Abstract
Background
The natural history and management of pancreatic cysts, especially for branch duct intraductal papillary mucinous neoplasms (BD-IPMNs), remains uncertain. We developed evidence-based nomograms to assist with clinical decision-making.
Methods
We used decision analysis with Markov modeling to compare competing management strategies in a patient with a pancreatic head cyst radiographically suggestive of BD-IPMN, including: (1) initial pancreaticoduodenectomy (PD), (2) yearly non-invasive radiographic surveillance, (3) yearly invasive surveillance with endoscopic ultrasound (EUS), and (4) “do nothing.” We derived probability estimates systematic literature review. The primary outcomes were overall and quality-adjusted survival. We depicted the results in a series of nomograms accounting for age, co-morbidities, and cyst size.
Results
Initial PD was the dominant strategy to maximize overall survival for any cyst >2cm, regardless of age or comorbidities. In contrast, surveillance was the dominant strategy for any lesion <1cm. However, when measuring quality-adjusted survival, the “do nothing” approach maximized quality of life for all cysts <3cm in patients aged <75. Once age exceeded 85 years, non-invasive surveillance dominated. Initial PD did not maximize quality of life in any age group or cyst size.
Concousions
Management of pancreatic cysts can be guided using novel Markov-based clinical nomograms, and depends on age, cyst size, comorbidities, and whether patients value overall survival vs. quality-adjusted survival. For patients focused on overall survival, regardless of quality of life, surgery is optimal for lesions >2cm. For patients focused on quality-adjusted survival, a 3cm threshold is more appropriate for surgery except for the extreme elderly.
Keywords: Pancreatic Cyst, IPMN, Decision Analysis, Nomogram
INTRODUCTION
The prevalence of pancreatic cysts has increased dramatically – a likely consequence of increased use and improved quality of abdominal imaging, coupled with the aging of the population. The management of a single isolated pancreatic cyst in an asymptomatic patient represents a clinical conundrum. Whereas intraductal papillary mucinous neoplasms (IPMN) or mucinous cystic neoplasms (MCNs) are considered at increased risk for malignant transformation, other cysts, including serous adenomas or pseudocysts, have no known malignant potential.1,2 Moreover, it is often difficult to prognosticate and predict the natural history of individual pancreatic cysts. This clinical uncertainty is distressing to patients and their providers who seek guidance in determining whether to do nothing, initiate invasive or non-invasive surveillance, or proceed directly to surgical resection – a seemingly draconian maneuver given the often times low pre-test likelihood for malignancy.3,4 Yet many patients are at risk for subsequent malignancy; the clinical decision cannot be taken lightly.
In particular, the incidence of IPMNs has increased 5-fold in the last decade.5,6 Both main duct (MD-IPMN) and branch duct (BD-IPMN) types are premalignant lesions, recognized histologically along a spectrum ranging from benign adenomas to invasive cancers.7-9 The rate of malignant transformation of MD-IPMN is considerably higher than BD-IPMNs.1,2,5,10-18 Because of the unpredictable and potentially less aggressive natural history of these BD-IPMN lesions, some argue in favor of surgical resection of advanced lesions, such as carcinoma in situ and invasive cancer, while continuing to survey patients with early lesions, such as adenomas.2,19,20
With current imaging techniques, including computerized tomographic (CT) scanning, magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) combined with pancreatic cyst fluid analysis, MD-IPMN and BD-IPMN can be diagnosed with an accuracy of 80%.21,22 However, our ability to reliably predict the underlying histology or rate of malignant transformation remains imperfect.10,13,21-26
In light of the diagnostic and prognostic uncertainty, international guidelines were developed in 2006 in Sendai, Japan to guide the clinician in operative and non-operative management of presumed IPMNs. However, for BD-IPMN surgical resection should only be considered if the size exceeds 3cm, the main pancreatic duct exceeds 6mm, there are mural nodules, or if there are related foregut symptoms. If these criteria are not met, then surveillance should be undertaken.12
However, these guidelines are imperfect for a variety of reasons. As there is a paucity of prospective natural history data studying pancreatic cysts, these guidelines rely predominantly on data gleaned from retrospective surgical data which carries its own biases.27-29 Similarly, the guidelines have not been able to account for evolutions in diagnostic technologies or our increased understanding of the natural history of pancreatic cysts. Moreover, the guidelines do not account for issues relating to operative mortality, patient age, functional status, or patient preference. Given the lack of prospective randomized data and the uncertainty about how to evaluate and manage patients with pancreatic cysts, we sought to better define the optimal management strategy for pancreatic cysts which are believed to be BD-IPMN through a decision analysis using currently available data.30
METHODS
Model Overview
Using decision analysis software (DATA 4.0, TreeAge Software, Inc., Williamstown, MA), we evaluated a hypothetical cohort of patients ranging from 65 to 85 years old with a variety of asymptomatic pancreatic cysts ranging from 0.5cm to >3cm lesions in the head of the pancreas. This location was chosen as it is the most common location for branch duct IPMNs, and because it represents a significant surgical challenge.5,10,19,31 Of note, our model does not apply to patients with symptoms that can be attributed to the pancreatic cyst. Decision-analysis is less applicable in symptomatic patients since competing non-invasive strategies are generally not recommended in the presence of attributable symptoms; guidelines recommend surgery over surveillance.
Although the base-case patient was considered to have likely BD-IPMN based on typical clinical features, we ensured that the patient was eligible to have other cystic lesions. This distinction acknowledges the clinical reality that the true cyst type is largely unknown with certainty at the time of diagnostic imaging; instead, physicians work with a range of pre-test likelihoods.21,22 Diagnostic certainty can only be established after surgery, yet the clinical challenge is whether to perform surgery in the first place. We explicitly developed our model to mimic this common yet complex clinical scenario. Patients entered the model with the possibility that at the time of diagnosis they could have a malignant IPMN, benign branch duct IPMN, a benign non-mucinous cyst, or pancreatic cancer with cystic degeneration. They then entered one of four competing strategies: (1) immediate pancreaticoduodenectomy (PD) followed by surveillance; (2) noninvasive surveillance with either MRI or CT followed by PD if malignant features develop (i.e. cyst >3cm; main pancreatic duct >6mm; presence of mural nodules)12; (3) invasive surveillance with EUS coupled with fine needle aspiration (FNA) followed by a PD if malignant features develop; or (4) “do nothing,” in which watchful waiting ensued without active surveillance, followed by PD only if cancer developed.
Using a Bayesian approach, the model incorporated the sensitivity and specificity of diagnostic testing along with the prevalence of underlying disease in the hypothetical cohort. In each strategy, if the type of lesion were not already malignant at baseline, then the patient assumed a probability of subsequent malignant transformation. The model accounted for false positive, false negative, true positive, and true negative diagnostic pathways. This created a range of clinical scenarios, including both appropriate and inappropriate PD or surveillance. This Bayesian approach was incorporated into all arms of the model except the “do nothing” approach, where active diagnostic surveillance was not employed. In this strategy, only the development of cancer led to definitive evaluation and potential surgical intervention. To test a variety of age ranges, we ran separate models to estimate outcomes in 65, 75, and 85-year-old base-case patients.
Competing Strategies
Our model included 4 competing strategies, described below. These strategies incorporated over 50 probability estimates governing relevant clinical probabilities in the management of IPMN, benign pancreatic cysts, and pancreatic cancer (Table 2). To derive these estimates, we performed a systematic search of MEDLINE from 1966 to July of 2007 using pre-specified phrases and key words, and limited our data abstractions of English-language. When data were not available, we relied on expert consensus opinion to inform our base-case estimates, and conducted sensitivity analyses over a wide range of values. All Markov transition probabilities were expressed as annual probabilities.
Table 2.
Probability Estimates
| Variable | Weighted Mean | Range in Literature | Range in Sensitivity Analysis | Source |
|---|---|---|---|---|
| Probability that a benign cyst grows | 0.035 | 0.035 | 0 – 0.1 | 50 |
| Probability of chronic complications after a pancreaticoduodenectomy | 0.194 | 0.15-0.30 | 0.06-0.30 | 8,43,51 |
| Probability of perioperative complications after a pancreaticoduodenectomy | 0.412 | 0.2-0.68 | 0.3-0.6 | 2,8,27,44,46,51-57 |
| Probability of death with a recurrent malignant IPMN following surgical resection | 0.842 | 0.80 – 1.0 | 0.3 – 0.9 | 19,27,58,59 |
| Probability of death with a recurrent pancreatic cancer following surgical resection | 0.9 | n/a | 0.3 -0.9 | 57,60-64* |
| Probability of developing symptoms with a benign cyst | 0.05 | n/a | 0.01-0.15 | 50* |
| Probability of developing symptoms with a benign IPMN | 0.05 | n/a | 0.01-0.15 | Expert opinion |
| Probability of developing symptoms with unrecognized malignant IPMN | 0.95 | n/a | 0.8-1.0 | Expert opinion |
| Probability of dying from adjuvant chemotherapy | 0.002 | 0 – 0.01 | 0 – 0.01 | 65-68 |
| Probability of dying from an EUS-FNA | 0.0001 | 0 – 0.002 | 0 – 0.01 | 67,69-72 |
| Probability of dying from a malignant IPMN without treatment | 0.6 | n/a | 0.4 – 0.8 | Expert opinion |
| Probability of dying from pancreatic cancer without treatment | 0.9 | n/a | 0.8 -1.0 | Expert opinion |
| Probability of dying from a pancreaticoduodenectomy | 0.064 | 0 – 0.07 | 0.01 – 0.2 | 2-4,27,51,52,54,55,57,59,73-75 |
| Probability that a malignant IPMN found in the “do nothing” strategy is operable | 0.15 | n/a | 0 – 0.2 | Expert opinion |
| Probability that a malignant IPMN will return post pancreaticoduodenectomy | 0.17 | 0.11 –0.99 | 0 – 0.6 | 33, 32, 84, 10, 12, 14, 15, 20, 24, 25, 29 |
| Probability that pancreatic cancer will return post pancreaticoduodenectomy | 0.24 | n/a | 0 -0.6 | Expert opinion |
| Probability that a pancreatic cancer found in the “do nothing” strategy is operable | 0.1 | n/a | 0.01-0.3 | Expert opinion |
| Probability that a CT of a benign IPMN will demonstrate a true negative result | 0.99 | 0.72-0.92 | 0.5 -1.0 | 44,49,76* |
| Probability that a CT of a malignant IPMN will demonstrate a true positive result | 0.8 | 0.72 -0.92 | 0.5 -1.0 | 44,49,76 |
| Probability of a EUS-FNA of a benign IPMN demonstrating a true negative result | 0.99 | 0.75-1.0 | 0.5 -1.0 | 14,17,44,48,49,77* |
| Probability that a EUS-FNA of a malignant IPMN will demonstrate a true positive result | 0.86 | 0.75 – 1.0 | 0.5 – 1.0 | 14,17,44,48,49,77 |
| Quality of life (utility) of chemotherapy for malignant IPMN or pancreatic cancer | 0.62 | n/a | 0.4 – 0.9 | 38* |
| Quality of life (utility) of chronic complications from a pancreaticoduodenectomy | 0.65 | 0.42 – 1.0 | 0.4 - 0.9 | 34-37 |
| Quality of life (utility)of perioperative complications from a pancreaticoduodenectomy | 0.50 | 0.42 – 1.0 | 0.4 - 0.9 | 34-37 |
| Quality of life (utility) of developing inoperable malignant IPMN or pancreatic cancer | 0.65 | n/a | 0.4 -0.9 | 38* |
| Quality of life (utility) of undergoing invasive surveillance | 0.98 | n/a | 0.5 – 1.0 | 78* |
| Quality of life (utility) of undergoing noninvasive surveillance | 0.98 | n/a | 0.5 – 1.0 | 78 |
| Quality of life (utility) of having been cured of cancer without any complications | 0.99 | 0.42 – 1.0 | 0.5 – 1.0 | 34-37 |
| Quality of life (utility) of developing recurrent malignant IPMN or pancreatic cancer | 0.68 | 0.42 – 1.0 | 0.4 – 0.8 | 38* |
| Quality of life (utility) of undergoing a pancreaticoduodenectomy with no complications | 0.98 | 0.42 – 1.0 | 0.5 – 1.0 | 34-37,79 |
Represents probabilities where a combination of available data and expert opinion were used to generate the specific probabilities
“Noninvasive Surveillance” Strategy
Patients in this strategy entered into a Markov model with annual radiographic surveillance with either abdominal CT scan or MRI. The patients either developed malignant transformation of their lesion, or did not. In both instances, the images either revealed evidence of malignant features, or did not. This yielded four diagnostic pathways: (1) imaging demonstrated a true positive where a malignant process is appropriately identified; (2) a false negative where a malignant process is missed; (3) a false positive where a benign process is mistakenly identified as having worrisome features; or (4) a true negative where a benign process is appropriately identified as benign. Figure 1 depicts an example of one of the many different Markov states.
Figure 1.
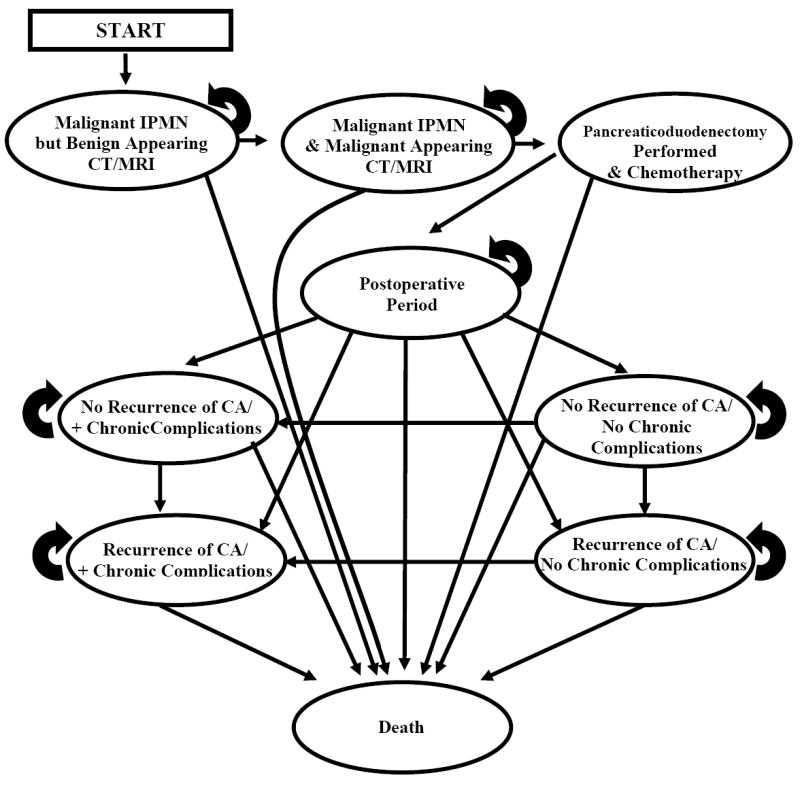
Example Markov State Diagram. Patients in the model cycled between health states according to annual probability estimates. The model included a wide variety of possible movements across the competing strategies. As an example, the diagram below demonstrates the possible state paths for patients undergoing non-invasive surveillance in a patient with an underlying, unrecognized, malignant IPMN.
“Invasive Surveillance” Strategy
Patients in this strategy entered annual surveillance with repeated EUS examinations with or without fine needle aspiration (FNA). This arm was identical to the “noninvasive surveillance” strategy except in two regards: (1) the invasive strategy included a measureable yet low risk of complications from EUS-FNA, including mortality; and (2) EUS-FNA had a small yet measurable improvement in sensitivity and specificity for detecting malignant features compared to CT or MRI – a consequence of improved diagnostic accuracy from cyst fluid analysis and careful evaluation of cyst wall characteristics.
“Initial Pancreaticoduodenectomy (PD)” Strategy
In this strategy all patients progressed directly to PD regardless of presence versus absence of malignant features. The model accounted for perioperative morbidity and mortality from PD – a function of surgical experience and patient comorbidities. Patients found to have malignant IPMN or pancreatic cancer subsequently received adjuvant chemotherapy – itself associated with morbidity and mortality.
“Do Nothing” Strategy
In this strategy all patients had no further active surveillance or workup performed after identification of their lesion. Patients were re-evaluated only if cancer developed. Once cancer developed all patients were considered for PD. The percentage of those who were operable candidates was lower than in the surveillance arms because of the delay in diagnosis. Patients who were resectable then entered Markov cycles identical to the original “initial PD” strategy.
Conditional Probabilities
We assumed that patients could harbor any of several potential underlying diagnoses, including malignant IPMN, benign branch duct IPMN, a benign non-mucinous cyst, or pancreatic cancer with cystic degeneration. Because the risk of malignancy correlates with cyst size, we established a series of probabilities conditional on cyst size. Using logic nodes, in which probabilities are conditional on concurrently measured variables (in this case cyst size), we conditionally linked size-specific data (i.e. rate of malignant transformation, baseline risk of underlying prevalent malignancy, etc.) to cyst size (Table 1). In addition, we varied these parameters over a wide range in sensitivity analyses.
Table 1.
Probability Estimates for BD-IPMNs of Varying Sizes
| Size < 1 | Size 1 to <2cm | Size 2 to <3cm | Size ≥ 3cm | |
|---|---|---|---|---|
| % Benign Cysts 41 | 9% | 9% | 9% | 9% |
| % Benign BD-IPMN 2,12,15,27,31,39,40,42-47 | 89% | 86% | 79% | 70% |
| % Malignant BD -IPMN 2,12,15,27,31,39,40,42-47 | 1% | 3% | 11% | 20% |
| % Pancreatic CA* | 1% | 1% | 1% | 1% |
| Rate of Benign to Malignant Transformation per Year43,48,49 | 0.001% | 1% | 1.7% | 3% |
Outcomes
We performed a decision analysis to evaluate two outcomes: unadjusted life years (LY), which tracks overall survival, independent of quality of life and morbidity, and quality-adjusted life-years (QALYs), a standard metric in decision models that accounts for both quantity of life (i.e. overall survival), and quality of life, as measured by utilities. We did not incorporate costs into the model as our objective was to focus solely on effectiveness, not cost-effectiveness. The purpose of our clinical nomograms, described below, is to assist patients and physicians with understanding how their decisions affect overall survival and quality of life – not the economic costs of competing decisions.
Utilities
To calculate QALYs, we incorporated a range of relevant health state utilities, or health related quality of life estimates, based on previously published health related quality of life data.32,33 The utilities related to those undergoing PD were based on four studies.34-37 No studies evaluated utilities for the short and long-term complications of PD, as was required in our model. Therefore, we extrapolated utilities from related data from other surgeries and health states, and used sensivitity analysis to test these estimates over a wide range of values. We were unable to identify validated utilities for pancreatic cancer or malignant IPMN. Therefore, we extrapolated data from breast cancer studies to estimate utilities for undergoing chemotherapy, inoperable cancer, and recurrent cancer.38 Because breast cancer is a potentially curable disease while malignant IPMN and pancreatic cancer carry worse survival rates, we lowered the respective utilities for each variable in our base-case model and performed sensitivity analysis over a wide range of estimates.
Sensitivity Analyses
Table 2 lists the base-case probability estimates with respective ranges. To test the influence of all variables on the model results, we performed a multivariable sensitivity analysis (“tornado analysis”) to help identify the most influential variables. We then performed 1-way sensitivity analysis on all variables and 2-way sensitivity analyses on the most influential variables. We present the 1-way analyses stratified by 3 age groups: 65, 75, and 85 year old. We present the 2-way analyses visually as age-stratified “nomograms” to assist decision-makers with identifying strategies that optimize outcomes under varying clinical circumstances.
Monte Carlo Simulations
Whereas 1-way and 2-way analyses provide information regarding the robustness of a model, they are inadequate to fully simulate real-world conditions. To acknowledge the reality that each individual carries a unique composition of clinical probabilities, we conducted a probabilistic (Monte Carlo) simulation under the assumption that all variables were triangular in distribution. We evaluated a series of Monte Carlo simulations stratified by age and cyst size, using 1000 trials per simulation. We report the absolute number of patients (per 1000) for which each competing strategies maximizes outcomes.
RESULTS
Table 3 displays the results of the base-case analyses, stratified by pancreatic cyst size (1cm, 2cm, and 3c,) and age (65, 75, and 85 years). The model revealed that the optimal strategy is conditional upon several factors, including patient age, cyst size, and whether the patient values overall survival or quality-adjusted survival. In patients who value overall survival regardless of quality of life, surgical resection with initial PD was the dominant strategy for any cyst size ≥2cm, even after considering the perioperative risks and the possibility that the cyst is not malignant. However, in patients who seek to maximize quality-adjusted survival (not just overall survival), the “do nothing” strategy maximized QALYs across all age groups for any cyst <3cm. Notably, the absolute differences in quality-adjusted survival were small across all groups with cyst sizes <3cm. However, for cysts >3cm, surgical resection dominated in patients 65-75 years – both for overall survival, as above, and quality-adjusted survival. As the age of the patient advanced to 85 years with cysts >3cm, surveillance then became the dominate strategy.
Table 3.
Results of Base-Case Analyses. The table depicts a visual heuristic to help identify the optimal strategy by patient age, cyst size, and patient preference for unadjusted vs. quality adjusted survival. Shading demonstrates the degree of superiority over the competing strategies. Each number represents the length of discounted years that a patient will live, on average, with each individual strategy. For instance, if an 85 year old patient has a 3cm cyst, then a pancreaticoduodenectomy (Whipple) adds a modest 0.356 years over the other strategies. However, if quality of life is desired over unadjusted survival, then the invasive surveillance strategy is superior, although it provides a minimal benefit of 0.030 years of quality adjusted life compared to the next closest competitor.
| Life Years | Quality Adjusted Life Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Cyst Size (cm) | Do Nothing | Non-Invasive | Invasive | Whipple | Do Nothing | Non-Invasive | Invasive | Whipple |
| 65 | 1 | 12.713 | 12.760 | 12.763 | 12.733 | 12.546 | 12.180 | 12.196 | 11.449 |
| 2 | 10.353 | 11.556 | 11.571 | 12.090 | 11.105 | 10.966 | 10.992 | 10.810 | |
| 3 | 9.713 | 10.099 | 10.129 | 12.090 | 9.361 | 9.497 | 9.534 | 10.810 | |
| 75 | 1 | 8.786 | 8.810 | 8.813 | 8.615 | 8.677 | 8.469 | 8.480 | 7.646 |
| 2 | 8.005 | 8.143 | 8.156 | 8.284 | 7.830 | 7.776 | 7.794 | 7.298 | |
| 3 | 4.883 | 4.960 | 4.968 | 4.969 | 6.814 | 6.941 | 6.968 | 7.298 | |
| 85 | 1 | 5.256 | 5.265 | 5.267 | 5.084 | 5.195 | 5.092 | 5.098 | 4.387 |
| 2 | 4.883 | 4.960 | 4.968 | 4.969 | 4.774 | 4.760 | 4.770 | 4.248 | |
| 3 | 4.449 | 4.598 | 4.613 | 4.969 | 4.278 | 4.366 | 4.381 | 4.248 | |
| Shading | Strength of Recommendation | ||||||||
| Strong superiority over other strategies (yields > 1 additional years of life) | |||||||||
| Modest superiority over other strategies (yields ≥ 0.3 additional years of life) | |||||||||
| Minimal superiority over other strategies (yields < 0.3 additional years of life) | |||||||||
Sensitivity Analysis
Table 4 lists the results of the sensitivity analyses for 65, 75, and 85 year old patients with a <1cm suspected IPMN. The most influential variables were the annual rate of malignant transformation of a sub-centimeter IPMN, prevalence of underlying malignant IPMN at baseline, mortality related to untreated malignant IPMN, and surgical morality related to a PD. For example, if a 65 year old patient had a rate of malignant transformation exceeding 1% per year, a pre-test likelihood of underlying malignancy exceeding 4.5%, or a perioperative mortality rate below 6.4%, then surgery became the dominant strategy. Non-invasive and invasive surveillance were nearly equivocal. However, if the mortality rate for EUS-FNA exceeded 0.01%, despite its better sensitivity and specificity, it became inferior to CT/MRI.
Table 4.
Sensitivity Analysis for a 65 year old with <1cm presumed Branch-Duct (BD) IPMN.
| Variable | Base Case Estimate | 65 year-old Threshold | 75 year-old Threshold | 85 year-old Threshold | Explanation |
|---|---|---|---|---|---|
| Annual probability of incident cancer in a <1cm BD-IMPN | 0.1% | 1% | 2.0% | 3% | If the annual rate of malignant transformation of a benign IPMN exceeds threshold then surgery is superior to surveillance |
| Baseline probability of prevalent cancer in a <1cm BD-IPMN | 1% | 4.5% | 6.0% | 7.5% | Once the prevalent rate of cancer exceeds threshold surgery is superior to surveillance |
| Annual death rate from untreated malignant IPMN | 60% | 65% | 69% | 73% | If the annual mortality rate for an untreated malignant IPMN exceeds threshold then surgery is superior to surveillance |
| Perioperative mortality with pancreaticoduodenectomy | 6.4% | 6% | 3.8% | 2.0% | When the mortality rate of a Pancreaticoduodenectomy is below threshold then a Pancreaticoduodenectomy is the superior strategy. |
| Peri-procedural mortality with EUS-FNA | 0.01% | 0.01% | 0.01% | 0.01% | If the mortality rate with EUS-FNA increases above threshold then noninvasive surveillance becomes the superior strategy over invasive surveillance. |
Clinical Nomograms
Figure 2 and Figure 3 demonstrate nomograms to assist decision-makers with selecting between competing strategies. The nomograms plot cyst size against perioperative mortality, itself a function of age and comorbidities. Figure 2 depicts the data using life years as the outcome of interest, and Figure 3 depicts the data using QALYs as the outcome. Refer to the figure legends for further details regarding nomogram interpretation.
Figure 2.
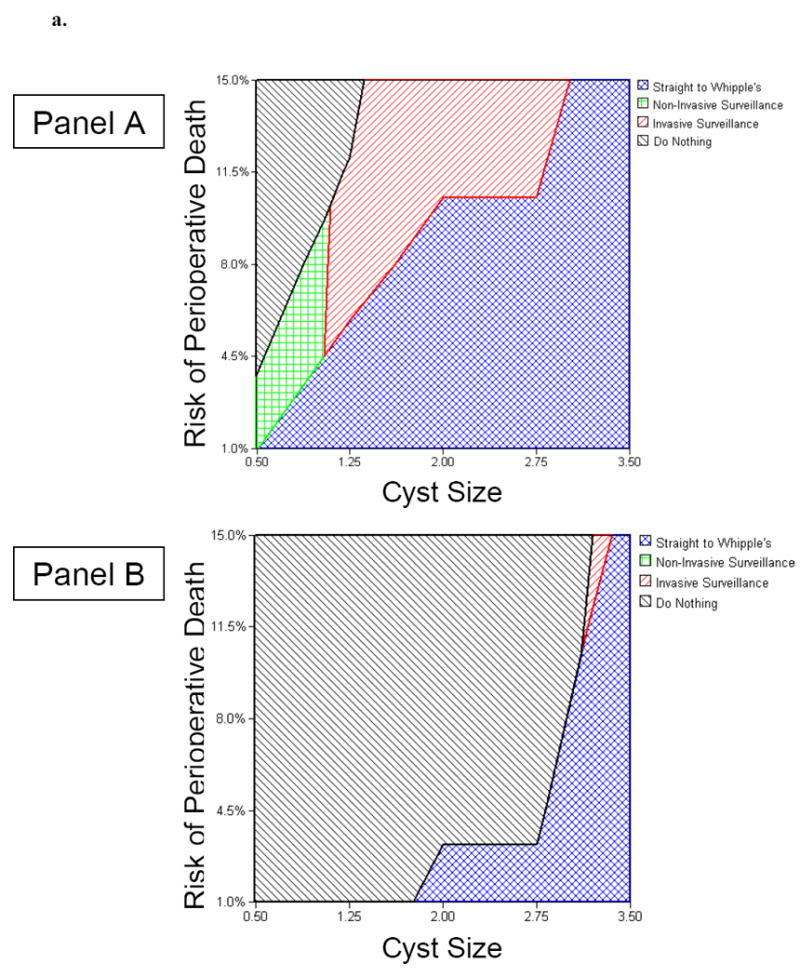
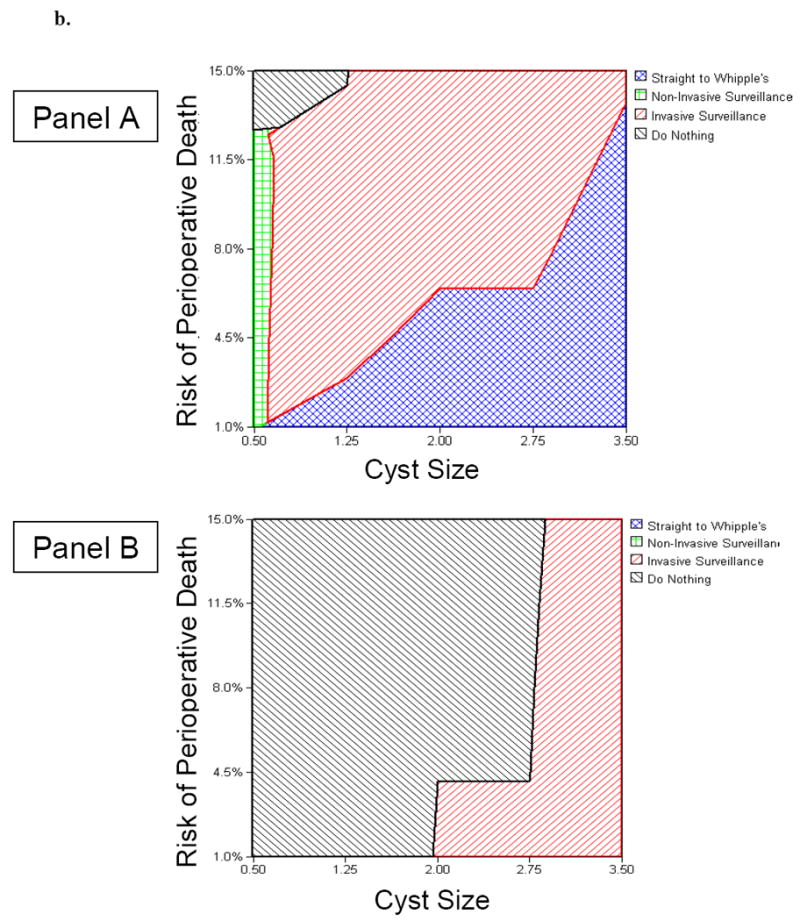
Clinical Nomograms to Guide Decision-Making in a Hypothetical 65-Year-Old Patient with Suspected BD-IPMN (Figure 2a) and a Hypothetical 85-Year-Old Patient with Suspected BD-IPMN (Figure 2b).
Panel A. Nomogram for a patient focused primarily on maximizing overall survival, independent of quality of life. Panel B. Nomogram for a patient focused on maximizing quality-adjusted survival.
Figure 2a. For example, for a 65 year old patient with a 2cm cyst and an estimated 5% risk of perioperative mortality from a Whipple operation, surgery maximizes overall survival, yet doing nothing maximizes quality-adjusted survival. However, if the cyst exceeds 3cm in size, then surgery is warranted in both instances (see text for details).
Figure 2b.For example, for an 85 year old patient with a 2cm cyst and an estimated 8% risk of perioperative mortality from a Whipple operation, surgery maximizes overall survival, yet doing nothing maximizes quality-adjusted survival. However, if perioperative mortality exceeded 13%, then surgery would never be warranted for this patient (see text for details).
Monte Carlo Simulations
Table 5 displays the results of the Monte Carlo simulations stratified by patient age, cyst size, and patient preference for unadjusted survival versus quality adjusted survival. Each analysis lists the results for a hypothetical cohort of 1000 patients, and provides the absolute number of patients for which each competing strategy maximizes outcomes. The preferred strategy mirrors the findings in Table 3.
Table 5.
Results of Monte Carlo Simulations. The table provides a visual heuristic with a similar interpretation as Table 3. For each simulation there are 1000 hypothetical patients subjected to the competing strategies. The results provide the absolute number of patients that would optimally benefit from each competing strategy, stratified by patient age, cyst size, and patient preference for unadjusted vs. quality adjusted survival. Shading demonstrates the degree of superiority over the competing strategies. For instance, for 1000 patients who are 85 years of age with a 3cm cyst, Whipple is the optimal strategy for 823 patients, invasive surveillance is optimal for 162 patients, and “do nothing” is optimal for only 15 patients.
| Life Years | Quality Adjusted Life Years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Cyst Size (cm) | Do Nothing | Non-Invasive | Invasive | Whipple | Do Nothing | Non-Invasive | Invasive | Whipple |
| 65 | 1 | 56 | 116 | 530 | 298 | 903 | 0 | 97 | 0 |
| 2 | 0 | 0 | 110 | 890 | 767 | 52 | 179 | 2 | |
| 3 | 0 | 0 | 0 | 1000 | 0 | 0 | 0 | 1000 | |
| 75 | 1 | 62 | 0 | 885 | 53 | 871 | 59 | 70 | 0 |
| 2 | 0 | 0 | 548 | 552 | 819 | 0 | 181 | 0 | |
| 3 | 0 | 0 | 98 | 902 | 0 | 0 | 104 | 896 | |
| 85 | 1 | 922 | 0 | 88 | 0 | 1000 | 0 | 0 | 0 |
| 2 | 79 | 0 | 722 | 199 | 894 | 0 | 106 | 0 | |
| 3 | 15 | 0 | 162 | 823 | 12 | 460 | 528 | 0 | |
DISCUSSION
The optimal management of pancreatic cysts remains uncertain and challenging. To date, no randomized prospective trials have been carried out for this disease. It is critical for providers and patients to have evidence-based guidance when selecting between competing management strategies to optimize individualized care. Therefore, we conducted a comprehensive, evidence-based Markov model to help inform decision making in this uncertain area.
Our model has five key findings: First, for patients primarily focused on maximizing survival, regardless of quality of life, a 2cm size threshold appears optimal for proceeding to surgery – this is smaller than the 3cm threshold supported by the Sendai guidelines. Second, for patients focused on optimizing both quantity and quality of life, either the “do nothing” or surveillance strategy appear optimal for any patient with a <3cm lesion who is between 65-75 years of age. Moreover, if quality of life is the outcome of interest, then no lesion in a patient over 85 years of age should undergo resection. Third, the optimal strategy for any given patient varies depending on surgical morbidity, age, cyst size, and whether the patient values overall survival or quality-adjusted survival – factors balanced in our clinical nomograms. Fourth, our findings emphasize that future research should evaluate three key variables which are pivotal in the understanding of this disease process: annual rate of malignant transformation of a benign IPMN; prevalence of malignant IPMN in a cystic lesion presumed to be a BD-IPMN; and natural history of malignant IPMN which does not undergo treatment. Last, given the importance of quality of life in guiding decision-making, future research should better define and validate health utilities relevant to the management of pancreatic cysts.
The Sendai guidelines serve as the template for which most providers manage this disease.12 Our model parallels these guidelines for cysts <1 cm and ≥3cm. However, despite these similarities, our model deviates from the guidelines. For lesions ≥2 cm we find that surgery is the dominant strategy for maximizing overall life expectancy (in contrast to quality-adjusted life expectancy). This suggestion of decreasing the size cutoff to ≥2cm is not a novel one, having been suggested by data from a recent retrospective study.39 In addition, our model varies from the guidelines when accounting for quality of life – a factor not explicitly acknowledged by the Sendai document. We find that surgery remains the superior strategy for maximizing quality of life in patients who are 65 and 75 years of age with ≥3cm cysts, but conclude that patients >85 years old have improved quality of life when managed with surveillance. This is likely because the poor quality of life experienced postoperatively often outweighs the minimal benefit derived from surgical resection in this population.
Our nomograms are novel tools which may allow patients and providers to identify specific strategies that optimize outcomes while accounting for cyst size and predicted surgical risk. For instance, the nomograms indicate that a 65-year-old patient with an estimated 8% surgical mortality who has a 2cm presumed BD-IPMN in the head of pancreas should choose surgery to maximize overall survival. However, if the same patient were 85 year old, then the nomogram recommends surveillance in lieu of surgery. This is similar to the management of prostate cancer in the elderly, where “watchful waiting” is often more appropriate than radical prostatectomy.5
Our study has limitations. First, as with any decision model, it is difficult to accurately capture the complexities of everyday clinical decision-making. Second, our model does not examine combinations of both EUS-FNA and CT/MRI. Since invasive surveillance with EUS+FNA currently offers the greatest sensitivity and specificity for the detection of radiologically malignant features, it is not surprising that it always dominates over the non-invasive strategy with CT or MRI. Third, while our model finds the “do nothing” strategy maximizes quality of life for patients with cysts <3cm, this must be interpreted with caution. The dominance of “do nothing” only has minimal superiority over the surveillance strategies. Therefore, as many patients and physicians may not feel comfortable “doing nothing,” our data demonstrate that surveillance is a reasonable approach and still superior to initial surgery. Fourth, this model focuses on pancreatic cysts arising in the head of the pancreas; it does not apply to cysts arising in the body or tail, or to patients with multiple cysts or symptomatic cysts. As surgical management of isolated pancreatic body or tail cysts allows for less morbid surgery, initial surgical intervention might become the favored approach in these patients. Finally, our model is limited in its ability to accurately capture all factors that drive quality of life, including patient willingness to undergo surgery and fear of underlying malignancy. These factors are difficult to reliably capture in a computerized decision analysis. The usual approach to capturing this information in decision analysis, where tenable, is to account for quality of life decrements related to fear and concern. Our model does incorporate a wide range of utilities for both the outcomes and process of care engendered by the competing strategies. For example, we account for the quality of life decrement of watchful waiting, keeping in mind that not undergoing surgery also leaves some patients with quality of life decrements.
In summary, our model further validates many of the recommendations of the Sendai guidelines. However, it also deviates from the guidelines by suggesting that a 2cm threshold may be appropriate for surgery in patients who value overall survival regardless of quality of life. For patients focused on both quantity and quality of life, the 3cm threshold appears optimal. Additionally, the model provides novel insight into decisions based on quality of life and provides a nomogram for factoring patient specific surgical risks and cyst size into the decision making process.
Acknowledgments
Grant support: Dr. Spiegel is supported by a Veteran’s Affairs Health Services Research and Development (HSR&D) Career Development Transition Award (RCD 03-179-2), and by the CURE Digestive Disease Research Center (NIH 2P30 DK 041301-17). Dr Farrell is supported by a NIH K12 Career Development Award.
Footnotes
Authors’ Role in Manuscript: Brennan Spiegel and Benjamin Weinberg were involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis, and study supervision. James Farrell and James Tomlinson were involved in analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Disclaimer: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veteran Affairs.
No conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–7. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. IPMN of the pancreas: an updated experience. Ann Surg. 2004;239:788–97. doi: 10.1097/01.sla.0000128306.90650.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tran KT, Smeenk HG, van Eijck CH, et al. Pylorus preserving pancreaticoduodenectomy versus standard Whipple procedure: a prospective, randomized, multicenter analysis of 170 patients with pancreatic and periampullary tumors. Ann Surg. 2004;240:738–45. doi: 10.1097/01.sla.0000143248.71964.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeo CJ, Abrams RA, Grochow LB, et al. Pancreaticoduodenectomy for pancreatic adenocarcinoma: postoperative adjuvant chemoradiation improves survival. Ann Surg. 1997;225:621–33. doi: 10.1097/00000658-199705000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goh BK, Tan YM, Cheow PC, et al. Cystic lesions of the pancreas: an appraisal of an aggressive resectional policy adopted at a single institution during 15 years. Am J Surg. 2006;192:148–54. doi: 10.1016/j.amjsurg.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 6.Tang RS, Weinberg B, Dawson DW, et al. Evaluation of the guidelines for management of pancreatic branch-duct IPMN. Clin Gastroenterol Hepatol. 2008;6:815–9. doi: 10.1016/j.cgh.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Kloppel G, S E, Longnecker DS, editors. Histological typing of tumors of the exocrine pancreas. Springer-Verlag: World Health Organization: International Histological Classification of Tumors; Berlin, Germany: 1996. [Google Scholar]
- 8.Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92:547–56. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 9.Irie H, Yoshimitsu K, Aibe H, et al. Natural history of pancreatic IPMT of branch duct type: follow-up study by MRCP. J Comput Assist Tomogr. 2004;28:117–22. doi: 10.1097/00004728-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto S, Horton KM, Lawler LP, et al. IPMN of the pancreas: can benign lesions be differentiated from malignant lesions with multidetector CT? Radiographics. 2005;25:1451–68. doi: 10.1148/rg.256055036. [DOI] [PubMed] [Google Scholar]
- 11.Terris B, Ponsot P, Paye F, et al. IPMT of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–7. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of IPMN and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi K, Ogawa Y, Chijiiwa K, et al. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996;171:427–31. doi: 10.1016/S0002-9610(97)89624-9. [DOI] [PubMed] [Google Scholar]
- 14.Kubo H, Chijiiwa Y, Akahoshi K, et al. IPMT of the pancreas: differential diagnosis between benign and malignant tumors by endoscopic ultrasonography. Am J Gastroenterol. 2001;96:1429–34. doi: 10.1111/j.1572-0241.2001.03794.x. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type IPMN of the pancreas. J Clin Gastroenterol. 2003;36:261–5. doi: 10.1097/00004836-200303000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Kobari M, Egawa S, Shibuya K, et al. IPMT of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999;134:1131–6. doi: 10.1001/archsurg.134.10.1131. [DOI] [PubMed] [Google Scholar]
- 17.Doi R, Fujimoto K, Wada M, et al. Surgical management of IPMT of the pancreas. Surgery. 2002;132:80–5. doi: 10.1067/msy.2002.125386. [DOI] [PubMed] [Google Scholar]
- 18.Kitagawa Y, Unger TA, Taylor S, et al. Mucus is a predictor of better prognosis and survival in patients with IPMT of the pancreas. J Gastrointest Surg. 2003;7:12–8. doi: 10.1016/S1091-255X(02)00152-X. [DOI] [PubMed] [Google Scholar]
- 19.Chari ST, Yadav D, Smyrk TC, et al. Study of recurrence after surgical resection of IPMN of the pancreas. Gastroenterology. 2002;123:1500–7. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 20.Yamaguchi T, Baba T, Ishihara T, et al. Long-term follow-up of IPMN of the pancreas with ultrasonography. Clin Gastroenterol Hepatol. 2005;3:1136–43. doi: 10.1016/s1542-3565(05)00756-1. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhari VV, Raman SS, Vuong NL, et al. Pancreatic cystic lesions: discrimination accuracy based on clinical data and high resolution CT features. J Comput Assist Tomogr. 2007;31:860–7. doi: 10.1097/RCT.0b013e318039b277. [DOI] [PubMed] [Google Scholar]
- 22.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Kawamoto S, Lawler LP, Horton KM, et al. MDCT of IPMN of the pancreas: evaluation of features predictive of invasive carcinoma. AJR Am J Roentgenol. 2006;186:687–95. doi: 10.2214/AJR.04.1820. [DOI] [PubMed] [Google Scholar]
- 24.Procacci C, Carbognin G, Biasiutti C, et al. IPMT of the pancreas: spectrum of CT and MR findings with pathologic correlation. Eur Radiol. 2001;11:1939–51. doi: 10.1007/s003300100823. [DOI] [PubMed] [Google Scholar]
- 25.Cellier C, Cuillerier E, Palazzo L, et al. IPMT of the pancreas: accuracy of preoperative computed tomography, ERCP and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998;47:42–9. doi: 10.1016/s0016-5107(98)70297-4. [DOI] [PubMed] [Google Scholar]
- 26.Sugiyama M, Atomi Y. IPMT of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998;228:685–91. doi: 10.1097/00000658-199811000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez JR, Salvia R, Crippa S, et al. Branch-duct IPMN: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–9. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raimondo M, Tachibana I, Urrutia R, et al. Invasive cancer and survival of IPMT of the pancreas. Am J Gastroenterol. 2002;97:2553–8. doi: 10.1111/j.1572-0241.2002.06022.x. [DOI] [PubMed] [Google Scholar]
- 29.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct IPMN of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–85. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegel BM, Targownik LE, Kanwal F, et al. The quality of published health economic analyses in digestive diseases: a systematic review and quantitative appraisal. Gastroenterology. 2004;127:403–11. doi: 10.1053/j.gastro.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki Y, Atomi Y, Sugiyama M, et al. Cystic neoplasm of the pancreas: a Japanese multiinstitutional study of IPMT and mucinous cystic tumor. Pancreas. 2004;28:241–6. doi: 10.1097/00006676-200404000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Schmier J, Elixhauser A, Halpern MT. Health-related quality of life evaluations of gastric and pancreatic cancer. Hepatogastroenterology. 1999;46:1998–2004. [PubMed] [Google Scholar]
- 33.Park SM, Park MH, Won JH, et al. EuroQol and survival prediction in terminal cancer patients: a multicenter prospective study in hospice-palliative care units. Support Care Cancer. 2006;14:329–33. doi: 10.1007/s00520-005-0889-1. [DOI] [PubMed] [Google Scholar]
- 34.Behrman SW, Mulloy M. Total pancreatectomy for the treatment of chronic pancreatitis: indications, outcomes, and recommendations. Am Surg. 2006;72:297–302. [PubMed] [Google Scholar]
- 35.McLeod RS, Taylor BR, O’Connor BI, et al. Quality of life, nutritional status, and gastrointestinal hormone profile following the Whipple procedure. Am J Surg. 1995;169:179–85. doi: 10.1016/s0002-9610(99)80129-9. [DOI] [PubMed] [Google Scholar]
- 36.Billings BJ, Christein JD, Harmsen WS, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9:1059–66. doi: 10.1016/j.gassur.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Scheingraber S, Scheingraber T, Brauckhoff M, et al. Comparison between a general and a disease-specific health-related quality-of-life questionnaire in patients after pancreatic surgery. J Hepatobiliary Pancreat Surg. 2005;12:290–7. doi: 10.1007/s00534-005-0973-4. [DOI] [PubMed] [Google Scholar]
- 38.Lidgren M, Wilking N, Jonsson B, et al. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–81. doi: 10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 39.Jang JY, Kim SW, Lee SE, et al. Treatment guidelines for branch duct type IPMN of the pancreas: when can we operate or observe? Ann Surg Oncol. 2008;15:199–205. doi: 10.1245/s10434-007-9603-5. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt CM, White PB, Waters JA, et al. IPMN: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–51. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 41.Hammel PPP, Palazzo L. Frequency of Lesions Leading to Erroneous Diagnosis of IPMT of the Pancreas. Gastroenterology. 2007;132:A–761. [Google Scholar]
- 42.Jang JY, Kim SW, Ahn YJ, et al. Multicenter analysis of clinicopathologic features of IPMT of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12:124–32. doi: 10.1245/ASO.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Tada M, Kawabe T, Arizumi M, et al. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265–70. doi: 10.1016/j.cgh.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 44.Zamora C, Sahel J, Cantu DG, et al. IPMT of the pancreas: report of a case series and review of the literature. Am J Gastroenterol. 2001;96:1441–7. doi: 10.1111/j.1572-0241.2001.03689.x. [DOI] [PubMed] [Google Scholar]
- 45.Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in IPMT of the pancreas. Br J Surg. 2003;90:1244–9. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 46.Salvia R, Crippa S, Falconi M, et al. Branch-duct IPMN of the pancreas: to operate or not to operate? Gut. 2007;56:1086–90. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelaez-Luna M, Chari ST, Smyrk TC, et al. Do consensus indications for resection in branch duct IPMN predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–64. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 48.Levy P, Jouannaud V, O’Toole D, et al. Natural history of IPMT of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol. 2006;4:460–8. doi: 10.1016/j.cgh.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 49.Kobayashi G, Fujita N, Noda Y, et al. Mode of progression of IPMT of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005;40:744–51. doi: 10.1007/s00535-005-1619-7. [DOI] [PubMed] [Google Scholar]
- 50.Tseng JF, Warshaw AL, Sahani DV, et al. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–9. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Angelica M, Brennan MF, Suriawinata AA, et al. IPMN of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–8. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Diener MK, Knaebel HP, Heukaufer C, et al. A systematic review and meta-analysis of pylorus-preserving versus classical pancreaticoduodenectomy for surgical treatment of periampullary and pancreatic carcinoma. Ann Surg. 2007;245:187–200. doi: 10.1097/01.sla.0000242711.74502.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wade TP, el-Ghazzawy AG, Virgo KS, et al. The Whipple resection for cancer in U.S. Department of Veterans Affairs Hospitals. Ann Surg. 1995;221:241–8. doi: 10.1097/00000658-199503000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imaizumi T, Hanyu F, Harada N, et al. Extended radical Whipple resection for cancer of the pancreatic head: operative procedure and results. Dig Surg. 1998;15:299–307. doi: 10.1159/000018642. [DOI] [PubMed] [Google Scholar]
- 55.Schafer M, Mullhaupt B, Clavien PA. Evidence-based pancreatic head resection for pancreatic cancer and chronic pancreatitis. Ann Surg. 2002;236:137–48. doi: 10.1097/00000658-200208000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226:248–57. doi: 10.1097/00000658-199709000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maire F, Hammel P, Terris B, et al. Prognosis of malignant IPMT of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–22. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive IPMN of the pancreas. Am J Surg. 2005;189:632–6. doi: 10.1016/j.amjsurg.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Lin PW, Lin YJ. Prospective randomized comparison between pylorus-preserving and standard pancreaticoduodenectomy. Br J Surg. 1999;86:603–7. doi: 10.1046/j.1365-2168.1999.01074.x. [DOI] [PubMed] [Google Scholar]
- 60.Benassai G, Mastrorilli M, Quarto G, et al. Survival after pancreaticoduodenectomy for ductal adenocarcinoma of the head of the pancreas. Chir Ital. 2000;52:263–70. [PubMed] [Google Scholar]
- 61.Geer RJ, Brennan MF. Prognostic indicators for survival after resection of pancreatic adenocarcinoma. Am J Surg. 1993;165:68–72. doi: 10.1016/s0002-9610(05)80406-4. [DOI] [PubMed] [Google Scholar]
- 62.Millikan KW, Deziel DJ, Silverstein JC, et al. Prognostic factors associated with resectable adenocarcinoma of the head of the pancreas. Am Surg. 1999;65:618–23. [PubMed] [Google Scholar]
- 63.Tsao JI, Rossi RL, Lowell JA. Pylorus-preserving pancreatoduodenectomy. Is it an adequate cancer operation. Arch Surg. 1994;129:405–12. doi: 10.1001/archsurg.1994.01420280081010. [DOI] [PubMed] [Google Scholar]
- 64.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–31. doi: 10.1097/00000658-199506000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stocken DD, Buchler MW, Dervenis C, et al. Meta-analysis of randomised adjuvant therapy trials for pancreatic cancer. Br J Cancer. 2005;92:1372–81. doi: 10.1038/sj.bjc.6602513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. Jama. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 67.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358:1576–85. doi: 10.1016/s0140-6736(01)06651-x. [DOI] [PubMed] [Google Scholar]
- 68.Glimelius B, Hoffman K, Sjoden PO, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 69.Eloubeidi MA, Gress FG, Savides TJ, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385–9. doi: 10.1016/s0016-5107(04)01714-6. [DOI] [PubMed] [Google Scholar]
- 70.Eloubeidi MA, Chen VK, Eltoum IA, et al. EUS guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–8. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 71.Bournet B, Migueres I, Delacroix M, et al. Early morbidity of EUS: 13 years’ experience at a referral center. Endoscopy. 2006;38:349–54. doi: 10.1055/s-2005-921173. [DOI] [PubMed] [Google Scholar]
- 72.O’Toole D, Palazzo L, Arotcarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–4. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 73.Cuillerier E, Cellier C, Palazzo L, et al. Outcome after surgical resection of IPMT and mucinous tumors of the pancreas. Am J Gastroenterol. 2000;95:441–5. doi: 10.1111/j.1572-0241.2000.01764.x. [DOI] [PubMed] [Google Scholar]
- 74.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–9. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.White R, D’Angelica M, Katabi N, et al. Fate of the remnant pancreas after resection of noninvasive IPMN. J Am Coll Surg. 2007;204:987–93. doi: 10.1016/j.jamcollsurg.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 76.Taouli B, Vilgrain V, Vullierme MP, et al. IPMT of the pancreas: helical CT with histopathologic correlation. Radiology. 2000;217:757–64. doi: 10.1148/radiology.217.3.r00dc24757. [DOI] [PubMed] [Google Scholar]
- 77.Soweid A, Azar C, Labban B. Endosonographic evaluation of IPMT of the pancreas. Jop. 2004;5:258–65. [PubMed] [Google Scholar]
- 78.Mackenzie R, Hollingworth W, Dixon AK. Quality of life assessments in the evaluation of magnetic resonance imaging. Qual Life Res. 1994;3:29–37. doi: 10.1007/BF00647846. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen TC, Sohn TA, Cameron JL, et al. Standard vs. radical pancreaticoduodenectomy for periampullary adenocarcinoma: a prospective, randomized trial evaluating quality of life in pancreaticoduodenectomy survivors. J Gastrointest Surg. 2003;7:1–8. doi: 10.1016/s1091-255x(02)00187-7. [DOI] [PubMed] [Google Scholar]


