Abstract
Some studies indicate that small intestinal bacterial overgrowth (SIBO), as measured by hydrogen breath tests (HBT), is more prevalent in patients with irritable bowel syndrome (IBS) vs. matched controls without IBS. Although the data are conflicting, this observation has led to the hypothesis that SIBO may be a primary cause of IBS. Yet, it remains unclear whether SIBO is truly fundamental to the pathophysiology of IBS, or is instead a mere epiphenomenon or bystander of something else altogether. We hypothesize that SIBO might be a byproduct of the disproportionate use of proton pump inhibitors (PPIs) in IBS, as follows: (1) IBS patients are more likely than controls to receive PPI therapy; (2) PPI therapy may promote varying forms of SIBO by eliminating gastric acid; and (3) existing studies linking SIBO to IBS have not adjusted for or excluded the use of PPI therapy. When linked together, these premises form the basis for a simple and testable hypothesis: the relationship between SIBO and IBS may be confounded by PPIs. Our article explores these premises, lays out the argument supporting this “PPI hypothesis,” discusses potential implications, and outlines next steps to further investigate this possibility.
SMALL INTESTINAL BACTERIAL OVERGROWTH (SIBO) AND IRRITABLE BOWEL SYNDROME (IBS)
IBS is a condition of unknown etiology that presents with a recurrent abdominal pain or discomfort along with abnormalities in stool frequency or form (1). Because the symptoms of IBS overlap with SIBO (diarrhea, constipation, bloating, gas, and pain), it has been hypothesized that many patients with IBS have underlying SIBO (2-6). In fact, some believe that the diagnosis of IBS should be questioned in patients not found to have SIBO by diagnostic testing, or in those patients failing to respond to appropriate antibiotic theory (7). This causal theory has gained traction with the publication of studies indicating that SIBO, as measured by imperfect surrogate tests (8) (e.g., glucose hydrogen breath test [GHBT], lactulose HBT, jejunal aspirate), is more prevalent in patients with IBS than matched controls. For example, Pimentel et al. reported that 84% of IBS patients are LHBT-positive compared to only 20% of healthy controls (6). Notably, other investigators have not detected a significant difference in LHBT positivity between the groups (9, 10). More recently, Posserud and colleagues found no difference in jejunal aspirate yield between IBS and control patients when adopting the 105 colony forming units (CFU) threshold, but did find that mildly increased bacterial counts (using a lower 103 CFU cut off) were more common in IBS than controls (10). Kassinen et al. found that fecal microbiota of IBS subjects (as measured by DNA fingerprinting) differed significantly from healthy subjects, although there was no reported difference in the overall bacterial count (11). The relationship between IBS and SIBO is further supported by the data that IBS patients treated with a short course of antibiotics are more likely to experience symptom improvement versus patients receiving placebo (12). Although some investigators contend that this effect is based on the eradication of SIBO in IBS, the theory remains to be definitively proven.
COULD SIBO BE AN EPIPHENOMENON OF ANOTHER UNMEASURED FACTOR IN IBS?
Although there are data to support the relationship between SIBO and IBS, the SIBO theory is potentially limited in its ability to fully explain other proposed models of IBS, such as the biopsychosocial, visceral sensitivity, inflammatory, or neurohormonal models, among others (13). For example, it remains unclear how IBS symptoms could improve with non-pharmacological interventions (14-26), yet at the same time, be a fundamentally infectious disease. The observation that a subset of IBS patients consistently benefits from the non-pharmacological therapies distinguishes it from a condition like pneumonia and suggests that a cause-and-effect disease paradigm remains elusive. In other words, it seems unlikely that antibiotics alone will provide an answer for more than a subgroup of IBS patients. The lack of a strong relationship between SIBO and IBS is also supported by the observations that an antibiotic treatment in patients with IBS can relieve symptoms in a subset of patients without SIBO (27). Moreover, there are conflicting data that the eradication of SIBO correlates with symptom relief. Thus, the beneficial effects of antibiotics in some patients may not be related to the eradication of SIBO, but instead may be from another mechanism such as an antimicrobial effect on pathogenic organisms in the gut (27). As the SIBO hypothesis does not appear to unify multiple competing hypotheses, it raises the question of whether SIBO is truly fundamental to the pathophysiologic basis of IBS, or whether SIBO is a mere bystander, or even an epiphenomenon of other processes. The lack of consistency in the data linking SIBO to IBS raises the possibility that some other factor may be operating in the background. In other words, when data fail to converge in support of a hypothesis, it is reasonable to consider whether the null hypothesis is true instead, and that variations in the data merely reflect variations in other factors extrinsic to the relationship being tested. Although the inconsistent results linking SIBO to IBS could be a consequence of varying study methodologies, different local SIBO prevalence or disparate definitions of IBS, it may also simply reflect the presence of an external risk factor for SIBO that travels along with IBS but is not, in fact, intrinsic to IBS at all. In other words, some as yet unmeasured factor might confound the relationship between IBS and SIBO.
COULD IBS BE LINKED TO SIBO THROUGH PROTON PUMP INHIBITORS (PPIs)?
One simple and prevalent variable may fit the bill: PPIs. We hypothesize that the relationship between IBS and SIBO could potentially be confounded by the use of PPIs, as follows: (1) IBS patients are more likely than controls to receive PPI therapy (2), PPI therapy may promote varying forms of SIBO by eliminating gastric acid, and (3) the existing studies linking SIBO to IBS have not adjusted for or excluded the use of PPI therapy. When linked together, these premises form the basis for a simple and testable hypothesis: the relationship between SIBO and IBS may be confounded by PPIs (Fig. 1). We explore each premise in more detail below.
Figure 1.
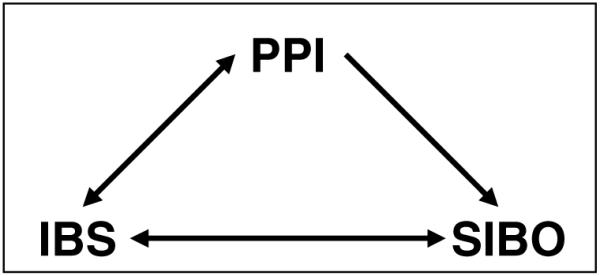
Hypothesized triangle of confounding among irritable bowel syndrome (IBS), small intestinal bacterial overgrowth (SIBO), and proton pump inhibitor (PPI) use. The observed relationship between IBS and SIBO might be explained by PPIs as PPI use is common in IBS patients, and PPIs might even induce some IBS symptoms (see text). In addition, PPIs are associated with SIBO (see text). Thus, PPIs meet the basic criterion for a confounder as they are bidirectionally associated to the purported risk factor (IBS), and unidirectionally associated to the outcome (SIBO).
IBS Patients Are More Likely Than Controls to Receive Long-Term PPI Therapy
Up to 40% of patients with IBS have comorbid gastro-esophageal reflux disease (GERD) (28), and 30–50% have overlapping dyspepsia (29, 30). Conversely, one-half of patients with GERD have comorbid IBS (28, 31). Because patients with IBS are more likely to have GERD and dyspepsia versus matched controls, they are also more likely to receive PPI therapy. Moreover, overuse of PPI therapy is common, and is often triggered by an unexplained abdominal pain. Because IBS patients have a long-standing and often difficult-to-treat abdominal pain, coupled with the fact that GERD and dyspepsia commonly overlap, chronic PPI therapy in IBS patients is extremely common in everyday clinical practice. This is supported by a recent cohort study revealing that 44% of patients with IBS were receiving a PPI (32)—a percentage that is much higher than non-IBS healthy controls, most of whom do not take regular PPI therapy. Thus, it is notable that IBS and PPI use are inexorably linked because IBS patients are highly enriched with PPI users.
PPI Therapy Can Promote SIBO
The high prevalence of PPI use in IBS would be irrelevant if PPI use were not, in fact, related to the outcome of interest—SIBO. However, PPIs are potent antisecretories, and hypochlorhydria is a risk factor for SIBO (33). The existence of gastric acid has a teleological explanation in that it serves as the primary defense against enteric infection. Thus, it comes as no surprise that removing this natural defense inevitably leads to perturbations in enteric flora—some clinically significant, some not. It has long been established that PPI therapy can alter gastric, duodenal, and intestinal bacterial profiles. For example, Thorens et al. randomized 47 patients with peptic ulcer to receive 4 wk of cimetidine versus omeprazole, and subsequently, cultured duodenal juice obtained during follow-up endoscopy (34). The authors found a higher incidence of bacterial overgrowth in the omeprazole arm (53% vs 17%). This finding was duplicated by Fried et al., who further demonstrated that PPI-related SIBO was due to both oral and colonic-type bacteria—not merely oral flora alone (35). Theisen and colleagues found that the suppression of gastric acid with omeprazole led to a high prevalence of SIBO, which, in turn, led to a markedly increased concentration of unconjugated bile acids (36). Moreover, Lewis et al. documented that omeprazole-related SIBO was associated with shorter intestinal transit times (37). These studies suggest that PPI-related SIBO could potentially lead to symptoms of IBS, such as diarrhea, as a result of an increased osmotic load from bile acids coupled with more rapid intestinal transit. It is notable that the most common side effects of PPIs include abdominal pain, bloating, flatulence, constipation, and diarrhea—symptoms that overlap with IBS and occur in up to 5% of PPI users.
Very few studies have investigated the relationship between PPI use and SIBO in IBS. Recently, Majewski and colleagues reported data on a cohort of 204 patients with IBS undergoing GHBT for SIBO, some of whom were receiving concurrent PPI therapy (32). The authors found that PPI use was higher in GHBT-positive patients (48% on a PPI) compared to GHBT-negative patients (39% on a PPI). Although this difference was not statistically significant (P = 0.2), the study was not powered to measure the impact of PPI therapy on GHBT results, nor did it measure the dose–response relationship to compare amount and duration of PPI exposure to GHBT positivity. However, the study provides initial pilot data, with a numerical trend supporting a potential relationship, and emphasizes the need to perform a larger study to overcome a potential type II error. More-over, recent data indicate that, among patients with GHBT positivity (including patients with IBS) receiving rifaximin for eradication, the re-growth of SIBO is independently predicted by the use of concurrent PPI therapy (38). Thus, not only might PPI therapy lead to SIBO in some patients with IBS, but also the recurrence of SIBO following antibiotic therapy might be accelerated in the setting of PPI therapy. In other words, so long as the risk factor for SIBO is present, the condition may recur despite temporary removal with antibiotics.
In considering this line of inquiry, it is important to distinguish the varying types of enteric infections related to PPI therapy. Skeptics might contend that PPIs are unlikely to confound the relationship between IBS and SIBO, chiefly because infectious complications are rare events. Indeed, the evidence-based reviews conclude that PPI-related bacterial overgrowth infrequently leads to clinically important disease (34, 39). However, these reviews have focused on overt infections such as Shigella, Salmonella, Yersinia, and Clostridium difficile (C. difficile) colitis—not merely HBT positivity. It is possible that a broader spectrum of PPI effects exists, akin to an “iceberg phenomenon,” with rare but observable events above the waterline and common yet covert events below the waterline (Fig. 2). According to this model, PPIs can cause rare yet clinically dramatic enteric infections (e.g., C. difficile colitis) that are detected above the waterline. These “JAMA-worthy events” (40) are truly rare (far below 1%), but are proof of principle that profound acid suppression can meaningfully alter the enteric flora in susceptible individuals. But what about the 5% of patients that develop IBS-type symptoms after initiation of PPI therapy? These patients may have underlying SIBO with resulting dyspepsia, abdominal pain, bloating, flatulence, diarrhea, and/or constipation—still a rare group, but an intriguing group because of the overlap between IBS and PPI-related symptoms. And below this group might reside a much larger population of PPI users with altered intestinal flora, but without clinically overt symptoms. This group might only be detected with HBT, or with highly sensitive tests for altered enteric flora (e.g., stool DNA fingerprinting). Given what we know about the profound impact of PPI therapy on gastric acid secretion, coupled with the knowledge that hypochlorhydria can alter the enteric flora, it is not hard to imagine that a highly tuned assay like stool DNA fingerprinting might detect minor differences in the flora between PPI users and non-PPI users.
Figure 2.
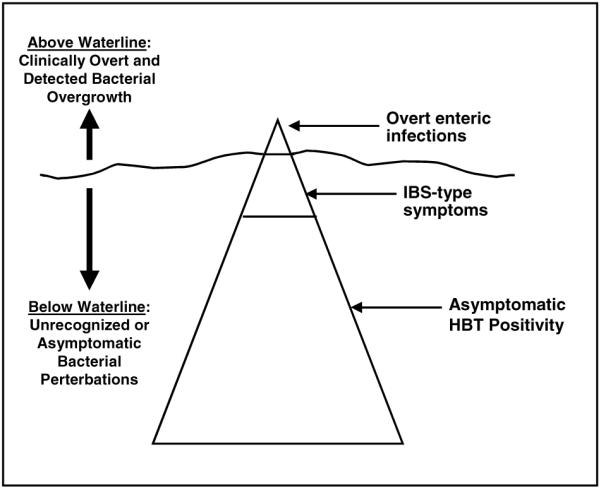
Proposed iceberg of PPI-related bacterial dysregulation. Among patients with PPI-related enteric infections, there is a small group with rare but observable events that occur above the waterline (e.g., C. difficile colitis), and a larger group with common yet covert events below the waterline. See text for details.
Studies Linking SIBO to IBS Have Not Excluded PPI Users
This line of inquiry would be moot if the existing studies linking SIBO to IBS had excluded PPI users. That is, if the linkage were found in the absence of PPI use, then it would systematically exclude PPI exposure as a confounding influence. However, the studies reporting higher rates of SIBO in IBS, including those using HBT (3-6), jejunal aspirate (9), and DNA fingerprinting (10), have not explicitly excluded or adjusted for PPI users. Moreover, none of the studies report the prevalence of PPI exposure in the IBS versus healthy control groups, making it impossible to judge whether PPI exposure could have played any role in influencing the results. This appears to be an important oversight as PPI use is highly prevalent in patients with IBS and is a known risk factor for SIBO. Yet, the published studies are meticulous about excluding other risk factors that are rare in IBS, such as cirrhosis, inflammatory bowel disease, and connective tissue disorders, among others (11). It could be argued that of all the potential confounders, short of previous antibiotic therapy, the use of PPIs should be considered among the most important potential confounders, given its high prevalence in the target population and its association with the development of SIBO.
IMPLICATIONS AND NEXT STEPS
The “PPI hypothesis” remains untested. Nonetheless, if it were true, then it would suggest that SIBO may not be fundamental to the pathophysiology of IBS, and instead may some times be a mere byproduct of treatment with PPIs. It would not indicate that PPIs cause IBS. Instead, it would suggest that PPIs might exacerbate IBS or merely alter the intestinal flora in a subclinical manner. This, in turn, could yield a “red herring” of HBT positivity, which might be falsely interpreted as causal of IBS when it is instead a mere bystander. In addition, it would not definitively prove that IBS is unrelated to SIBO, but would suggest that the studies demonstrating higher rates of SIBO in IBS versus healthy controls would need repeating with careful exclusion or adjustment for PPI status. This would also apply to randomized controlled trials of antibiotic therapy in IBS, where the benefits of active treatment might simply reflect, at least in part, a temporary reversal of PPI-related symptoms superimposed on underlying IBS.
Future research should include a prospective evaluation to measure the dose–response relationship between PPI exposure and SIBO in patients with IBS. If that were positive, the additional work might also include a randomized withdrawal study in IBS patients on PPI therapy (in the absence of concurrent acid-peptic disorders otherwise warranting PPIs). If there were a meaningful change in the bowel symptoms between patients switching to placebo versus those staying on active PPI, then it would indicate that PPIs play some role in exacerbating or propagating IBS symptoms and suggest that PPI withdrawal should be considered prior to initiating antibiotics.
Although this PPI hypothesis might seem naïve or overly simplistic, it is worth recalling the wisdom of Sir William Ockham, who surmised even in the 14th century that, among competing solutions to a problem, the solution with the fewest steps, postulates, or entities is generally preferred. In other words, as complexity rises, so does burden of proof. We believe this theory is both tenable and testable.
Acknowledgments
Financial support: Dr. Brennan M.R. Spiegel is supported by a Veteran’s Affairs Health Services Research and Development (HSR&D) Career Development Award (RCD 03–179–2), the CURE Digestive Disease Research Center (NIH 2P30 DK 041301–17), and NIH Center Grant 1 R24 AT002681-NCCAM. Dr. Lin Chang is supported by a National Institutes of Health SCOR grant (P50 DK64539) and a NIAMS grant (AR46122–01).
Footnotes
CONFLICT OF INTEREST--Guarantor of the article: Brennan Spiegel, M.D., M.S.H.S.
Potential competing interests: The opinions and assertions contained herein are the sole views of the authors and are not to be construed as official or as reflecting the views of the Department of Veteran Affairs.
REFERENCES
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Lin HC. Small intestinal bacterial overgrowth: A frame-work for understanding irritable bowel syndrome. JAMA. 2004;292:852–8. doi: 10.1001/jama.292.7.852. [DOI] [PubMed] [Google Scholar]
- 3.Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000;95:3503–6. doi: 10.1111/j.1572-0241.2000.03368.x. [DOI] [PubMed] [Google Scholar]
- 4.Lupascu A, Gabrielli M, Lauritano EC, et al. Hydrogen glucose breath test to detect small intestinal bacterial overgrowth: A prevalence case-control study in irritable bowel syndrome. Aliment Pharmacol Ther. 2005;22:1157–60. doi: 10.1111/j.1365-2036.2005.02690.x. [DOI] [PubMed] [Google Scholar]
- 5.Nucera G, Gabrielli M, Lupascu A, et al. Abnormal breath tests to lactose, fructose and sorbitol in irritable bowel syndrome may be explained by small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2005:1391–5. doi: 10.1111/j.1365-2036.2005.02493.x. [DOI] [PubMed] [Google Scholar]
- 6.Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome. A double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2003;98:412–9. doi: 10.1111/j.1572-0241.2003.07234.x. [DOI] [PubMed] [Google Scholar]
- 7.Pimentel M. Help at last: The Cedars-Sinai protocol for treating IBS. In: Trivieri L, editor. A new IBS solution. Health Point Press; Sherman Oaks, CA: pp. 69–90. [Google Scholar]
- 8.Saad R, Chey WD. The role of breath tests in clinical GI practice. Gastroenterology. 2007;133:1763–2007. doi: 10.1053/j.gastro.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 9.Walters B, Vanner SJ. Detection of bacterial overgrowth in IBS using the lactulose H2 breath test: Comparison with 14C-D-xylose and healthy controls. Am J Gastroenterol. 2005;100:1566–70. doi: 10.1111/j.1572-0241.2005.40795.x. [DOI] [PubMed] [Google Scholar]
- 10.Posserud I, Stotzer PO, Bjornsson ES, et al. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut. 2007;56:802–8. doi: 10.1136/gut.2006.108712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kassinen A, Krogius-Kurikka L, Makivuollo H, et al. The fecal microbiota of IBS patients differs significantly from that of healthy subjects. Gastroenterology. 2007;133:24–33. doi: 10.1053/j.gastro.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel M, Park S, Mirocha J, et al. The effect of a non-absorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome. Ann Intern Med. 2006;145:557–63. doi: 10.7326/0003-4819-145-8-200610170-00004. [DOI] [PubMed] [Google Scholar]
- 13.Drossman D. Treatment for bacterial overgrowth in the irritable bowel syndrome. Ann Intern Med. 2006;145:1–3. doi: 10.7326/0003-4819-145-8-200610170-00012. [DOI] [PubMed] [Google Scholar]
- 14.Drossman DA, Toner BB, Whitehead WE, et al. Cognitive-behavioral therapy versus education and desipramine versus placebo for moderate to severe functional bowel disorders. Gastroenterology. 2003;125:19–31. doi: 10.1016/s0016-5085(03)00669-3. [DOI] [PubMed] [Google Scholar]
- 15.Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consult Clin Psychol. 1994;62:576–82. doi: 10.1037//0022-006x.62.3.576. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy TM, Chalder T, McCrone P, et al. Cognitive behavioural therapy in addition to antispasmodic therapy for irritable bowel syndrome in primary care: Randomised controlled trial. Health Technol Assess. 2006;10:iii–iv. ix–x, 1–67. doi: 10.3310/hta10190. [DOI] [PubMed] [Google Scholar]
- 17.Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol. 1995;63:779–86. doi: 10.1037//0022-006x.63.5.779. [DOI] [PubMed] [Google Scholar]
- 18.Tkachuk GA, Graff LA, Martin GL, et al. Randomized controlled trial of cognitive-behavioral group therapy for irritable bowel syndrome in the medical setting. J Clin Psychol Med Settings. 2003;10:57–69. [Google Scholar]
- 19.Keefer L, Blanchard EB. The effects of relaxation response meditation on the symptoms of irritable bowel syndrome: Results of a controlled treatment study. Behav Res Ther. 2001;39:801–11. doi: 10.1016/s0005-7967(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 20.Lynch PM, Zamble E. A controlled behavioral treatment study of irritable bowel syndrome. Behav Ther. 1989;20:509–23. [Google Scholar]
- 21.Van Der Veek PP, van Rood YR, et al. Clinical trial: Short- and long-term benefit of relaxation training for irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26:943–52. doi: 10.1111/j.1365-2036.2007.03437.x. [DOI] [PubMed] [Google Scholar]
- 22.Blanchard EB, Schwarz SP, Suls JM, et al. Two controlled evaluations of multicomponent psychological treatment of irritable bowel syndrome. Behav Res Ther. 1992;30:175–89. doi: 10.1016/0005-7967(92)90141-3. [DOI] [PubMed] [Google Scholar]
- 23.Neff DF, Blanchard EB. A multi-component treatment for irritable bowel syndrome. Behav Ther. 1987;18:70–83. [Google Scholar]
- 24.Shaw G, Srivastava ED, Sadlier M, et al. Stress management for irritable bowel syndrome: A controlled trial. Digestion. 1991;50:36–42. doi: 10.1159/000200738. [DOI] [PubMed] [Google Scholar]
- 25.Guthrie E, Creed F, Dawson D, et al. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology. 1991;100:450–7. doi: 10.1016/0016-5085(91)90215-7. [DOI] [PubMed] [Google Scholar]
- 26.Spiegel BMR, Naliboff B, Mayer E, et al. The effectiveness of a model physician-patient relationship versus usual care in irritable bowel syndrome: A randomized controlled trial. Gastroenterology. 2006;130:A773. [Google Scholar]
- 27.Sharara AI, Aoun E, Abdul-Baki H, et al. A randomized double-blind placebo-controlled trial of rifaximin in patients with abdominal bloating and flatulence. Am J Gastroenterol. 2006;101:326–33. doi: 10.1111/j.1572-0241.2006.00458.x. [DOI] [PubMed] [Google Scholar]
- 28.Nastaskin I, Mehdikhani E, Conklin J, et al. Studying the overlap between IBS and GERD: A systematic review of the literature. Dig Dis Sci. 2006;51:2113–20. doi: 10.1007/s10620-006-9306-y. [DOI] [PubMed] [Google Scholar]
- 29.Locke GR, Zinsmeister AR, Fett SL, et al. Overlap of gastrointestional symptom complexes in a US community. Neurogastroenterol Motil. 2005;17:29–34. doi: 10.1111/j.1365-2982.2004.00581.x. [DOI] [PubMed] [Google Scholar]
- 30.Talley NJ, Dennis EH, Schettler-Duncan VA, et al. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–9. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 31.Nojkov B, Saad R, Adlis S, et al. Predictors of response to PPI therapy in patients with GERD: The influence of comorbid IBS and psychological disease. Aliment Pharmacol Ther. 2008;27:473–82. doi: 10.1111/j.1365-2036.2008.03596.x. [DOI] [PubMed] [Google Scholar]
- 32.Majewski M, McCallum RW. Results of small intestinal bacterial overgrowth testing in irritable bowel syndrome patients: Clinical profiles and effects of antibiotic trial. Adv Med Sci. 2007;52:139–42. [PubMed] [Google Scholar]
- 33.Williams C, McColl KE. Review article: Proton pump inhibitors and bacterial overgrowth. Aliment Pharmacol Ther. 2006;23:3–10. doi: 10.1111/j.1365-2036.2006.02707.x. [DOI] [PubMed] [Google Scholar]
- 34.Thorens J, Froehlichn F, Schwizer W, et al. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: A prospective randomised double blind study. Gut. 1996;39:54–9. doi: 10.1136/gut.39.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fried M, Siegrist H, Frei R, et al. Duodenal bacterial overgrowth during treatment in outpatients with omeprazole. Gut. 1994;35:23–6. doi: 10.1136/gut.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theisen J, Nehra D, Citron D, et al. Suppression of gastric acid secretion in patients with GERD results in gastric bacterial overgrowth and deconjugation of bile acids. J Gastrointest Surg. 2000;4:50–4. doi: 10.1016/s1091-255x(00)80032-3. [DOI] [PubMed] [Google Scholar]
- 37.Lewis SJ, Franco S, Young G, et al. Altered bowel function and duodenal bacterial overgrowth in patients treated with omeprazole. Aliment Pharmacol Ther. 1996;10:557–61. doi: 10.1046/j.1365-2036.1996.d01-506.x. [DOI] [PubMed] [Google Scholar]
- 38.Lauritano E, Gabrielli M, Scarpellini E, et al. High recurrence of small intestinal bacterial overgrowth after antibiotic treatment. DDW. 2007:AB S1203. [Google Scholar]
- 39.Laine L, Ahnen D, McClain C, et al. Review article: Potential GI effects of long-term acid suppression with PPIs. Aliment Pharmacol Ther. 2000;14:651–68. doi: 10.1046/j.1365-2036.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- 40.Dial S, Delaney JA, Barkun AN, et al. Use of gastric acid-suppressive agents and the risk of community-acquired Clostridium difficile-associated disease. JAMA. 2005;294:2989–95. doi: 10.1001/jama.294.23.2989. [DOI] [PubMed] [Google Scholar]


