the frequency of secondary saccades was not a consequence of consistent undershooting of the primary saccades because we did not find consistent undershooting in our sequential scanning task (Fig. S1). What else could be responsible? The increase in the frequency of secondary saccades at the larger separations could have been due to saccadic planning mechanisms that began to prepare a secondary saccade even before the error of the primary saccade was evaluated (e.g., Becker & Fuchs, 1969). This advanced preparation could reflect the fact that the saccadic system might be able to predict the need for a secondary saccade on the basis of internal knowledge that landing variability increases with increasing saccades size (Van Opstal & Van Gisbergen, 1989). Recent findings showing that the program of secondary saccades is not under voluntary control are also consistent with the idea that a primary saccade and the following secondary saccade are programmed as part of a single package (Ramakrishnan et al., 2010).
Despite the increased frequency of secondary saccades with the large target separations, the effectiveness of these saccades decreased as target separation increased. Specifically, the scatter of final landing positions (after secondary saccades) increased with target separation. This effect of target separation was unexpected because, presumably, subjects could have made additional secondary saccades to correct errors. This result implies that there was an adjustment in criterion for what defined an acceptable landing position, with the offset error criterion increasing for larger initial separations. When target separation increased, subjects increased the zone of acceptable landing locations, preferring instead to accomplish the task faster, rather than with better accuracy.
4.4. Overlap of the planning of primary and secondary saccades
The prior section suggested that the preparation of a primary saccade and the following secondary (corrective) saccade were part of a single package. This does not mean, however, that the preparation of a given primary saccade was forced to wait for completion of any prior secondary saccade. Preparation of the primary saccade could begin at least as early as when the preceding primary saccade arrived at or near the target. This would mean there would be overlap in the planning and execution of a secondary saccade to target N with the planning of the primary saccade to target N+1 (McPeek et al., 2000; Viviani & Swensson, 1982; Araujo et al., 2001; Becker & Jurgens, 1979; Caspi, Beutter & Eckstein , 2004; Theeuwes, Kramer, Hahn, Irwin & Zelinsky, 1999). This suggestion that overlap in the planning of saccades contributed to scanning of the saccadic sequences is supported by two pieces of evidence.
First, secondary saccades prolonged the dwell times between successive primary saccades by an amount (50-75 ms) that was not as long as the typical saccadic latency. These small increases show that the planning of primary and secondary saccades was not strictly serial. It was not strictly parallel (and independent) either, or there would be no increase at all. It is more likely that there was an overlap in the planning of primary and secondary saccades.
Second, overlap in the planning of saccades to two different targets (secondary saccade to target N and primary saccade to N+1) is supported by a comparison of two latencies: (1) the time between a secondary saccade and the subsequent primary saccade, and (2) the time between successive primary saccades in the absence of an intervening secondary saccade. If planning of the primary saccade to target N+1 did not start until the secondary saccade was completed, then these two latency intervals should be similar. They were not. Dwell times containing no secondary saccade were about 150-200 ms longer than the time between the secondary saccade and the following primary saccade (Fig. 6). This suggests that the planning of the primary saccade to target N+1 started before the completion of secondary saccades to target N.
Overlap in the planning of the primary and secondary saccades to different targets is an efficient attribute of saccadic planning systems because it reduces the cost in time of having to correct landing errors during performance of saccadic sequences. Overlapped planning has been discovered in the context of visual search (see references cited above). Our results suggest it may have wider applicability during saccadic scanning.
4.5. Implications for strategies of saccadic planning
A key component for understanding saccadic patterns in visual tasks is to understand the processing that occurs during the intervals between successive saccades. How much time is devoted to planning saccades, in contrast to identifying or analyzing foveal material? Our results, along with prior findings, suggest that a combination of strategic choices, as well as attributes of the saccadic system itself, act to minimize the time taken up by saccadic planning, freeing both time and resources for foveal processing or making longer-range behavioral decisions.
Insight into these strategies and attributes comes from both present and prior results. For example, when saccadic targets are surrounded by non-targets, the target is often reached more quickly with two saccades – an initial saccade to the middle of the entire configuration followed by a subsequent correction – rather than by a single saccade aimed directly to the target (Coëffé & O’Regan, 1987). Visual search shows comparable patterns. Specifically, initial saccades in search tasks do not always take into account visual cues that signal where targets might be found. As a result the initial saccade often heads to a useless location, to be followed by a secondary corrective saccade, often with a very short latency (Araujo et al., 2001; Caspi et al., 2004; Schnitzer & Kowler, 2006). Our present results are consistent with such a reliance on corrective saccades, rather than careful planning of the primary saccades. We found that the spatial precision of primary saccades was not improved by increased available dwell time prior to the primary saccade, leaving secondary saccades as the principal means of correcting landing errors. In some cases, even when secondary saccades were used, saccadic accuracy was sacrificed in order to maintain the pace of scanning.
These strategic choices outlined above show a bias toward making more saccades, rather than planning saccades more carefully. This strategy is facilitated by attributes of the saccadic system, including the ability to plan saccades predictively on the basis of global features of the display (e.g., target separation), and also the ability to plan multiple saccades concurrently (Becker & Jurgens, 1979; Viviani & Swensson, 1982; McPeek et al., 2000; Araujo et al., 2001). These attributes facilitate production of corrective saccades without devoting excessive time or cognitive effort.
This preference for multiple saccades and rapid scanning makes saccades different from many other motor activities, where the costs of moving to the wrong place, or the time taken up by corrections, may be high enough to encourage more careful selection and planning of primary movements (Meyer et al., 1988). People evidently do not spend much time deliberating where to look, or pondering how accurately to aim the saccade. Such efforts, as we have shown, would not necessarily be successful (more time does not ensure a more accurate landing position). In addition, producing saccadic corrections for errors is easy. These characteristics are the mark of a system designed to minimize planning and preparation time in favor of facilitating foveal explorations of multiple locations.
4.6. Future directions
These experiments were done with head movements restricted by bitebars or chinrests. Our results showed that adjustments to the accuracy of saccades made to scan a sequence of targets were carried out mainly by modulating the use of time-consuming secondary saccades. We did not find evidence for adjustments of the dwell times or latency intervals preceding primary saccades, nor of the average velocities of the primary saccades.
Adjustments to the velocities of primary saccades may, however, prove to be more important during saccadic sequences when the head is free to move. Head movements are unavoidable during saccadic tasks unless head supports are used (Epelboim, Steinman, Kowler, Edwards, Pizlo, Erkelens & Collewijn, 1995), even when gaze shifts are relatively small (Kowler et al., 1991). The extent of compensation for head movements by means of counter-rotations of the eye varies over an enormous range, in part as a response to task demands (Epelboim et al., 1997; Epelboim, 1998; Snyder et al., 2002), resulting in a wider range of gaze-shift velocities, durations and landing errors than found when the head is restrained. Whether such control of gaze shift dynamics is used strategically in various natural tasks to trade-off speed, accuracy, and the frequency of secondary saccades is an open and interesting question.
4.7. Summary
We found that performance of saccadic sequences showed a speed/accuracy trade-off in that conditions with higher difficulty levels (larger target separations and smaller sizes) took more time to perform. This result is in agreement with the predictions of Fitts’s Law. The aspect of saccadic performance that was predicted best by Fitts’s Index of Difficulty was the frequency of secondary saccades.
A higher level of accuracy of the sequential saccadic eye movements was achieved by use of secondary (corrective) saccades, rather than by attempts to take more time in order to aim the primary saccades more carefully. Analysis of timing of the saccades also showed that during the sequences, the planning of the secondary saccades and the next primary saccade could overlap. Due to the overlap in the preparation of saccades, the increase of the time between fixations of consecutive targets due to adding secondary saccades was relatively small compared to typical saccadic latencies. Nevertheless, secondary saccades led to increases in the total time required to complete the sequences because the overlap in programming of primary and secondary saccades was not complete. These results show that the partial parallel preparation of primary and secondary saccades is the factor that is most responsible for the speed-accuracy tradeoffs during the performance of saccadic sequences. Such effects would be difficult to detect in conventional single-target saccadic tasks, and support the implementation of scanning strategies that use available time for exploring more locations, rather than for the careful aiming of primary saccades.
Supplementary Material
Acknowledgements
Supported by NIH EY15522. We thank Zygmunt Pizlo, Manish Singh, Elizabeth Torres and Brian Schnitzer for helpful comments and discussions of this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrams RA, Meyer DE, Kornblum S. Speed and accuracy of saccadic eye movements: Characteristics of impulse variability in the oculomotor system. Journal of Experimental Psychology: Human perception and Performance. 1989;15:529–543. doi: 10.1037//0096-1523.15.3.529. [DOI] [PubMed] [Google Scholar]
- Al-Aidroos N, Fischer MH, Adam JJ, Pratt J. Structured perceptual arrays and the modulation of fitts’s law: examining saccadic eye movements. Journal of Motor Behavior. 2008;40:155–164. doi: 10.3200/JMBR.40.2.155-164. [DOI] [PubMed] [Google Scholar]
- Araujo C, Kowler E, Pavel M. Eye movements during visual search: The costs of choosing the optimal path. Vision Research. 2001;41:3613–3625. doi: 10.1016/s0042-6989(01)00196-1. [DOI] [PubMed] [Google Scholar]
- Becker W, Fuchs AF. Further properties of the human saccadic system: Eye movements and correction saccades with and without visual fixation points. Vision Research. 1969;9:1247–1258. doi: 10.1016/0042-6989(69)90112-6. [DOI] [PubMed] [Google Scholar]
- Becker W, Jürgens R. An analysis of the saccadic system by means of double step stimuli. Vision Research. 1979;19:967–83. doi: 10.1016/0042-6989(79)90222-0. [DOI] [PubMed] [Google Scholar]
- Caspi A, Beutter BR, Eckstein MP. The time course of visual information accrual guiding eye movement decisions. Proceedings of the National Academy of Sciences. 2004;101:13086–13090. doi: 10.1073/pnas.0305329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RHS, Williams M. Neural computation of log likelihood in control of saccadic eye movements. Nature. 1995;377:59–62. doi: 10.1038/377059a0. [DOI] [PubMed] [Google Scholar]
- Chen-Harris H, Joiner WM, Ethier V, Zee DS, Shadmehr R. Adaptive control of saccades via internal feedback. Journal of Neuroscience. 2008;28:2804–2813. doi: 10.1523/JNEUROSCI.5300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coëffé C, O’Regan JK. Reducing the influence of non-target stimuli on saccade accuracy: Predictability and latency effects. Vision Research. 1987;27:227–240. doi: 10.1016/0042-6989(87)90185-4. [DOI] [PubMed] [Google Scholar]
- Cohen EH, Schnitzer BS, Gersch TM, Singh M, Kowler E. The relationship between spatial pooling and attention in saccadic and perceptual tasks. Vision Research. 2007;47:1907–1923. doi: 10.1016/j.visres.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Erkelens CJ, Steinman RM. Binocular co-ordination of human horizontal saccadic eye movements. Journal of Physiology. 1988;404:157–182. doi: 10.1113/jphysiol.1988.sp017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn H, Kowler E. The significance of microsaccades for vision and oculomotor control. Journal of Vision. 2008;8(14:20):1–21. doi: 10.1167/8.14.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornsweet TN, Crane HD. Accurate two-dimensional eye tracker using first and fourth Purkinje images. Journal of the Optical Society of America. 1973;63:921–928. doi: 10.1364/josa.63.000921. [DOI] [PubMed] [Google Scholar]
- Crane JD, Steele CM. Accurate three-dimensional eyetracker. Applied Optics. 1978;17:691–705. doi: 10.1364/AO.17.000691. [DOI] [PubMed] [Google Scholar]
- Dick S, Ostendorf F, Kraft A, Ploner CJ. Saccades to spatially extended targets: the role of eccentricity. Neuroreport. 2004;15:453–456. doi: 10.1097/00001756-200403010-00014. [DOI] [PubMed] [Google Scholar]
- Epelboim J, Steinman RM, Kowler E, Edwards M, Pizlo Z, Erkelens CJ, Collewijn H. The function of visual search and memory in sequential looking tasks. Vision Research. 1995;35:3401–3422. doi: 10.1016/0042-6989(95)00080-x. [DOI] [PubMed] [Google Scholar]
- Epelboim J, Steinman RM, Kowler E, Pizlo Z, Erkelens CJ, Collewijn H. Gaze shift dynamics in two kinds of sequential looking tasks. Vision Research. 1997;37:2597–2607. doi: 10.1016/s0042-6989(97)00075-8. [DOI] [PubMed] [Google Scholar]
- Epelboim J. Gaze and retinal-image-stability in two kinds of sequential looking tasks. Vision Research. 1998;38:3773–3784. doi: 10.1016/s0042-6989(97)00450-1. [DOI] [PubMed] [Google Scholar]
- Frost D, Poppel E. Different programming modes of human saccadic eye movements as a function of stimulus eccentricity: Indications of a function subdivision of the visual field. Biological Cybernetics. 1976;23:39–48. doi: 10.1007/BF00344150. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47:381–391. [PubMed] [Google Scholar]
- Fitts PM, Peterson JR. Information capacity of discrete motor responses. Journal of Experimental Psychology. 1964;67:103–112. doi: 10.1037/h0045689. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Dosher B. Dynamic allocation of attention during sequences of saccades. Vision Research. 2004;44:1469–1483. doi: 10.1016/j.visres.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Gersch TM, Kowler E, Schnitzer BS, Dosher BA. Attention during sequences of saccades along marked and memorized paths. Vision Research. 2009;49:1256–1266. doi: 10.1016/j.visres.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends In Neurosciences. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grosjean M, Shiffrar M, Knoblich G. Fitts’s law holds for action perception. Psychological Science. 2007;18:95–99. doi: 10.1111/j.1467-9280.2007.01854.x. [DOI] [PubMed] [Google Scholar]
- Harwood MR, Madelain L, Krauzlis RJ, Wallman J. The spatial scale of attention strongly modulates saccade latencies. Journal of Neurophysiology. 2008;99:1743–1757. doi: 10.1152/jn.00589.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Wolpert DM. Signal-dependent noise determines motor planning. Nature. 1998;394:780–784. doi: 10.1038/29528. [DOI] [PubMed] [Google Scholar]
- Henson DB. Corrective saccades: effect of altering visual feedback. Vision Research. 1978;18:63–67. doi: 10.1016/0042-6989(78)90078-0. [DOI] [PubMed] [Google Scholar]
- Heywood S, Churcher J. Structure of the visual array and saccadic latency: Implications for oculomotor control. Quarterly Journal of Experimental Psychology. 1980;32:335–41. doi: 10.1080/14640748008401169. [DOI] [PubMed] [Google Scholar]
- Hooge IT, Erkelens CJ. Control of fixation duration in a simple search task. Perception Psychophysics. 1996;58:969–976. doi: 10.3758/bf03206825. [DOI] [PubMed] [Google Scholar]
- Hooge IT, Erkelens CJ. Adjustment of fixation duration in visual search. Vision Research. 1998;38:1295–1302. doi: 10.1016/s0042-6989(97)00287-3. [DOI] [PubMed] [Google Scholar]
- Hooge IT, Erkelens CJ. Peripheral vision and oculomotor control during visual search. Vision Research. 1999;39:1567–1575. doi: 10.1016/s0042-6989(98)00213-2. [DOI] [PubMed] [Google Scholar]
- Inhoff AW. Preparing sequences of saccades under choicereaction conditions. Effects of sequence length and context. Acta Psychologica. 1986;61:211–218. doi: 10.1016/0001-6918(86)90082-x. [DOI] [PubMed] [Google Scholar]
- Jin Z, Reeves A. Attentional release in the saccadic gap effect. Vision Research. 2009;49:2045–2055. doi: 10.1016/j.visres.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Kalesnykas RP, Hallett PE. Retinal eccentricity and the latency of eye saccades. Vision Research. 1994;34:517–531. doi: 10.1016/0042-6989(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Kapoula Z. Evidence for a range effect in the saccadic system. Vision Research. 1985;25:1155–1157. doi: 10.1016/0042-6989(85)90105-1. [DOI] [PubMed] [Google Scholar]
- Klapp ST. Feedback versus motor programming in the control of aimed movements. Journal of Experimental Psychology. 1975;104:147–153. [PubMed] [Google Scholar]
- Kowler E, Anton S. Reading twisted text: Implications for the role of saccades. Vision Research. 1987;27:45–60. doi: 10.1016/0042-6989(87)90142-8. [DOI] [PubMed] [Google Scholar]
- Kowler E, Pizlo Z, Zhu GL, Erkelens C, Steinman RM, Collewijn H. Coordination of head and eyes during the performance of natural (and unnatural) visual tasks. In: Berthoz A, Graf W, Vidal PP, editors. The Head-Neck Sensory Motor System. Oxford University Press; N.Y.: 1991. [Google Scholar]
- Kowler E, Blaser E. The accuracy and precision of saccades to small and large targets. Vision Research. 1995;35:1741–1754. doi: 10.1016/0042-6989(94)00255-k. [DOI] [PubMed] [Google Scholar]
- Lemij HP, Collewijn C. Differences in accuracy of human saccades between stationary and jumping targets. Vision Research. 1989;29:1737–1748. doi: 10.1016/0042-6989(89)90156-9. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Skavenski AA, Nakayama K. Concurrent processing of saccades in visual search. Vision Research. 2000;40:2499–2516. doi: 10.1016/s0042-6989(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Abrams RA, Kornblum S, Wright CE, Smith JEK. Optimality in human motor performance: Ideal control of rapid aimed movements. Psychological Review. 1988;95:340–370. doi: 10.1037/0033-295x.95.3.340. [DOI] [PubMed] [Google Scholar]
- Ottes FP, Van Gisbergen JAM, Eggermont JJ. Latency dependence of colour-based target vs nontarget information by the saccadic system. Vision Research. 1985;25:849–862. doi: 10.1016/0042-6989(85)90193-2. [DOI] [PubMed] [Google Scholar]
- Palmer J, Huk AC, Shadlen MN. The effect of stimulus strength on the speed and accuracy of a perceptual decision. Journal of Vision. 2005:376–404. doi: 10.1167/5.5.1. [DOI] [PubMed] [Google Scholar]
- Plamondon R, Alimi AM. Speed/accuracy tradeoffs in target-directed movements. Behavioral and Brain Science. 1997;20:279–349. doi: 10.1017/s0140525x97001441. [DOI] [PubMed] [Google Scholar]
- Pizlo Z, Rosenfeld A, Epelboim J. An exponential pyramid model of the time-course of size processing. Vision Research. 1995;35:1089–1107. doi: 10.1016/0042-6989(94)00195-r. [DOI] [PubMed] [Google Scholar]
- Pratt J, Dodd M, Welsh T. Growing older does not always mean moving slower: Examining aging and the saccadic motor system. Journal of Motor Behavior. 2006;38:373–382. doi: 10.3200/JMBR.38.5.373-382. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan A, Chokhandre S, Murthy A. Voluntary control of multisaccade gaze shifts during movement preparation and execution. Journal of Neurophysiology. 2010;103:2400–2416. doi: 10.1152/jn.00843.2009. [DOI] [PubMed] [Google Scholar]
- Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control both the direction and distance of pointing movements. Experimental Brain Research. 2005;162:458–473. doi: 10.1007/s00221-004-2064-1. [DOI] [PubMed] [Google Scholar]
- Schnitzer BS, Kowler E. Eye movements during multiple readings of the same text. Vision Research. 2006;46:1611–1632. doi: 10.1016/j.visres.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Effect of target size, luminance and color on monocular fixation. Journal of the Optical Society of America. 1965;55:1158–1165. [Google Scholar]
- Steinman RM, Haddad GM, Skavenski AA, Wyman D. Miniature Eye Movement. Science. 1973;181:810–819. doi: 10.1126/science.181.4102.810. [DOI] [PubMed] [Google Scholar]
- Snyder LH, Calton JL, Dickinson AR, Lawrence BM. Eye-hand coordination: saccades are faster when accompanied by a coordinated arm movement. Journal of Neurophysiology. 2002;87:2279–2286. doi: 10.1152/jn.00854.2001. [DOI] [PubMed] [Google Scholar]
- Stritzke M, Trommershäuser J, Gegenfurtner K. Effects of salience and reward information during saccadic decisions under risk. Journal of the Optical Society of America A. 2009;26:B1–B13. doi: 10.1364/JOSAA.26.0000B1. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Kramer AF, Hahn S, Irwin DE, Zelinsky GJ. Influence of attentional capture on oculomotor control. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:1595–1608. doi: 10.1037//0096-1523.25.6.1595. [DOI] [PubMed] [Google Scholar]
- Van Opstal AJ, Van Gisbergen JAM. Scatter in the metrics of saccades and properties of the collicular motor map. Vision Research. 1989;29:1183–1196. doi: 10.1016/0042-6989(89)90064-3. [DOI] [PubMed] [Google Scholar]
- Vishwanath D, Kowler E. Saccadic localization in the presence of cues to three-dimensional shape. Journal of Vision. 2004;4:445–458. doi: 10.1167/4.6.4. [DOI] [PubMed] [Google Scholar]
- Viviani P, Swensson RG. Saccadic eye movements to peripherally discriminated visual targets. Journal of experimental Psychology: Human Perception & Performance. 1982;8:113–126. doi: 10.1037//0096-1523.8.1.113. [DOI] [PubMed] [Google Scholar]
- Wright CE, Meyer DE. Conditions for a linear speed-accuracy trade-off in aimed movements. Quarterly Journal of Experimental Psychology. 1983;35A:279–296. doi: 10.1080/14640748308402134. [DOI] [PubMed] [Google Scholar]
- Wyman D, Steinman RM. Latency characteristics of small saccades. Vision Research. 1973;13:2165–2172. doi: 10.1016/0042-6989(73)90195-8. [DOI] [PubMed] [Google Scholar]
- Zingale C, Kowler E. Planning sequences of saccades. Vision Research. 1987;27:1327–1341. doi: 10.1016/0042-6989(87)90210-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.